Noninvasive Delineation of Glioma Infiltration with Combined 7T Chemical Exchange Saturation Transfer Imaging and MR Spectroscopy: A Diagnostic Accuracy Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. MR Imaging Protocol
2.3. PET Imaging Protocol
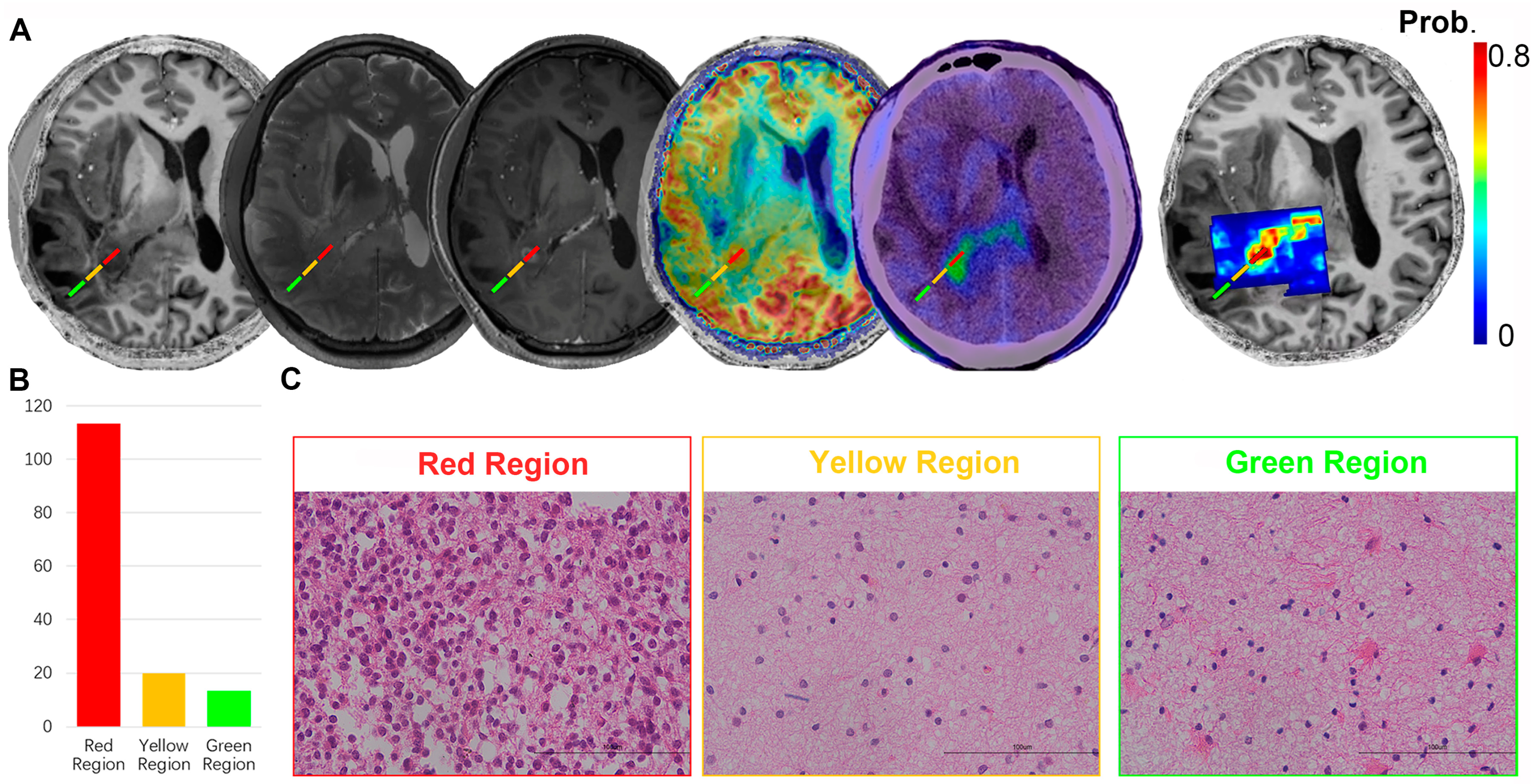
2.4. Surgery and Pathologic Evaluation
2.5. Reproducibility of Radiomics Feature Extraction
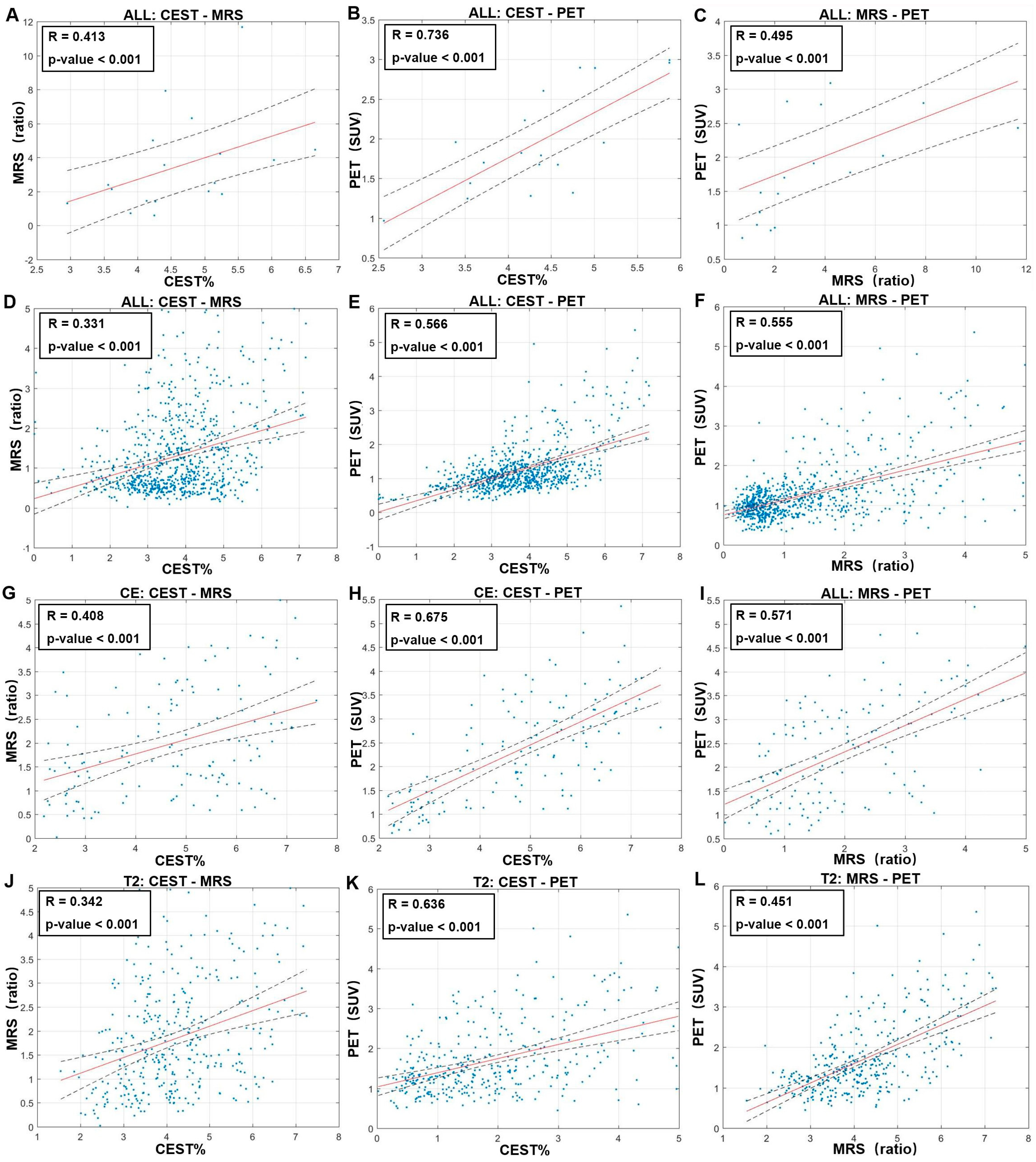
2.6. Imaging Analysis and Statistical Analysis
3. Results
3.1. Patient Demographics
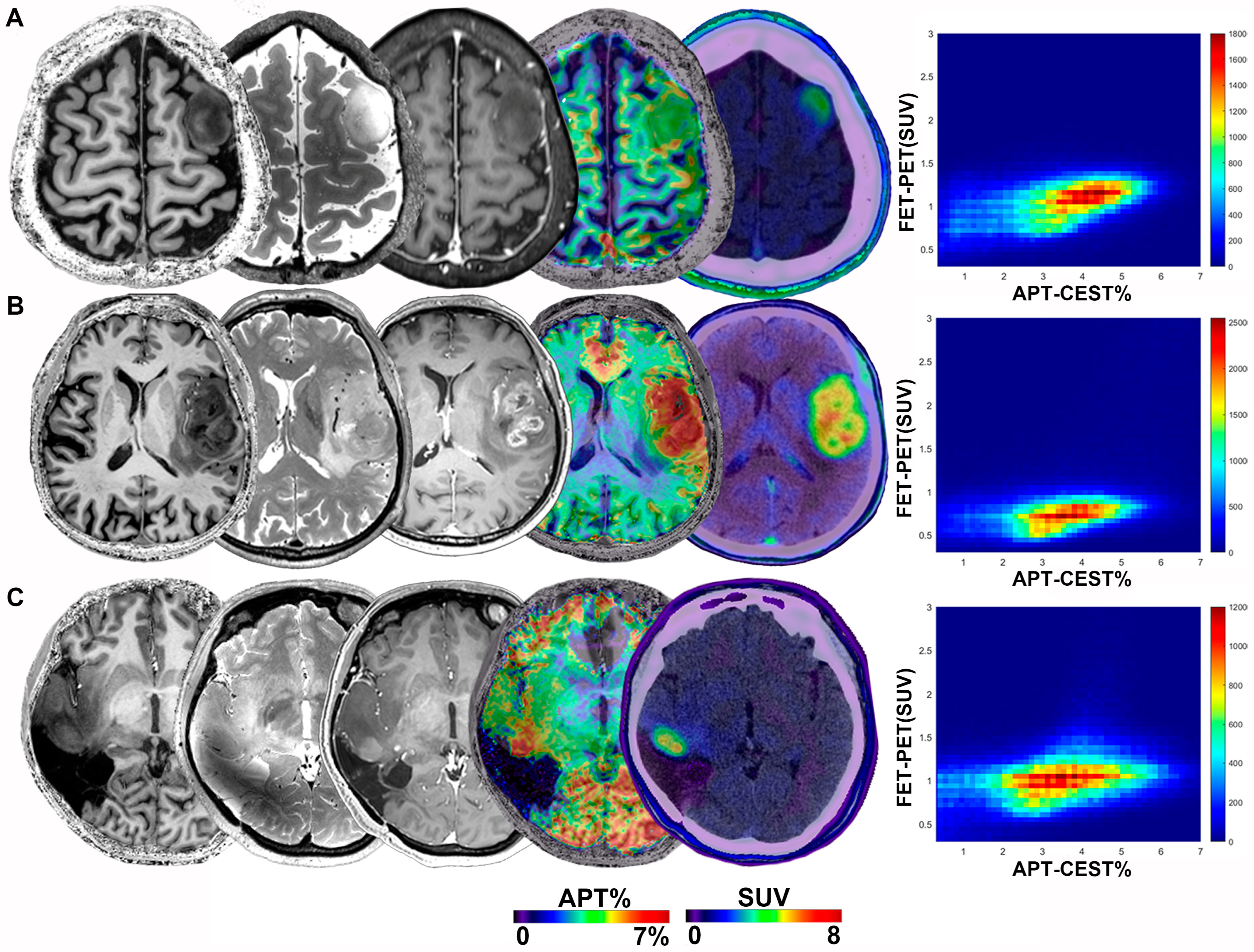
3.2. Glioma Manifestations on Imaging
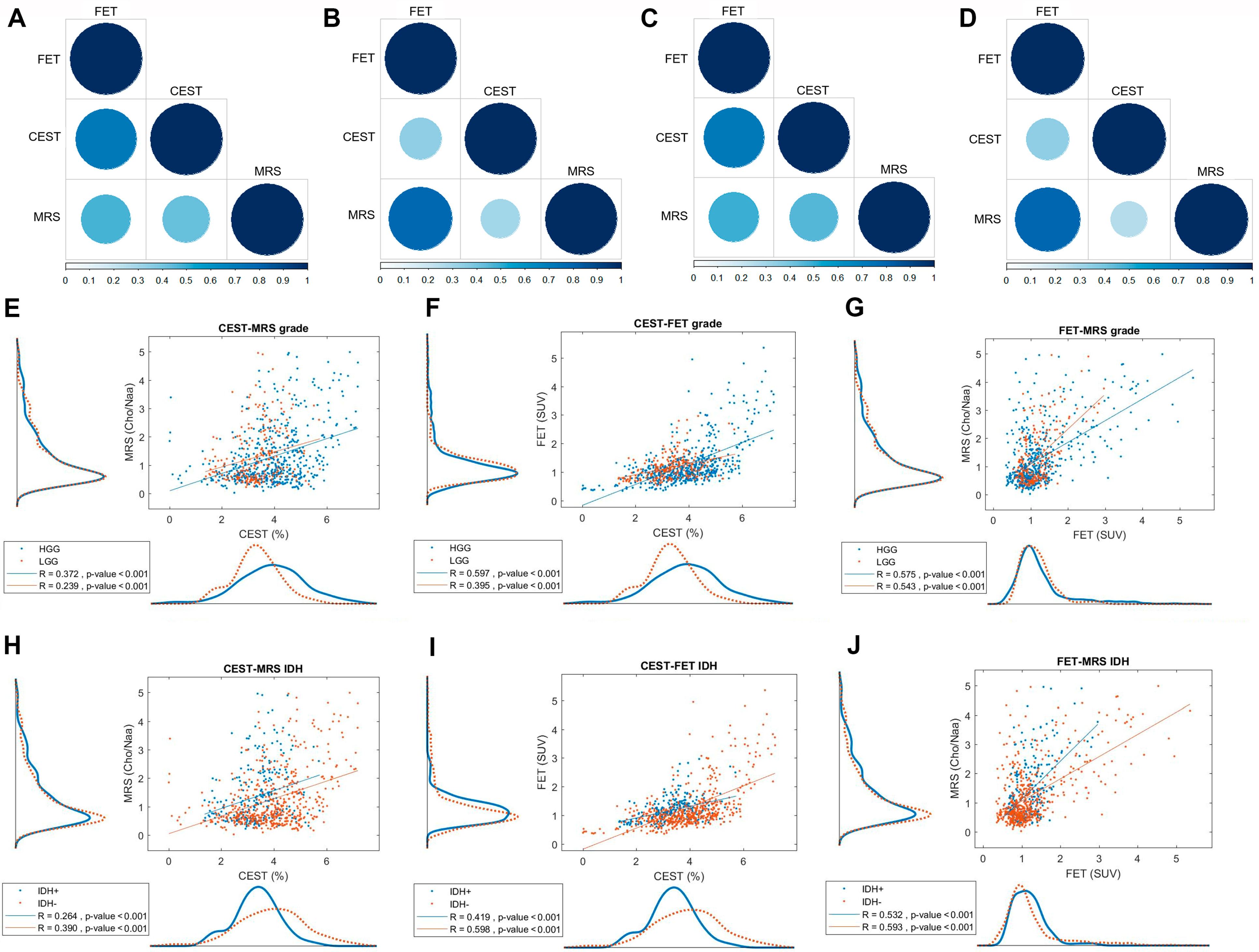
3.3. Tumor Grade and IDH Status
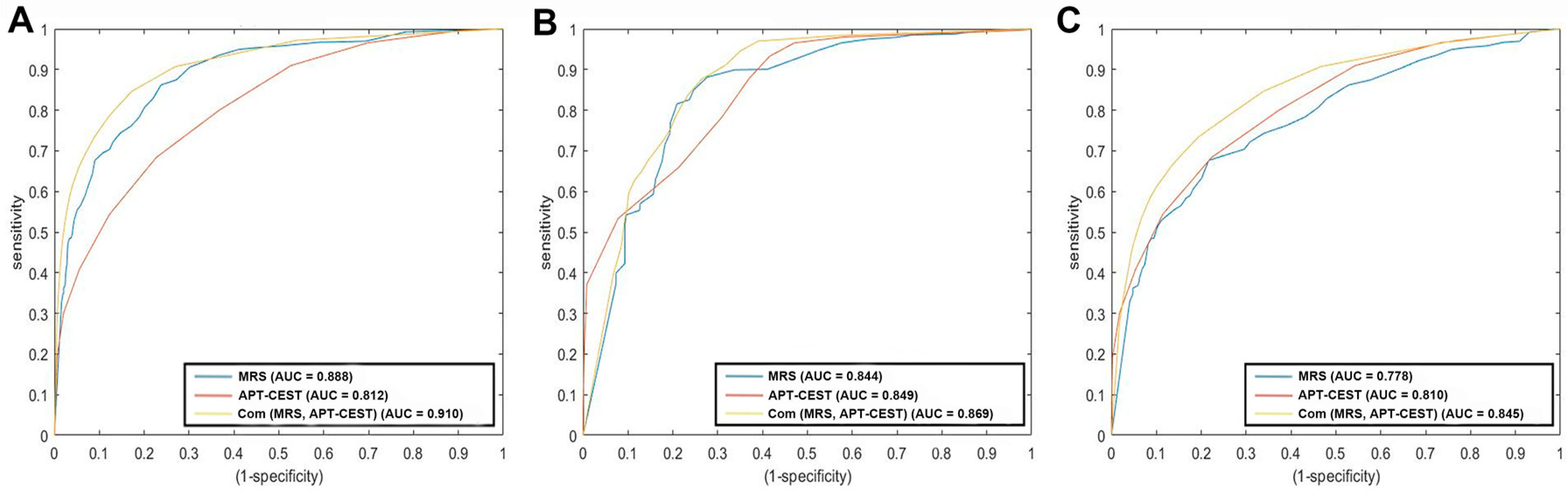
3.4. Diagnostic Accuracy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. Cbtrus statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2012-2016. Neuro-Oncology 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. Eano guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yue, Q.; Liu, Y.; Fan, D.; Fan, K.; Li, S.; Qian, J.; Han, L.; Fang, F.; Xu, F.; et al. Image-guided chemotherapy with specifically tuned blood brain barrier permeability in glioma margins. Theranostics 2018, 8, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Albert, N.L.; Weller, M.; Suchorska, B.; Galldiks, N.; Soffietti, R.; Kim, M.M.; la Fougere, C.; Pope, W.; Law, I.; Arbizu, J.; et al. Response assessment in neuro-oncology working group and european association for neuro-oncology recommendations for the clinical use of pet imaging in gliomas. Neuro Oncol. 2016, 18, 1199–1208. [Google Scholar] [CrossRef]
- Schiff, D.; Van den Bent, M.; Vogelbaum, M.A.; Wick, W.; Miller, C.R.; Taphoorn, M.; Pope, W.; Brown, P.D.; Platten, M.; Jalali, R.; et al. Recent developments and future directions in adult lower-grade gliomas: Society for neuro-oncology (sno) and european association of neuro-oncology (eano) consensus. Neuro Oncol. 2019, 21, 837–853. [Google Scholar] [CrossRef]
- Consolino, L.; Anemone, A.; Capozza, M.; Carella, A.; Irrera, P.; Corrado, A.; Dhakan, C.; Bracesco, M.; Longo, D.L. Non-invasive investigation of tumor metabolism and acidosis by mri-cest imaging. Front. Oncol. 2020, 10, 161. [Google Scholar] [CrossRef]
- Choi, Y.S.; Ahn, S.S.; Lee, S.K.; Chang, J.H.; Kang, S.G.; Kim, S.H.; Zhou, J. Amide proton transfer imaging to discriminate between low- and high-grade gliomas: Added value to apparent diffusion coefficient and relative cerebral blood volume. Eur. Radiol. 2017, 27, 3181–3189. [Google Scholar] [CrossRef]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting idh mutation status in grade ii gliomas using amide proton transfer-weighted (aptw) mri. Magn. Reson. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Jiang, S.; Eberhart, C.G.; Lim, M.; Heo, H.Y.; Zhang, Y.; Blair, L.; Wen, Z.; Holdhoff, M.; Lin, D.; Huang, P.; et al. Identifying recurrent malignant glioma after treatment using amide proton transfer-weighted mr imaging: A validation study with image-guided stereotactic biopsy. Clin. Cancer Res. 2019, 25, 552–561. [Google Scholar] [CrossRef]
- Paech, D.; Windschuh, J.; Oberhollenzer, J.; Dreher, C.; Sahm, F.; Meissner, J.E.; Goerke, S.; Schuenke, P.; Zaiss, M.; Regnery, S.; et al. Assessing the predictability of idh mutation and mgmt methylation status in glioma patients using relaxation-compensated multipool cest mri at 7.0 t. Neuro Oncol. 2018, 20, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhuang, D.X.; Yao, C.J.; Lin, C.P.; Wang, T.L.; Qin, Z.Y.; Wu, J.S. Metabolic approach for tumor delineation in glioma surgery: 3d mr spectroscopy image-guided resection. J. Neurosurg. 2016, 124, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Overcast, W.B.; Davis, K.M.; Ho, C.Y.; Hutchins, G.D.; Green, M.A.; Graner, B.D.; Veronesi, M.C. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on pet-mri imaging of malignant brain tumors. Curr. Oncol. Rep. 2021, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.; Ehses, P.; Scheffler, K. Snapshot-cest: Optimizing spiral-centric-reordered gradient echo acquisition for fast and robust 3d cest mri at 9.4 t. Nmr Biomed. 2018, 31, e3879. [Google Scholar] [CrossRef] [PubMed]
- Pauleit, D. O-(2-[18f]fluoroethyl)-l-tyrosine pet combined with mri improves the diagnostic assessment of cerebral gliomas. Brain 2005, 128, 678–687. [Google Scholar] [CrossRef]
- Maurer, G.D.; Brucker, D.P.; Stoffels, G.; Filipski, K.; Filss, C.P.; Mottaghy, F.M.; Galldiks, N.; Steinbach, J.P.; Hattingen, E.; Langen, K.J. (18)f-fet pet imaging in differentiating glioma progression from treatment-related changes: A single-center experience. J. Nucl. Med. 2020, 61, 505–511. [Google Scholar] [CrossRef]
- Unterrainer, M.; Vettermann, F.; Brendel, M.; Holzgreve, A.; Lifschitz, M.; Zahringer, M.; Suchorska, B.; Wenter, V.; Illigens, B.M.; Bartenstein, P.; et al. Towards standardization of (18)f-fet pet imaging: Do we need a consistent method of background activity assessment? EJNMMI Res. 2017, 7, 48. [Google Scholar] [CrossRef]
- Hua, T.; Zhou, W.; Zhou, Z.; Guan, Y.; Li, M. Heterogeneous parameters based on (18)f-fet pet imaging can non-invasively predict tumor grade and isocitrate dehydrogenase gene 1 mutation in untreated gliomas. Quant. Imaging Med. Surg 2021, 11, 317–327. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Z.; Wen, J.; Xie, F.; Zhu, Y.; Zhang, Z.; Xiao, J.; Chen, Y.; Li, M.; Guan, Y.; et al. A nomogram modeling (11)c-met pet/ct and clinical features in glioma helps predict idh mutation. Front. Oncol. 2020, 10, 1200. [Google Scholar] [CrossRef]
- Windschuh, J.; Zaiss, M.; Meissner, J.E.; Paech, D.; Radbruch, A.; Ladd, M.E.; Bachert, P. Correction of b1-inhomogeneities for relaxation-compensated cest imaging at 7 t. Nmr Biomed. 2015, 28, 529–537. [Google Scholar] [CrossRef]
- Schuenke, P.; Windschuh, J.; Roeloffs, V.; Ladd, M.E.; Bachert, P.; Zaiss, M. Simultaneous mapping of water shift and b1 (wasabi)-application to field-inhomogeneity correction of cest mri data. Magn. Reson. Med. 2017, 77, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Passow, J.E.; Lachance, D.H.; Molinaro, A.M.; Walsh, K.M.; Decker, P.A.; Sicotte, H.; Pekmezci, M.; Rice, T.; Kosel, M.L.; Smirnov, I.V.; et al. Glioma groups based on 1p/19q, idh, and tert promoter mutations in tumors. N. Engl. J. Med. 2015, 372, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Fu, Z.; Yang, C.; Zhang, K.; Jiang, S.; Lee, D.H.; Heo, H.Y.; Zhang, Y.; Cole, R.N.; Van Eyk, J.E.; et al. Assessing amide proton transfer (apt) mri contrast origins in 9 l gliosarcoma in the rat brain using proteomic analysis. Mol. Imaging Biol. 2015, 17, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yu, H.; Wang, X.; Lu, S.; Li, Y.; Feng, L.; Zhang, Y.; Heo, H.Y.; Lee, D.H.; Zhou, J.; et al. Molecular mri differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer mr imaging at 3 tesla. Eur. Radiol. 2016, 26, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Joo, B.; Han, K.; Ahn, S.S.; Choi, Y.S.; Chang, J.H.; Kang, S.G.; Kim, S.H.; Zhou, J.; Lee, S.K. Amide proton transfer imaging might predict survival and idh mutation status in high-grade glioma. Eur. Radiol. 2019, 29, 6643–6652. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 who classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Patel, S.H.; Poisson, L.M.; Brat, D.J.; Zhou, Y.; Cooper, L.; Snuderl, M.; Thomas, C.; Franceschi, A.M.; Griffith, B.; Flanders, A.E.; et al. T2-flair mismatch, an imaging biomarker for idh and 1p/19q status in lower-grade gliomas: A tcga/tcia project. Clin. Cancer Res. 2017, 23, 6078–6085. [Google Scholar] [CrossRef]
- Han, Z.; Chen, Q.; Zhang, L.; Mo, X.; You, J.; Chen, L.; Fang, J.; Wang, F.; Jin, Z.; Zhang, S.; et al. Radiogenomic association between the t2-flair mismatch sign and idh mutation status in adult patients with lower-grade gliomas: An updated systematic review and meta-analysis. Eur. Radiol. 2022, 32, 5339–5352. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, H.S.; Park, K.J.; Kim, S.J.; Kim, J.H.; Smith, S.A. Pre- and posttreatment glioma: Comparison of amide proton transfer imaging with mr spectroscopy for biomarkers of tumor proliferation. Radiology 2016, 278, 514–523. [Google Scholar] [CrossRef]
- Nagashima, H.; Tanaka, K.; Sasayama, T.; Irino, Y.; Sato, N.; Takeuchi, Y.; Kyotani, K.; Mukasa, A.; Mizukawa, K.; Sakata, J.; et al. Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for idh1 mutant glioma. Neuro Oncol. 2016, 18, 1559–1568. [Google Scholar] [PubMed]
- Leao, D.J.; Craig, P.G.; Godoy, L.F.; Leite, C.C.; Policeni, B. Response assessment in neuro-oncology criteria for gliomas: Practical approach using conventional and advanced techniques. AJNR Am. J. Neuroradiol. 2020, 41, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Hu, S.; Huang, F.; Wang, X.; Guo, L.; Quan, X.; Wang, S.; Zhou, J. Mr imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 2010, 51, 616–622. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, P.; Lam, W.W.; Xu, J.; Knutsson, L.; Stanisz, G.J. Magnetization transfer contrast and chemical exchange saturation transfer mri. Features and analysis of the field-dependent saturation spectrum. Neuroimage 2018, 168, 222–241. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lucas, H.C., Jr.; Shmueli, G. Research Commentary—Too Big to Fail: Large Samples and the p-Value Problem. Inf. Syst. Res. 2013, 24, 906–917. [Google Scholar] [CrossRef]
- Armocida, D.; Pesce, A.; Palmieri, M.; D’Andrea, G.; Salvati, M.; Santoro, A.; Frati, A. Periventricular zone involvement as a predictor of survival in glioblastoma patients: A single centre cohort-comparison investigation concerning a distinct clinical entity. Interdiscip. Neurosurg. 2021, 25, 101185. [Google Scholar] [CrossRef]
| All Patients | WHO II | WHO III | WHO IV | ||
|---|---|---|---|---|---|
| No. of patients | |||||
| 18 | 4 | 5 | 9 | ||
| Age (yrs) | |||||
| Mean | 49.5 | 43 | 40.4 | 57.4 | |
| Range | 28–75 | 32–65 | 28–47 | 36–75 | |
| Gender | |||||
| Male | 11 (61.11%) | 2 (50%) | 2 (40%) | 7 (77.78%) | |
| Female | 7 (38.89%) | 2 (50%) | 3 (60%) | 2 (22.23%) | |
| Position | |||||
| Frontal Lobe | 6 (33.33%) | 2 (50%) | 2 (40%) | 2 (22.22%) | |
| Parietal Lobe | 1 (5.56%) | 1 (25%) | 0 | 0 | |
| Occipital Lobe | 0 | 0 | 0 | 0 | |
| Temporal and Insular Lobe | 9 (50%) | 1 (25%) | 3 (60%) | 5 (55.56%) | |
| Others | 2 (11.11%) | 0 | 0 | 2 (22.22%) | |
| IDH status | |||||
| Wildtype | 13 (72.22%) | 0 | 5 (100%) | 8 (88.89%) | |
| Mutant | 5 (27.78%) | 4 (100%) | 0 | 1 (11.11%) | |
| Mean | Std | p-Value | ||
|---|---|---|---|---|
| APT-CEST (%) | HGG | 3.923 | 1.239 | <0.001 |
| LGG | 3.317 | 0.868 | ||
| IDH mutant | 3.358 | 0.846 | <0.001 | |
| IDH wildtype | 3.932 | 1.264 | ||
| FET-PET (SUV) | HGG | 1.272 | 0.763 | 0.037 |
| LGG | 1.161 | 0.422 | ||
| IDH mutant | 1.184 | 0.412 | 0.115 | |
| IDH wildtype | 1.266 | 0.780 | ||
| MRS (CNR) | HGG | 1.295 | 1.023 | 0.889 |
| LGG | 1.284 | 0.967 | ||
| IDH mutant | 1.360 | 1.012 | 0.183 | |
| IDH wildtype | 1.258 | 1.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Yu, Y.; Guo, Y.; Chu, Y.; Chang, J.; Hsu, Y.; Liebig, P.A.; Xiong, J.; Yu, W.; Feng, D.; et al. Noninvasive Delineation of Glioma Infiltration with Combined 7T Chemical Exchange Saturation Transfer Imaging and MR Spectroscopy: A Diagnostic Accuracy Study. Metabolites 2022, 12, 901. https://doi.org/10.3390/metabo12100901
Yuan Y, Yu Y, Guo Y, Chu Y, Chang J, Hsu Y, Liebig PA, Xiong J, Yu W, Feng D, et al. Noninvasive Delineation of Glioma Infiltration with Combined 7T Chemical Exchange Saturation Transfer Imaging and MR Spectroscopy: A Diagnostic Accuracy Study. Metabolites. 2022; 12(10):901. https://doi.org/10.3390/metabo12100901
Chicago/Turabian StyleYuan, Yifan, Yang Yu, Yu Guo, Yinghua Chu, Jun Chang, Yicheng Hsu, Patrick Alexander Liebig, Ji Xiong, Wenwen Yu, Danyang Feng, and et al. 2022. "Noninvasive Delineation of Glioma Infiltration with Combined 7T Chemical Exchange Saturation Transfer Imaging and MR Spectroscopy: A Diagnostic Accuracy Study" Metabolites 12, no. 10: 901. https://doi.org/10.3390/metabo12100901
APA StyleYuan, Y., Yu, Y., Guo, Y., Chu, Y., Chang, J., Hsu, Y., Liebig, P. A., Xiong, J., Yu, W., Feng, D., Yang, B., Chen, L., Wang, H., Yue, Q., & Mao, Y. (2022). Noninvasive Delineation of Glioma Infiltration with Combined 7T Chemical Exchange Saturation Transfer Imaging and MR Spectroscopy: A Diagnostic Accuracy Study. Metabolites, 12(10), 901. https://doi.org/10.3390/metabo12100901










