Revisiting Quantification of Phenylalanine/Tyrosine Flux in the Ochronotic Pathway during Long-Term Nitisinone Treatment of Alkaptonuria
Abstract
1. Introduction
2. Materials and Methods
2.1. Procedures
2.2. Chemical Analyses
2.3. Statistical Analysis
3. Results
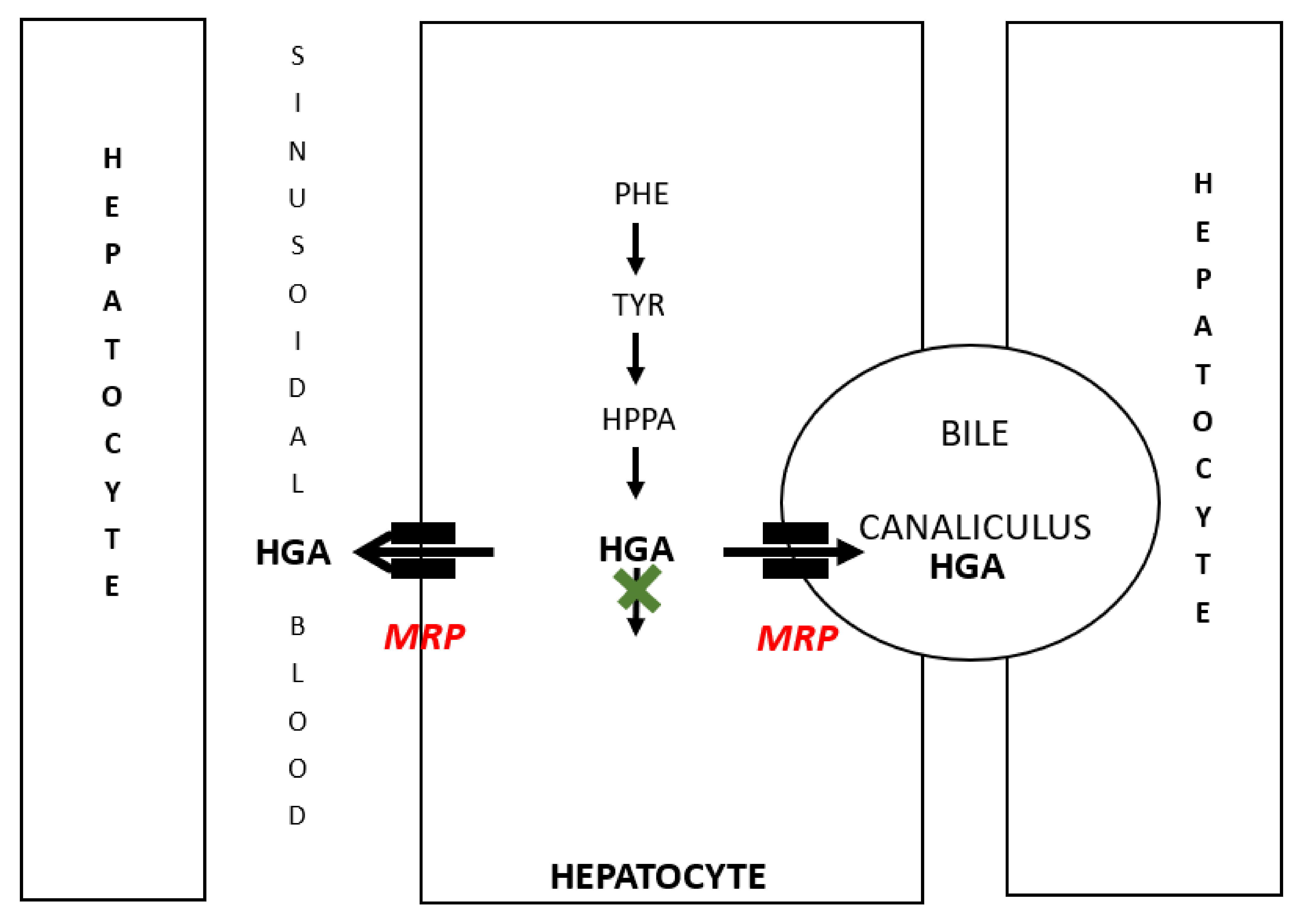
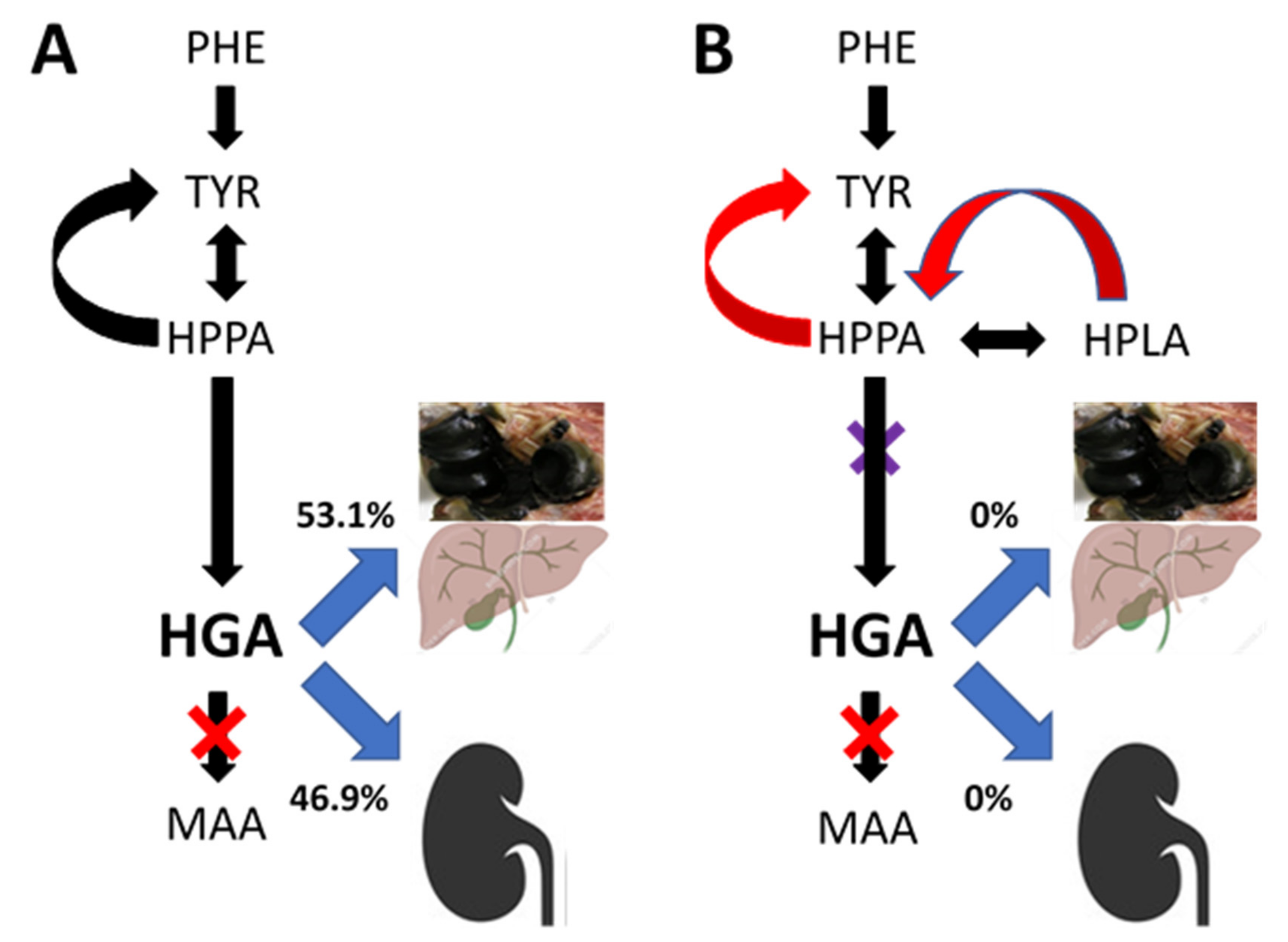
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranganath, L.R.; Norman, B.P.; Gallagher, J.A. Ochronotic pigmentation is caused by homogentisic acid and is the key event in Alkaptonuria leading to the destructive consequences of the disease—A review. J. Inherit. Metab. Dis. 2019, 42, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Virchow, R. Ein Fall von allgemeiner Ochronose der Knorpel und knorplahnlichen Theiie. Arch. Pathol. Anat. Physiol. Klin. Med. 1866, 37, 212–219. [Google Scholar]
- Albrecht, H. Ueber Ochronose. Z Heilkd/k 1902, 23, 366–378. [Google Scholar]
- O’Brien, W.M.; La Du, B.N.; Bunim, J.J. Biochemical, pathologic and clinical aspects of alcaptonuria, ochronosis and ochronotic arthropathy: Review of world literature (1584–1962). Am. J. Med. 1963, 34, 813–838. [Google Scholar] [CrossRef]
- La Du, B.N.; Zannoni, V.G.; Laster, L.; Seegmiller, J.E. The nature of the defect in tyrosine metabolism in alcaptonuria. J. Biol. Chem. 1958, 230, 251–260. [Google Scholar] [CrossRef]
- Phornphutkul, C.; Introne, W.J.; Perry, M.B.; Bernardini, I.; Murphey, M.D.; Fitzpatrick, D.L.; Anderson, P.D.; Huizing, M.; Anikster, Y.; Gerber, L.H.; et al. Natural history of Alkaptonuria. N. Engl. J. Med. 2002, 347, 2111–2121. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.S.; Milan, A.M.; Hughes, A.T.; Dutton, J.J.; Ranganath, L.R. Serum concentrations and urinary excretion of homogentisic acid and tyrosine in normal subjects. Clin. Chem. Lab. Med. 2015, 53, e81–e83. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Boyde, A.; Wilson, P.J.M.; Jarvis, J.C.; Davidson, J.S.; Hunt, J.A.; Ranganath, L.R.; Gallagher, J.A. The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Care Res. 2011, 63, 3887–3896. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Jarvis, J.C.; Gallagher, J.A. Recent advances in management of alkaptonuria. J. Clin. Pathol. 2013, 66, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Cox, T. Natural History of Alkaptonuria Revisited: Analysis based on scoring systems. J. Inherit. Metab. Dis. 2011, 34, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.J.; Keenan, C.M.; Sutherland, H.; Wilson, P.J.; Wlodarski, B.; Taylor, A.M.; Williams, D.P.; Ranganath, L.R.; Gallagher, J.A.; Jarvis, J.C. Ochronotic osteoarthropathy in a mouse model of alkaptonuria, and its inhibition by nitisinone. Ann. Rheum. Dis. 2014, 73, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.M.; Preston, A.J. Nitisinone Arrests but Does Not Reverse Ochronosis in Alkaptonuric Mice. JIMD Rep. 2015, 24, 45–50. [Google Scholar] [PubMed]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Khedr, M.; Davison, A.S.; Wilson, P.J.; Dillon, J.P.; West, E.; Gallagher, J.A. Reversal of ochronotic pigmentation in alkaptonuria following nitisinone therapy: Analysis of data from the United Kingdom National Alkaptonuria Centre. JIMD Rep. 2020, 55, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Khedr, M.; Cooper, M.S.; Hughes, A.T.; Milan, A.M.; Davison, A.S.; Norman, B.P.; Sutherland, H.; Jarvis, J.C.; Fitzgerald, R.; Markinson, L.; et al. Nitisinone causes acquired tyrosinosis in alkaptonuria. J. Inherit. Metab. Dis. 2020, 43, 1014–1023. [Google Scholar] [CrossRef]
- Ranganath, L.R.; Psarelli, E.E.; Arnoux, J.B.; Braconi, D.; Briggs, M.; Bröijersén, A.; Loftus, N.; Bygott, H.; Cox, T.F.; Davison, A.S.; et al. Suitability of Nitisinone in Alkaptonuria 2 (SONIA 2)—A randomised study on the efficacy and safety of nitisinone in alkaptonuria. Lancet Diabetes Endocrinol. 2020, 8, 762–772. [Google Scholar] [CrossRef]
- Ranganath, L.; Khedr, M.; Milan, A.; Davison, A.; Hughes, A.; Usher, J.; Taylor, S.; Loftus, N.; Daroszewska, A.; West, E.; et al. Nitisinone arrests ochronosis and decreases rate of progression of Alkaptonuria: Evaluation of the effect of nitisinone in the United Kingdom National Alkaptonuria Centre. Mol. Genet. Metab. 2018, 125, 127–134. [Google Scholar] [CrossRef]
- Milan, A.M.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Rovensky, J.; Psarelli, E.E.; Cox, T.F.; Rhodes, N.P.; Gallagher, J.A.; Ranganath, L.R. Unmasking nature: Quantification of tyrosine flux in the ochronotic pathway during nitisinone treatment of Alkaptonuria. Sci. Rep. 2019, 9, 10024. [Google Scholar] [CrossRef]
- Hughes, A.T.; Milan, A.M.; Shweihdi, E.; Gallagher, J.A.; Ranganath, L.R. Method development and validation for analysis of phenylalanine, 4-hydroxyphenyllactic acid and 4-hydroxyphenylpyruvic acid in serum and urine. JIMD Rep. 2022, 63, 341–350. [Google Scholar] [CrossRef]
- Hughes, A.T.; Milan, A.M.; Christensen, P.; Ross, G.; Davison, A.S.; Gallagher, J.A.; Dutton, J.J.; Ranganath, L.R. Urine homogentisic acid and tyrosine: Simultaneous analysis by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 963, 106–112. [Google Scholar] [CrossRef]
- Hughes, A.T.; Milan, A.M.; Davison, A.S.; Christensen, P.; Ross, G.; Gallagher, J.A.; Dutton, J.J.; Ranganath, L.R. Serum markers in alkaptonuria: Simultaneous analysis of homogentisic acid, tyrosine and nitisinone by liquid chromatography tandem mass spectrometry. Ann. Clin. Biochem. 2015, 52, 597–605. [Google Scholar] [CrossRef]
- Madsen, B.W.; Everett, A.W.; Sparrow, M.P.; Fowkes, N.D. Linear kinetic model to estimate protein synthesis after (14C)Tyrosine infusion in dogs. FEBS Lett. 1977, 79, 313–316. [Google Scholar] [CrossRef]
- Deurenberg, P.; Wolde-Gebriel, Z.; Schouten, F.J. Validity of Predicted Total Body Water and Extracellular Water Using Multifrequency Bioelectrical Impedance in an Ethiopian Population. Ann. Nutr. Metab. 1995, 39, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Dutton, J.J.; Fitzgerald, R.; Briggs, M.C.; Bygott, H.; Psarelli, E.E.; Cox, T.F.; Gallagher, J.A.; et al. Suitability Of Nitisinone In Alkaptonuria 1 (SONIA 1): An international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann. Rheum. Dis. 2016, 75, 362–367. [Google Scholar] [PubMed]
- Helliwell, T.R.; Gallagher, J.A.; Ranganath, L. Alkaptonuria—A review of surgical and autopsy pathology. Histopathology 2008, 53, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, V.G.; Lomtevas, N.; Goldfinger, S. Oxidation of homogentisic acid to ochronotic pigment in connective tissue. Biochim. Biophys. Acta 1969, 177, 94–105. [Google Scholar] [CrossRef]
- Chow, W.Y.; Norman, B.P.; Roberts, N.B.; Ranganath, L.R.; Teutloff, C.; Bittl, R.; Duer, M.J.; Gallagher, J.A.; Oschkinat, H. Pigmentation chemistry and radical-based collagen degradation in alkaptonuria and osteoarthritic cartilage. Angew. Chem. Int. Ed. 2020, 59, 11937–11942. [Google Scholar] [CrossRef] [PubMed]
- Lustberg, T.J.; Schulman, J.D.; Seegmuller, J.E. Decreased binding of (14)C-homogentisic acid induced by ascorbic acid in connective tissues of rats with experimental alkaptonuria. Nature 1970, 228, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Barshop, B.; Nyhan, W.L.; Leslie, J.; Seegmiller, J.E.; Gruber, H.; Garst, M.; Winter, S.; Michals, K.; Matalon, R. Effects of ascorbic acid in alkaptonuria: Alterations in benzoquinone acetic acid and an ontogenic effect in infancy. Pediatr. Res. 1989, 26, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Kroll, T.; Prescher, M.; Smits, S.H.J.; Schmitt, L. Structure and Function of Hepatobiliary ATP Binding Cassette Transporters. Chem. Rev. 2021, 121, 5240–5288. [Google Scholar] [CrossRef] [PubMed]
- Kullak-Ublick, G.A. Regulation of organic anion and drug transporters of the sinusoidal membrane. J. Hepatol. 1999, 31, 563–573. [Google Scholar] [CrossRef]
- Shugarts, S.; Benet, L.Z. The Role of Transporters in the Pharmacokinetics of Orally Administered Drugs. Pharm. Res. 2009, 26, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.R.; Milan, A.M.; Hughes, A.T.; Khedr, M.; Davison, A.S.; Shweihdi, E.; Norman, B.P.; Hughes, J.H.; Bygott, H.; Luangrath, E.; et al. Homogentisic acid is not only eliminated by glomerular filtration and tubular secretion but also produced in the kidney in alkaptonuria—Increase in circulating homogentisic acid in alkaptonuria with ageing is due to decreased renal clearance. J. Inherit. Metab. Dis. 2020, 43, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Bülow, C.; Rosenberg, J. Intrahepatisk galdesten hos en patient med alkaptonuri [Intrahepatic gallstones in patient with alkaptonuria]. Ugeskr Laeger 2009, 22, 2198–2199. [Google Scholar]
- Cox, T.F.; Ranganath, L.R. A quantitative assessment of alkaptonuria: Testing the reliability of two disease severity scoring systems. J. Inherit. Metab. Dis. 2011, 34, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, L.; Milan, A.; Hughes, A.; Davison, A.; Khedr, M.; Norman, B.; Bou-Gharios, G.; Gallagher, J.; Gornall, M.; Jackson, R.; et al. Characterization of changes in the tyrosine pathway by 24-hour profiling during nitisinone treatment in alkaptonuria. Mol. Genet. Metab. Rep. 2022, 30, 100846. [Google Scholar] [CrossRef]
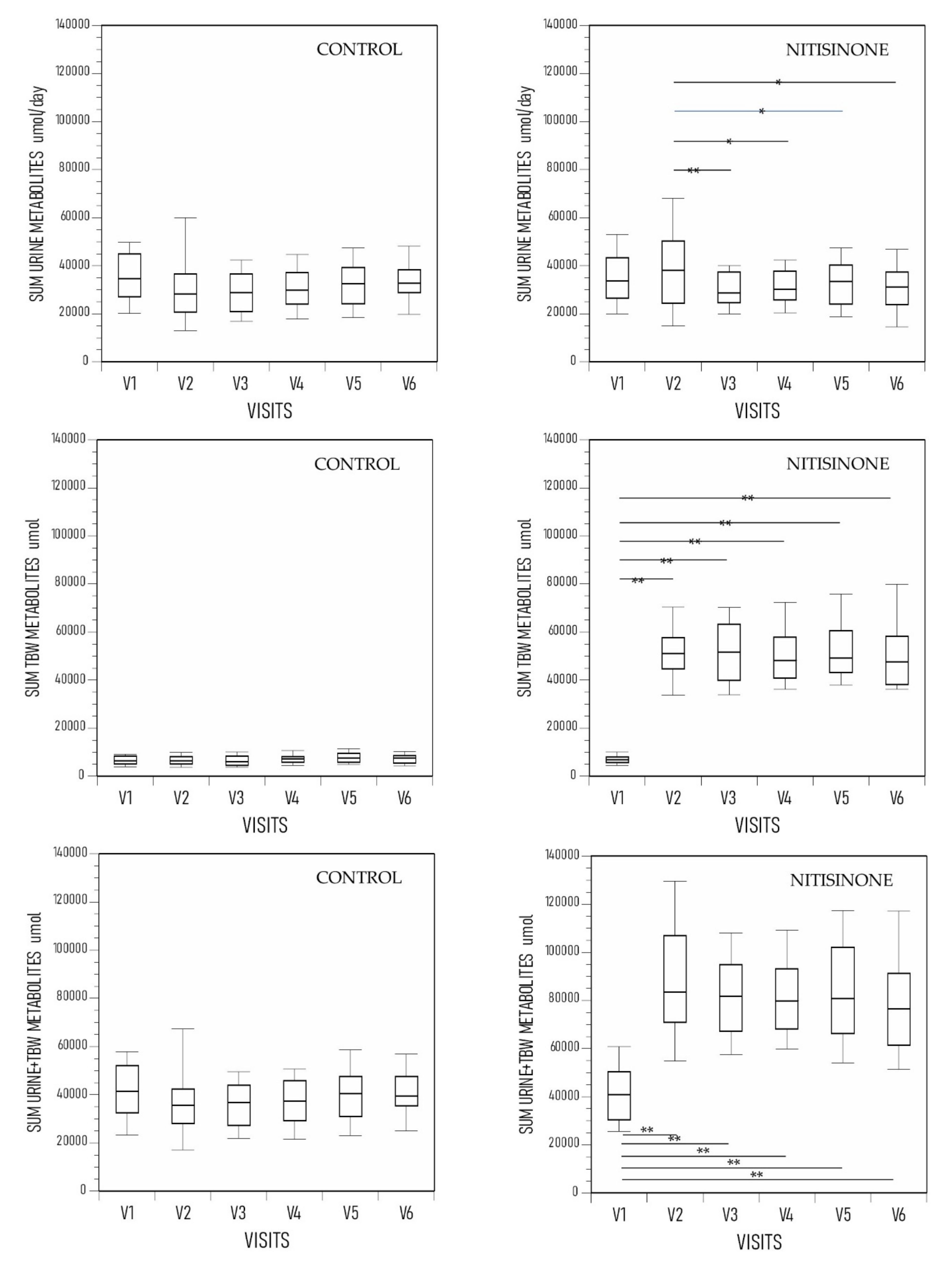
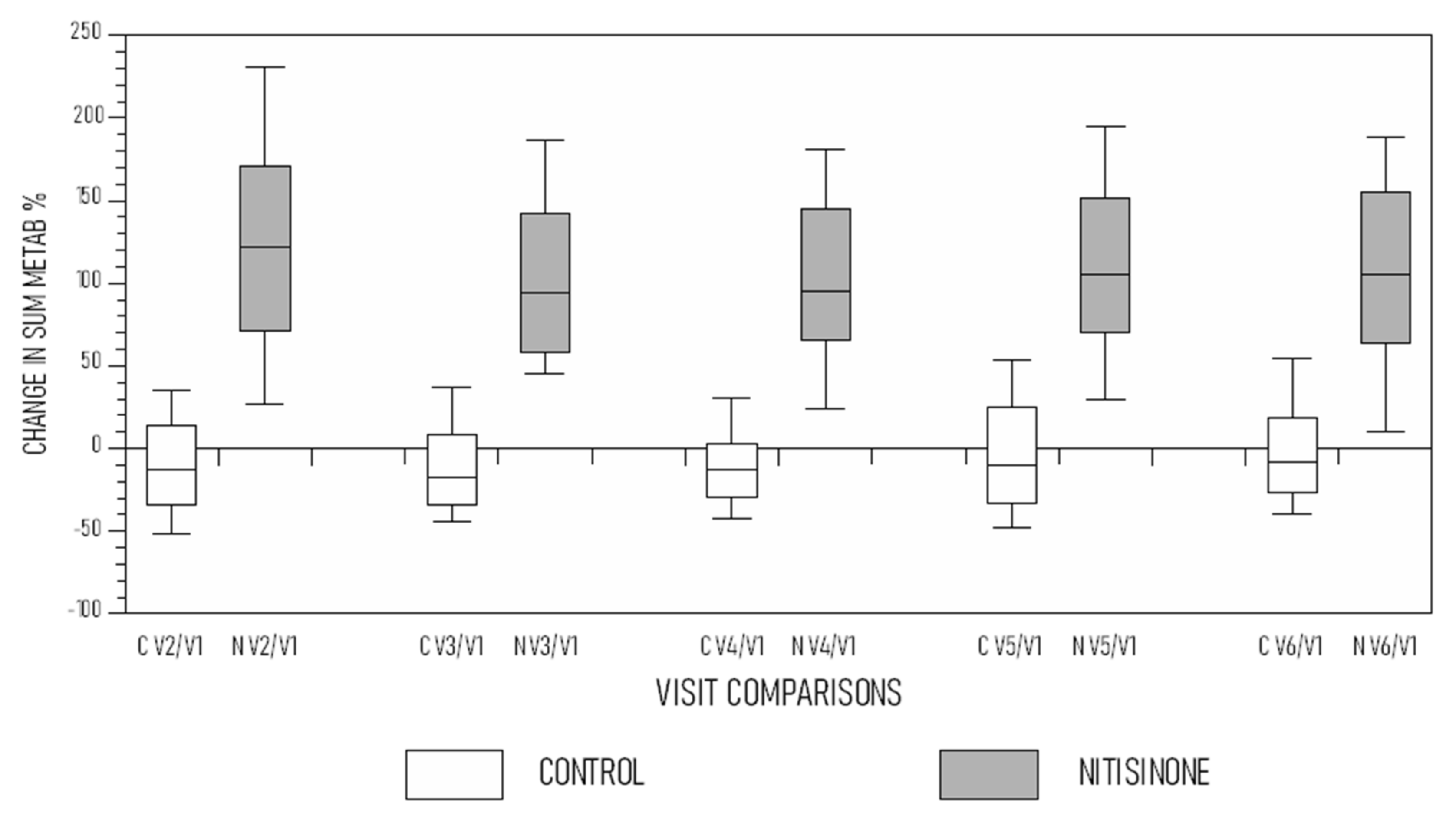
| Age (Years) | Weight (kg) | SUM METAB (µmol/L) | Change SUM METAB % | cAKUSSI | mAKUSSI | OCH SC | |
|---|---|---|---|---|---|---|---|
| Control group | |||||||
| V1 | 47.6 ± 10.1 | 74.1 ± 15.5 | 42,716 ± 15,212 | 80.4 ± 33.3 † | 54.0 ± 24.9 * | 18.0 ± 10.9 | |
| V2 | 48.1 ± 10.0 | 74.7 ± 15.6 | 38,829 ± 19,257 | −9.1 ± 36.5 | |||
| V3 | 48.8 ± 10.0 | 74.3 ± 15.6 | 36,485 ± 12,065 | −9.0 ± 37.0 | 79.2 ± 34.8 | 54.8 ± 25.6 | 17.8 ± 10.9 |
| V4 | 49.8 ± 10.1 | 76.0 ± 15.7 | 37,503 ± 10,428 | −11.8 ± 28.2 | 83.1 ± 33.9 | 57.2 ± 26.1 | 17.8 ± 10.7 |
| V5 | 50.6 ± 10.1 | 74.9 ± 14.6 | 40,699 ± 13,268 | 0.9 ± 47.3 | 90.1 ± 37.2 | 63.4 ± 30.3 | 19.4 ± 10.7 |
| V6 | 51.6 ± 10.1 | 73.8 ± 15.0 | 41,020 ± 11,724 | 0.2 ± 38.6 | 95.7 ± 36.4 | 67.0 ± 29.9 | 20.3 ± 11.2 |
| Nitisinone group | |||||||
| V1 | 49.0 ± 11.3 | 74.8 ± 14.8 | 42,268 ± 14,177 **** | 86.9 ± 34.6 | 56.6 ± 27.2 | 21.4 ± 10.7 | |
| V2 | 49.2 ± 11.3 | 75.7 ± 15.1 | 91,145 ± 29,629 | 131 ± 106 | |||
| V3 | 49.8 ± 11.3 | 78.1 ± 15.0 | 82,204 ± 20,743 | 110 ± 86 | 84.2 ± 34.0 | 57.2 ± 27.3 | 20.9 ± 11.1 |
| V4 | 50.8 ± 11.3 | 78.6 ± 15.3 | 82,343 ± 21,724 | 110 ± 85 | 91.1 ± 35.6 | 62.1 ± 28.3 | 21.1 ± 10.6 |
| V5 | 51.3 ± 11.3 | 78.6 ± 15.9 | 84,610 ± 22,801 | 111 ± 64 | 88.8 ± 38.8 | 61.2 ± 31.1 | 20.2 ± 10.8 |
| V6 | 52.6 ± 11.4 | 78.4 ± 16.1 | 80,874 ± 24,976 | 105 ± 65 | 94.8 ± 37.3 | 66.4 ± 31.2 | 20.3 ± 10.8 |
| uHGA24 | 36,361 umol or 6.1 g per day |
| All HGA equivalents pre-NIT in TBW and 24-h urine | 42,268 umol or 7.1 g per day or 46.4% total daily PHE/TYR flux |
| All HGA equivalents during NIT | 91,145 umol or 15.32 g per day |
| Additional HGA equivalents during NIT | 8.21 g or 53.6% total daily PHE/TYR flux per day |
| Additional HGA equivalents during NIT per year (365 days) | 2997 g or 3.0 kg per year |
| Additional HGA equivalents during NIT per 10 years | 30 kg per 10 years |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranganath, L.R.; Hughes, A.T.; Davison, A.S.; Khedr, M.; Imrich, R.; Rudebeck, M.; Olsson, B.; Norman, B.P.; Bou-Gharios, G.; Gallagher, J.A.; et al. Revisiting Quantification of Phenylalanine/Tyrosine Flux in the Ochronotic Pathway during Long-Term Nitisinone Treatment of Alkaptonuria. Metabolites 2022, 12, 920. https://doi.org/10.3390/metabo12100920
Ranganath LR, Hughes AT, Davison AS, Khedr M, Imrich R, Rudebeck M, Olsson B, Norman BP, Bou-Gharios G, Gallagher JA, et al. Revisiting Quantification of Phenylalanine/Tyrosine Flux in the Ochronotic Pathway during Long-Term Nitisinone Treatment of Alkaptonuria. Metabolites. 2022; 12(10):920. https://doi.org/10.3390/metabo12100920
Chicago/Turabian StyleRanganath, Lakshminarayan R., Andrew T. Hughes, Andrew S. Davison, Milad Khedr, Richard Imrich, Mattias Rudebeck, Birgitta Olsson, Brendan P. Norman, George Bou-Gharios, James A. Gallagher, and et al. 2022. "Revisiting Quantification of Phenylalanine/Tyrosine Flux in the Ochronotic Pathway during Long-Term Nitisinone Treatment of Alkaptonuria" Metabolites 12, no. 10: 920. https://doi.org/10.3390/metabo12100920
APA StyleRanganath, L. R., Hughes, A. T., Davison, A. S., Khedr, M., Imrich, R., Rudebeck, M., Olsson, B., Norman, B. P., Bou-Gharios, G., Gallagher, J. A., & Milan, A. M. (2022). Revisiting Quantification of Phenylalanine/Tyrosine Flux in the Ochronotic Pathway during Long-Term Nitisinone Treatment of Alkaptonuria. Metabolites, 12(10), 920. https://doi.org/10.3390/metabo12100920








