Impact of Micro- and Nanoplastics on Mitochondria
Abstract
1. Introduction
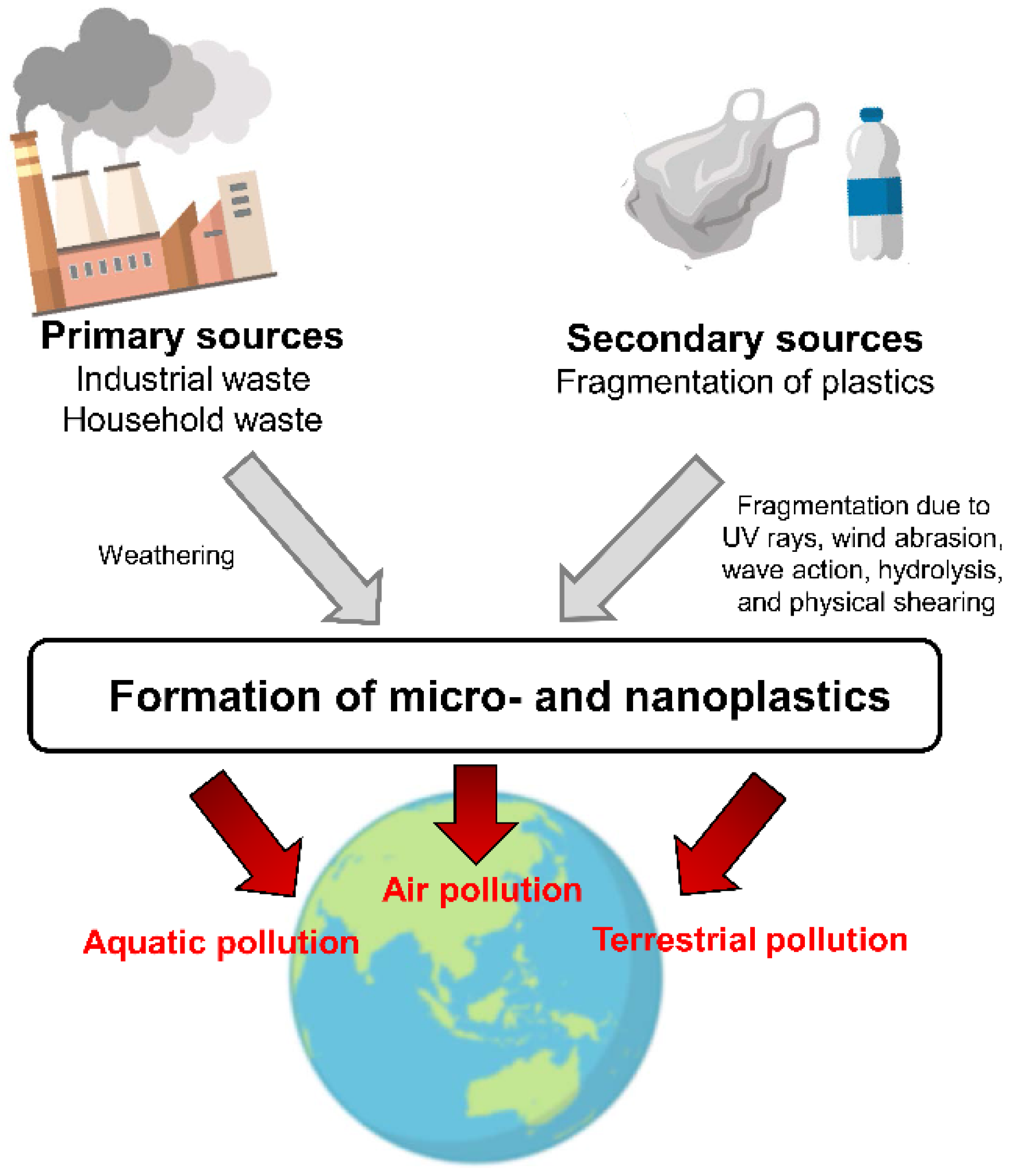
2. Environmental Contaminants: Plastics in the Environment
2.1. Micro- and Nanoplastics
2.2. Environmental Pollution with Microplastics
2.3. Microplastics in the Food Chains and Food
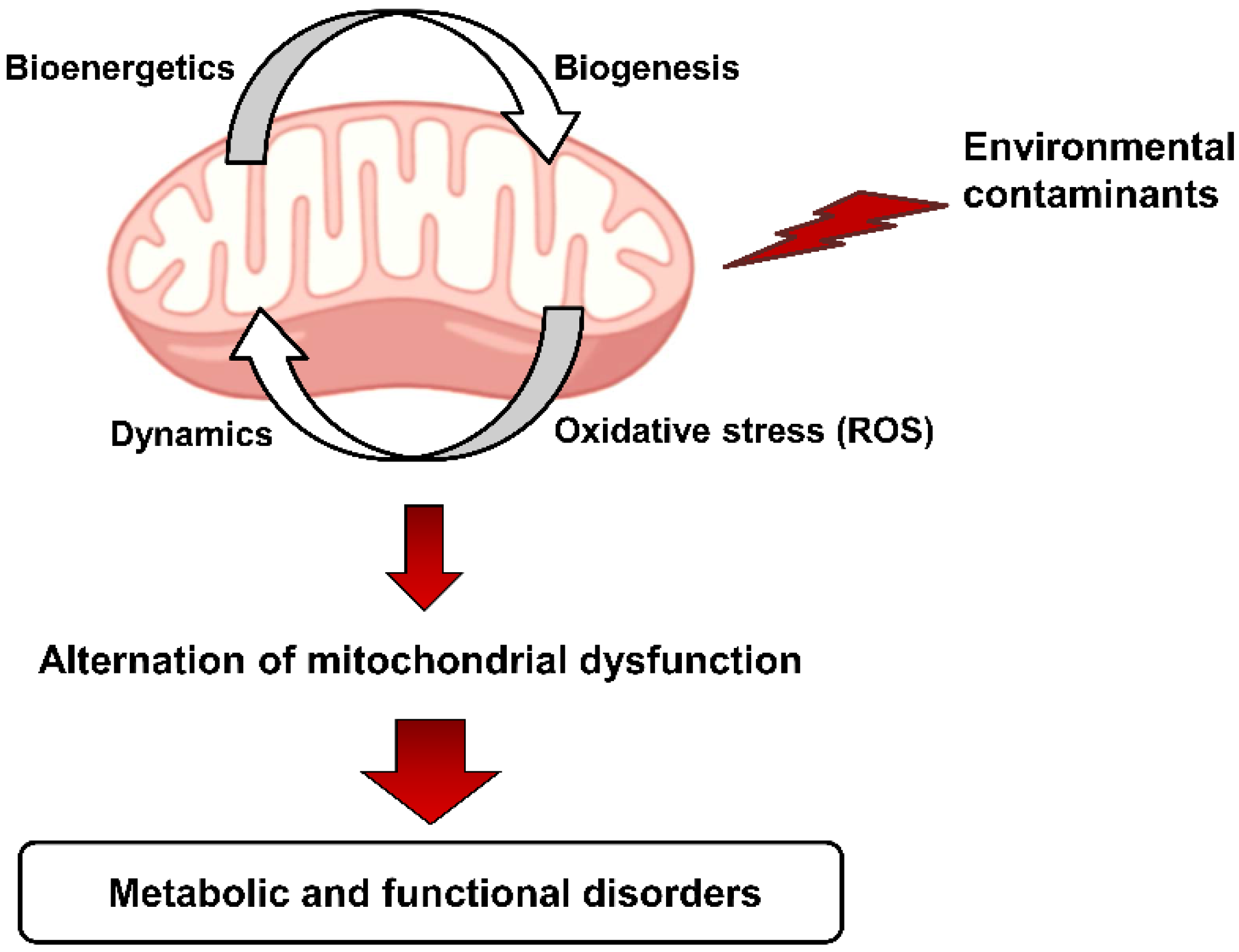
3. Toxicities of Micro- and Nanoplastics on Mitochondria
3.1. Toxicities in Human Cells
3.2. Toxicities in Other Animal Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archibald, J.M. Endosymbiosis and Eukaryotic Cell Evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.W. Mitochondrial evolution. Cold Spring Harb. Perspect. Biol. 2012, 4, a011403. [Google Scholar] [CrossRef] [PubMed]
- Kurland, C.G.; Andersson, S.G.E. Origin and evolution of the mitochondrial proteome. Microbiol. Mol. Biol. Rev. 2000, 64, 786–820. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Kozlov, A.V.; Camara, A.K.S. Mitochondria in Health and Diseases. Cells 2020, 9, 1177. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Bio. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Ann. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Coskun, P.; Wyrembak, J.; Schriner, S.E.; Chen, H.W.; Marciniack, C.; LaFerla, F.; Wallace, D.C. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim. Biophys. Acta-Gen. Subj. 2012, 1820, 553–564. [Google Scholar] [CrossRef]
- Brandon, M.; Baldi, P.; Wallace, D.C. Mitochondrial mutations in cancer. Oncogene 2006, 25, 4647–4662. [Google Scholar] [CrossRef]
- Mercer, J.R.; Cheng, K.K.; Figg, N.; Gorenne, I.; Mahmoudi, M.; Griffin, J.; Vidal-Puig, A.; Logan, A.; Murphy, M.P.; Bennett, M. DNA Damage Links Mitochondrial Dysfunction to Atherosclerosis and the Metabolic Syndrome. Circ. Res. 2010, 107, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Hansford, R.G.; Hogue, B.A.; Mildaziene, V. Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J. Bioenerg. Biomembr. 1997, 29, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Fang, P.; Mai, J.T.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial Control of Cellular Life, Stress, and Death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Seol, D.W. The role of mitochondria in apoptosis. Bmb Rep. 2008, 41, 11–22. [Google Scholar] [CrossRef]
- Ott, M.; Amunts, A.; Brown, A. Organization and Regulation of Mitochondrial Protein Synthesis. Ann. Rev. Biochem. 2016, 85, 77–101. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef]
- Caston, R.A.; Demple, B. Risky repair: DNA-protein crosslinks formed by mitochondrial base excision DNA repair enzymes acting on free radical lesions. Free Radic. Biol. Med. 2017, 107, 146–150. [Google Scholar] [CrossRef]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Filograna, R.; Mennuni, M.; Alsina, D.; Larsson, N.G. Mitochondrial DNA copy number in human disease: The more the better? FEBS Lett. 2021, 595, 976–1002. [Google Scholar] [CrossRef]
- Duarte-Hospital, C.; Tete, A.; Brial, F.; Benoit, L.; Koual, M.; Tomkiewicz, C.; Kim, M.J.; Blanc, E.B.; Coumoul, X.; Bortoli, S. Mitochondrial Dysfunction as a Hallmark of Environmental Injury. Cells 2022, 11, 110. [Google Scholar] [CrossRef]
- Peters, A.; Nawrot, T.S.; Baccarelli, A.A. Hallmarks of environmental insults. Cell 2021, 184, 1455–1468. [Google Scholar] [CrossRef] [PubMed]
- Roubicek, D.A.; de Souza-Pinto, N.C. Mitochondria and mitochondrial DNA as relevant targets for environmental contaminants. Toxicology 2017, 391, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Dreier, D.A.; Mello, D.F.; Meyer, J.N.; Martyniuk, C.J. Linking Mitochondrial Dysfunction to Organismal and Population Health in the Context of Environmental Pollutants: Progress and Considerations for Mitochondrial Adverse Outcome Pathways. Environ. Toxicol. Chem. 2019, 38, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Parsai, K.; Raghuwanshi, P.; Ali, S.A.; Tiwari, V.; Bhargava, A.; Mishra, P.K. Emerging role of mitochondria in airborne particulate matter-induced immunotoxicity. Environ. Pollut. 2021, 270, 116242. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. T R Soc. B 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- PlasticsEurope. Annual Production of Plastics Worldwide from 1950 to 2020 (in Million Metric Tons). Statista 2021. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 23 August 2022).
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of benzo[a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Schirinzi, G.F.; Perez-Pomeda, I.; Sanchis, J.; Rossini, C.; Farre, M.; Barcelo, D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environ. Res. 2017, 159, 579–587. [Google Scholar] [CrossRef]
- Whitacre, D.M. Preface. In Reviews of Environmental Contamination and Toxicology Volume 228; Springer: Cham, Switzerland, 2014. [Google Scholar] [CrossRef]
- Lebordais, M.; Gutierrez-Villagomez, J.M.; Gigault, J.; Baudrimont, M.; Langlois, V.S. Molecular impacts of dietary exposure to nanoplastics combined with arsenic in Canadian oysters (Crassostrea virginica) and bioaccumulation comparison with Caribbean oysters (Isognomon alatus). Chemosphere 2021, 277, 130331. [Google Scholar] [CrossRef]
- Ricciardi, M.; Pironti, C.; Motta, O.; Miele, Y.; Proto, A.; Montano, L. Microplastics in the Aquatic Environment: Occurrence, Persistence, Analysis, and Human Exposure. Water 2021, 13, 973. [Google Scholar] [CrossRef]
- Matthews, S.; Mai, L.; Jeong, C.B.; Lee, J.S.; Zeng, E.Y.; Xu, E.G. Key mechanisms of micro- and nanoplastic (MNP) toxicity across taxonomic groups. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 247, 109056. [Google Scholar] [CrossRef] [PubMed]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful effects of the microplastic pollution on animal health: A literature review. PeerJ 2022, 10, e13503. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.M.; Arneborg, L.; Brostrom, G.; Almroth, B.C.; Gipperth, L.; Hassellov, M. The unaccountability case of plastic pellet pollution. Mar. Pollut. Bull. 2018, 129, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Lambert, S.; Sinclair, C.; Boxall, A. Occurrence, degradation, and effect of polymer-based materials in the environment. Rev. Environ. Contam. Toxicol. 2014, 227, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Global concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shim, W.J.; Kwon, J.H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Enders, K.; Lenz, R.; Stedmon, C.A.; Nielsen, T.G. Abundance, size and polymer composition of marine microplastics ≥10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar. Pollut. Bull. 2015, 100, 70–81. [Google Scholar] [CrossRef]
- Llorca, M.; Farre, M. Current Insights into Potential Effects of Micro-Nanoplastics on Human Health by in-vitro Tests. Front. Toxicol 2021, 3, 752140. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Erni-Cassola, G.; Zadjelovic, V.; Gibson, M.I.; Christie-Oleza, J.A. Distribution of plastic polymer types in the marine environment; A meta-analysis. J. Hazard. Mater. 2019, 369, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Sarijan, S.; Azman, S.; Said, M.I.M.; Jamal, M.H. Microplastics in freshwater ecosystems: A recent review of occurrence, analysis, potential impacts, and research needs. Environ. Sci. Pollut. R 2021, 28, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 1–22. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, X.H.; Fang, T.; Xu, P.; Zhu, L.X.; Li, D.J. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Vega, J.M.; Quej, V.K.; Chi, J.D.; del Cid, L.S.; Chi, C.; Segura, G.E.; Gertsen, H.; Salanki, T.; van der Ploeg, M.; et al. Field evidence for transfer of plastic debris along a terrestrial food chain. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Beriot, N.; Peek, J.; Zornoza, R.; Geissen, V.; Huerta Lwanga, E. Low density-microplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Sci. Total Environ. 2021, 755, 142653. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. Int. 2018, 25, 36046–36063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A Review of Microplastics in Table Salt, Drinking Water, and Air: Direct Human Exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef]
- Sivagami, M.; Selvambigai, M.; Devan, U.; Velangani, A.A.J.; Karmegam, N.; Biruntha, M.; Arun, A.; Kim, W.; Govarthanan, M.; Kumar, P. Extraction of microplastics from commonly used sea salts in India and their toxicological evaluation. Chemosphere 2021, 263, 128181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, S.; Duan, Z.; Wang, L. Pulmonary toxicology assessment of polyethylene terephthalate nanoplastic particles in vitro. Environ. Int 2022, 162, 107177. [Google Scholar] [CrossRef] [PubMed]
- Halimu, G.; Zhang, Q.R.; Liu, L.; Zhang, Z.C.; Wang, X.J.; Gu, W.; Zhang, B.W.; Dai, Y.M.; Zhang, H.W.; Zhang, C.G.; et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, H.; Wang, C.; Su, X.L.; Song, Y.; Wu, P.; Yang, Z.; Wong, M.H.; Cai, Z.; Zheng, C. Metabolomics Reveal Nanoplastic-Induced Mitochondrial Damage in Human Liver and Lung Cells. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Shen, R.; Yang, K.R.; Cheng, X.; Guo, C.L.; Xing, X.Q.; Sun, H.N.; Liu, D.S.; Liu, X.W.; Wang, D.G. Accumulation of polystyrene microplastics induces liver fibrosis by activating cGAS/STING pathway. Environ. Pollut. 2022, 300, 118986. [Google Scholar] [CrossRef]
- Wu, B.; Wu, X.M.; Liu, S.; Wang, Z.Z.; Chen, L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, J.; Ning, B.; Fan, L.; Sun, T.; Fang, Y.; Wu, J.; Li, S.; Duan, C.; Zhang, Y.; et al. Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere 2020, 254, 126788. [Google Scholar] [CrossRef]
- Wang, Y.L.; Lee, Y.H.; Hsu, Y.H.; Chiu, I.J.; Huang, C.C.; Huang, C.C.; Chia, Z.C.; Lee, C.P.; Lin, Y.F.; Chiu, H.W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 2021, 129, 57003. [Google Scholar] [CrossRef]
- Florance, I.; Chandrasekaran, N.; Gopinath, P.M.; Mukherjee, A. Exposure to polystyrene nanoplastics impairs lipid metabolism in human and murine macrophages in vitro. Ecotoxicol. Environ. Saf. 2022, 238, 113612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, J.; Huang, Q.; Xie, Y.; Wu, R.H.; Zhong, J.Y.; Deng, H.P. Multi-omics analysis reveals size-dependent toxicity and vascular endothelial cell injury induced by microplastic exposure in vivo and in vitro. Environ. Sci.-Nano. 2022, 9, 663–683. [Google Scholar] [CrossRef]
- Tang, Q.S.; Li, T.W.; Chen, K.Z.; Deng, X.Y.; Zhang, Q.; Tang, H.L.; Shi, Z.F.; Zhu, T.M.; Zhu, J.H. PS-NPs Induced Neurotoxic Effects in SHSY-5Y Cells via Autophagy Activation and Mitochondrial Dysfunction. Brain Sci. 2022, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Alavehzadeh, A.; Ramezani, M.; Pourahmad, J. Differences in sensitivity of human lymphocytes and fish lymphocytes to polyvinyl chloride microplastic toxicity. Toxicol. Ind. Health 2022, 38, 100–111. [Google Scholar] [CrossRef]
- Liu, T.; Hou, B.L.; Wang, Z.P.; Yang, Y.L. Polystyrene microplastics induce mitochondrial damage in mouse GC-2 cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, B.Y.; Zhang, B.W.; Ye, Y.Y.; Jiang, W. Polystyrene micro(nano)plastics damage the organelles of RBL-2H3 cells and promote MOAP-1 to induce apoptosis. J. Hazard. Mater. 2022, 438, 129550. [Google Scholar] [CrossRef]
- Li, Y.Q.; Xu, M.K.; Zhang, Z.C.; Halimu, G.; Li, Y.Q.; Li, Y.S.; Gu, W.; Zhang, B.W.; Wang, X.J.; Rocha-Santos, T.A.P. In vitro study on the toxicity of nanoplastics with different charges to murine splenic lymphocytes. J. Hazard. Mater. 2022, 424, 127508. [Google Scholar] [CrossRef]
- Merkley, S.D.; Moss, H.C.; Goodfellow, S.M.; Ling, C.L.; Meyer-Hagen, J.L.; Weaver, J.; Campen, M.J.; Castillo, E.F. Polystyrene microplastics induce an immunometabolic active state in macrophages. Cell Biol. Toxicol. 2022, 38, 31–41. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, X.; Qi, X.; Liu, X.; Zhang, Y.; Qiao, S.; Lin, H. Di-(2-Ethylhexyl) Phthalate and Microplastics Induced Neuronal Apoptosis through the PI3K/AKT Pathway and Mitochondrial Dysfunction. J. Agric. Food Chem. 2022. [Google Scholar] [CrossRef]
- Liang, B.X.; Huang, Y.J.; Zhong, Y.Z.; Li, Z.M.; Ye, R.Y.; Wang, B.; Zhang, B.L.; Meng, H.; Lin, X.; Du, J.X.; et al. Brain single-nucleus transcriptomics highlights that polystyrene nanoplastics potentially induce Parkinson’s disease-like neurodegeneration by causing energy metabolism disorders in mice. J. Hazard. Mater. 2022, 430, 128459. [Google Scholar] [CrossRef]
- Kantha, P.; Liu, S.T.; Horng, J.L.; Lin, L.Y. Acute exposure to polystyrene nanoplastics impairs skin cells and ion regulation in zebrafish embryos. Aquat. Toxicol. 2022, 248, 106203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Chen, C.X.; Li, M.T.; Ke, J.; Huang, Y.C.; Bian, Y.F.; Guo, S.F.; Wu, Y.; Han, Y.; Liu, M.Y. Neurodevelopmental Toxicity of Polystyrene Nanoplastics in Caenorhabditis elegans and the Regulating Effect of Presenilin. ACS Omega 2020, 5, 33170–33177. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Wei, Y.Z.; Sowers, J.R. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008, 102, 401–414. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2007; p. xxxvi. 851p. [Google Scholar]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Mailloux, R.J.; McBride, S.L.; Harper, M.E. Unearthing the secrets of mitochondrial ROS and glutathione in bioenergetics. Trends Biochem. Sci. 2013, 38, 592–602. [Google Scholar] [CrossRef]
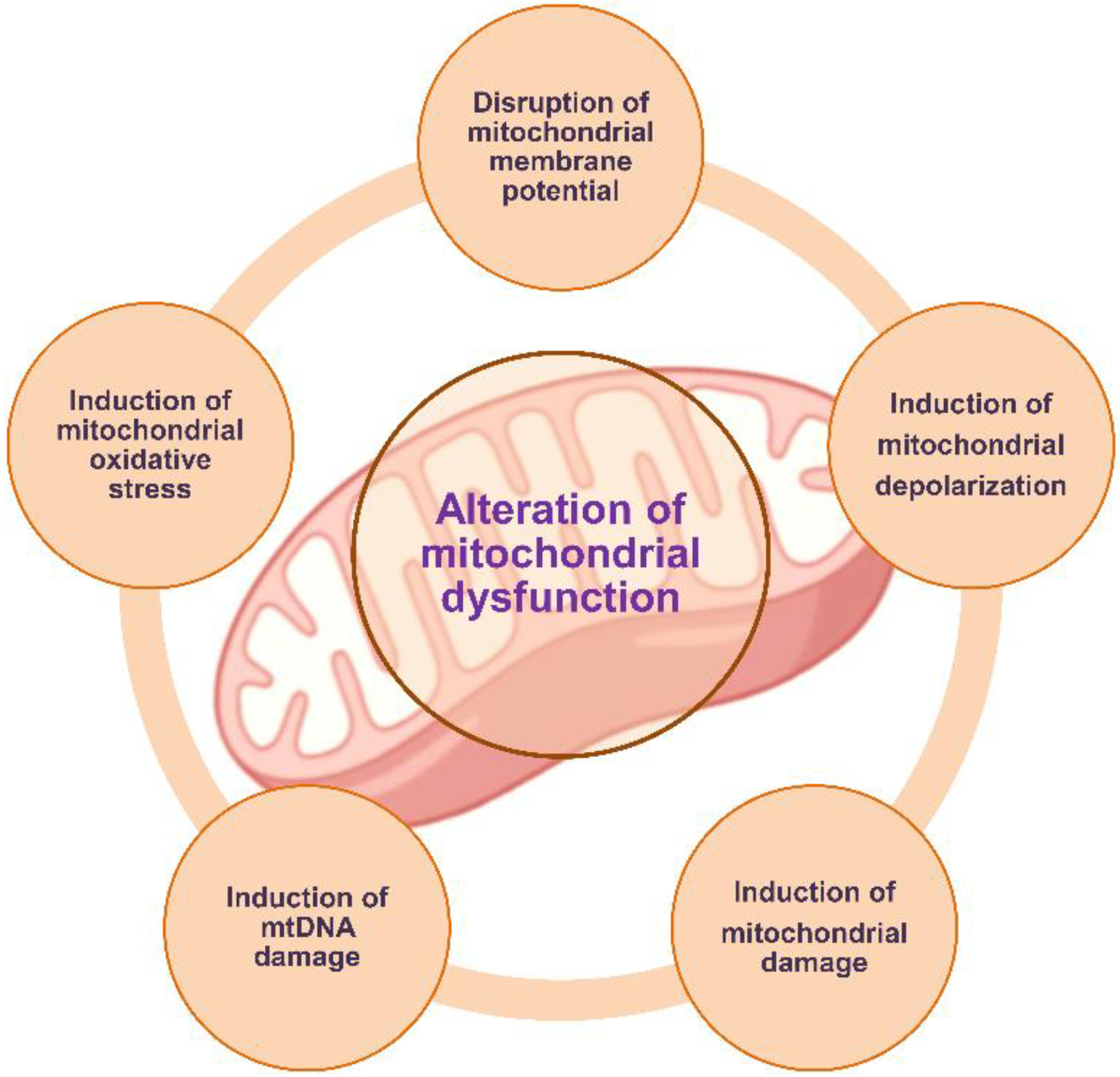
| Models | Mechanism | References |
|---|---|---|
| A549 (human alveolar epithelial cells) | Disruption of mitochondrial membrane potential | Zhang et al., 2022 [56] |
| Alteration of mitochondrial dysfunction | Halimu et al., 2022 [57] | |
| L02 (human hepatic cells) and BEAS-2B (human lung epithelial cells) | Alteration of mitochondrial dysfunction (disruption of mitochondrial membrane potential and suppression of mitochondrial respiration) | Lin et al., 2022 [58] |
| Human liver and mice liver cells | Induction of mtDNA damage | Shen et al., 2022 [59] |
| Caco-2 (human colon adenocarcinoma cells) | Induction of mitochondrial depolarization | Wu et al., 2019 [60] |
| Induction of mitochondrial depolarization | Wang et al., 2020 [61] | |
| HK-2 (human kidney proximal tubular epithelial cells) and in the kidneys of mice | Alteration of mitochondrial dysfunction | Wang et al., 2021 [62] |
| Human and murine macrophages | Disruption of mitochondrial membrane potential and induction of mitochondrial oxidative stress | Florance et al., 2022 [63] |
| HUVECs (human umbilical vein endothelial cells) | Alteration of mitochondrial dysfunction | Zhang et al., 2022 [64] |
| SHSY-5Y (human neuroblastoma cells) | Alteration of mitochondrial dysfunction | Tang et al., 2022 [65] |
| Human lymphocytes | Induction of mitochondrial damage | Salimi et al., 2022 [66] |
| GC-2 (mouse spermatocyte cells) | Induction of mitochondrial damage | Liu et al., 2022 [67] |
| RBL-2H3 (rat basophilic leukemia cells) | Induction of mitochondrial damage | Liu et al., 2022 [68] |
| Murine splenic lymphocytes | Disruption of mitochondrial membrane potential | Li et al., 2022 [69] |
| Murine macrophages | Reduction in mitochondrial respiration | Merkley et al., 2022 [70] |
| NS20Y (mouse neuroblastoma cells) | Alteration of mitochondrial dysfunction | Zhang et al., 2022 [71] |
| Mouse brain | Alteration of mitochondrial dysfunction | Liang et al., 2022 [72] |
| Zebrafish embryos | Induction of mitochondrial damage | Kantha et al., 2022 [73] |
| Caenorhabditis elegans | Induction of mitochondrial damage | Liu et al., 2020 [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.E.; Yi, Y.; Moon, S.; Yoon, H.; Park, Y.S. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites 2022, 12, 897. https://doi.org/10.3390/metabo12100897
Lee SE, Yi Y, Moon S, Yoon H, Park YS. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites. 2022; 12(10):897. https://doi.org/10.3390/metabo12100897
Chicago/Turabian StyleLee, Seung Eun, Yoojung Yi, Sangji Moon, Hyunkyung Yoon, and Yong Seek Park. 2022. "Impact of Micro- and Nanoplastics on Mitochondria" Metabolites 12, no. 10: 897. https://doi.org/10.3390/metabo12100897
APA StyleLee, S. E., Yi, Y., Moon, S., Yoon, H., & Park, Y. S. (2022). Impact of Micro- and Nanoplastics on Mitochondria. Metabolites, 12(10), 897. https://doi.org/10.3390/metabo12100897







