Integrated Analysis of Gut Microbiome and Lipid Metabolism in Mice Infected with Carbapenem-Resistant Enterobacteriaceae
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Bacteria
2.2. Animals
2.3. Biochemical Analyses
2.4. Metagenomics
2.5. Metabolomics
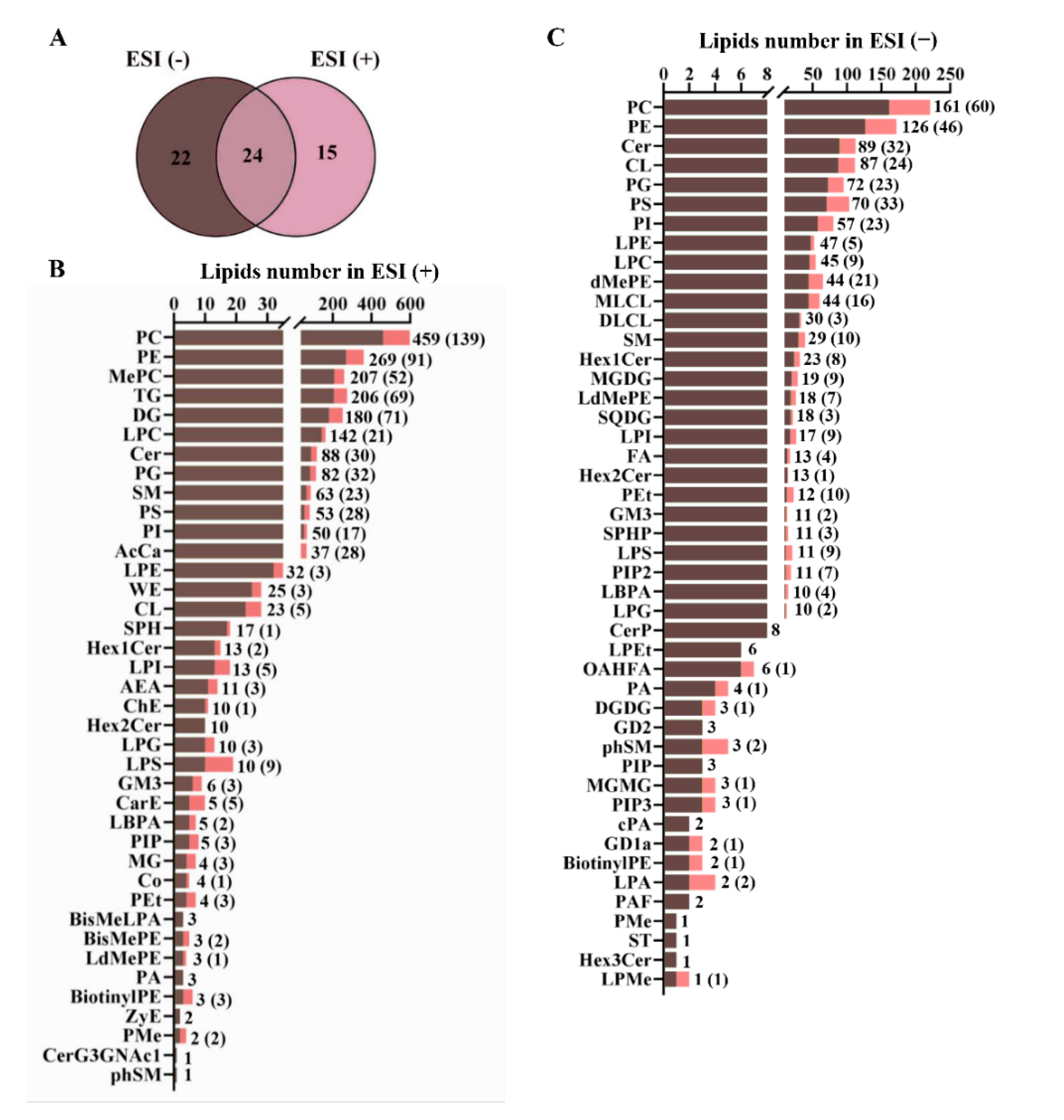
2.6. Lipidomics
2.6.1. Sample Preparation
2.6.2. LC-MS/MS Method for Lipid Analysis
2.6.3. Lipids Identification
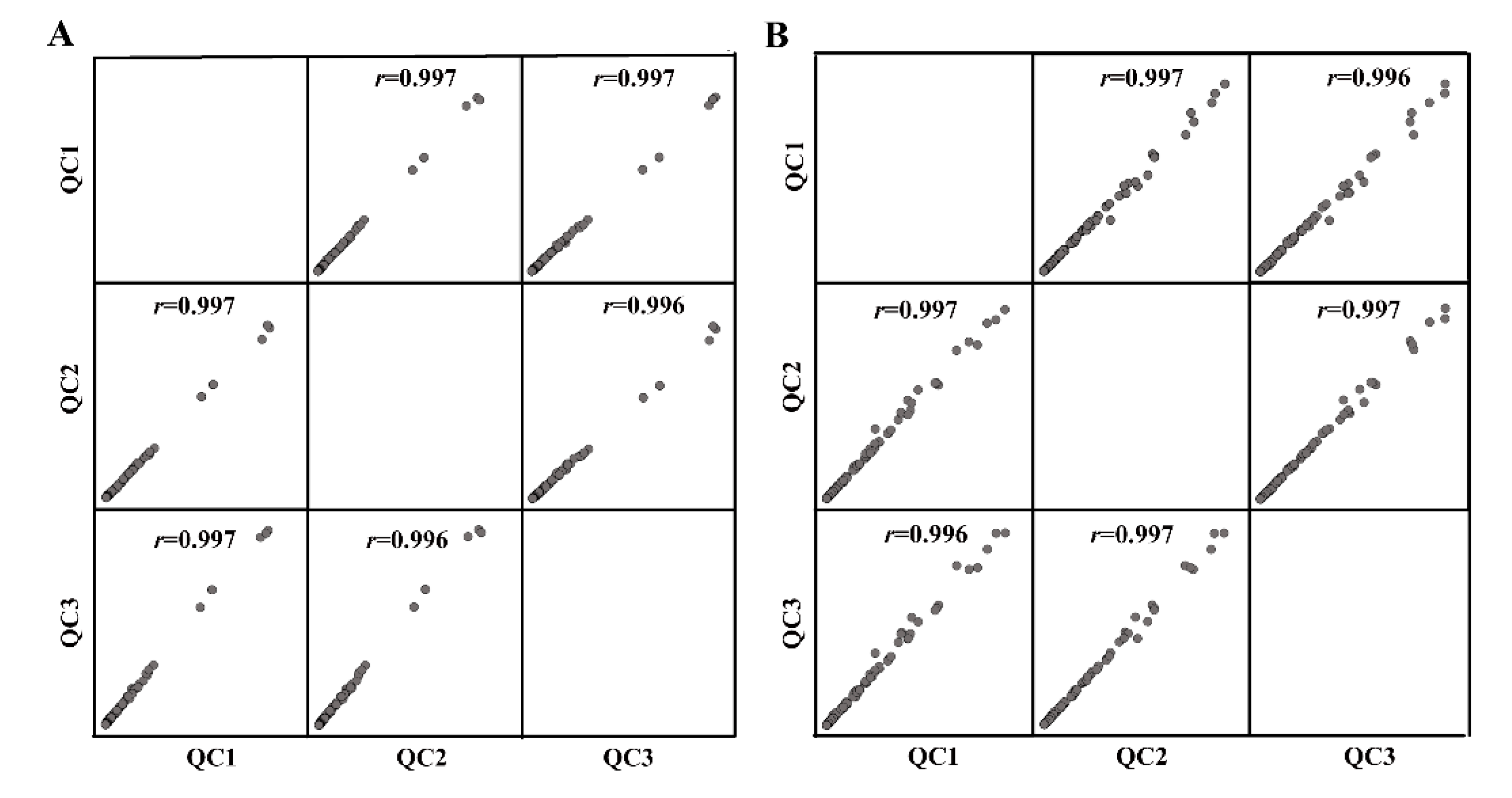
2.7. Statistics
2.8. Network Analysis and Potential Targets Prediction
3. Results
3.1. Hepatic Lipid Profile Analysis
3.2. Gut Microbiota Composition Analysis
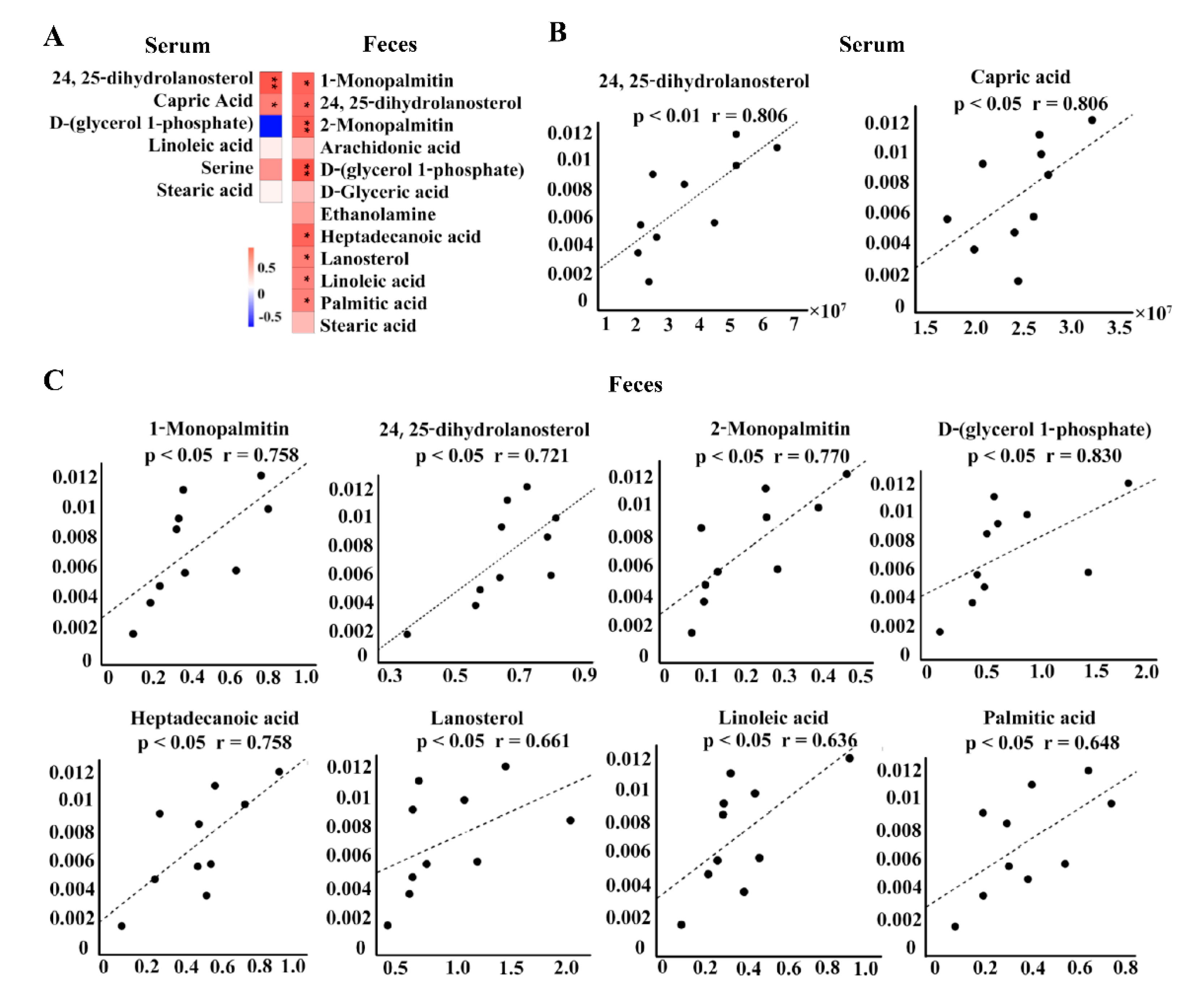
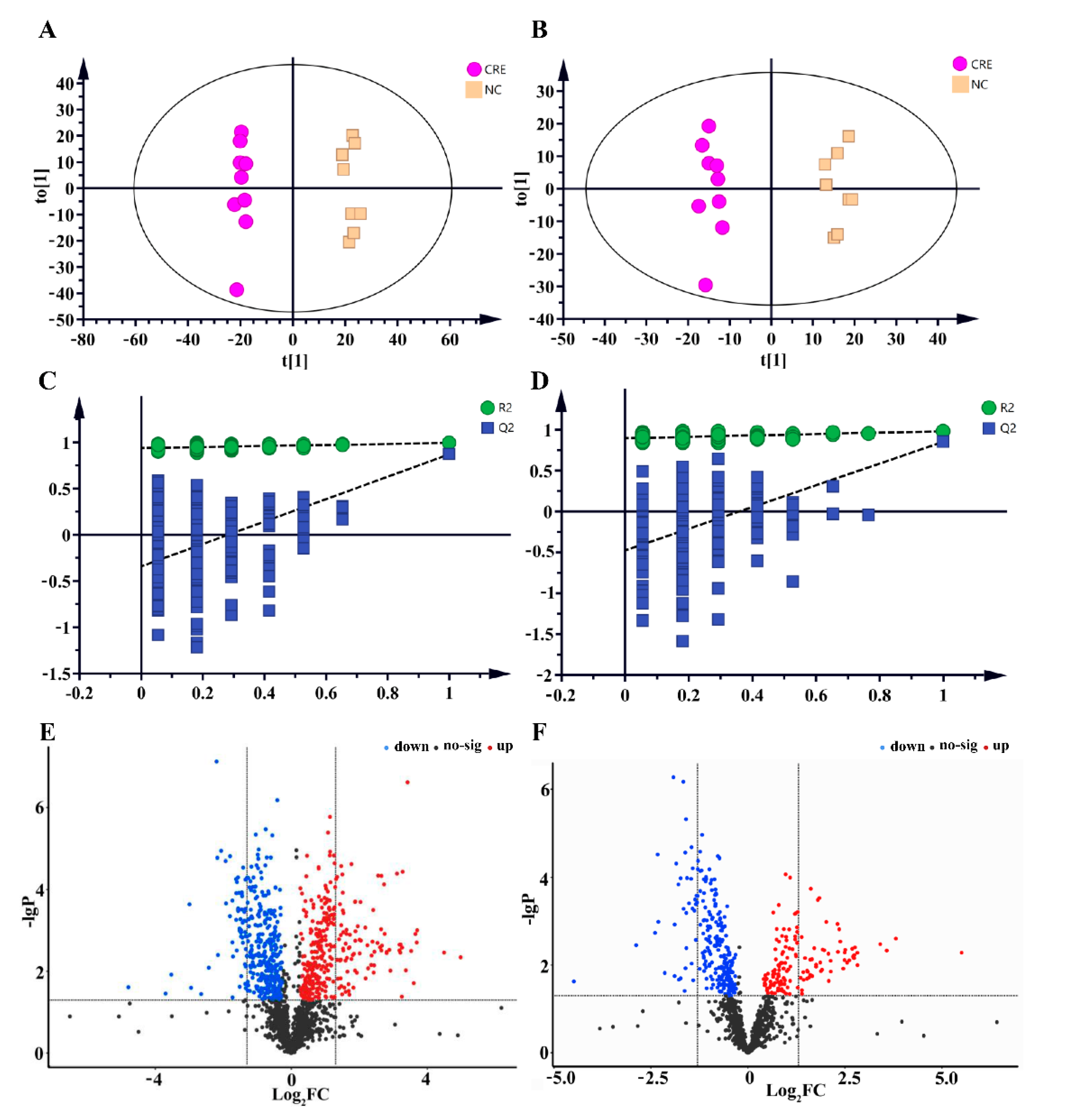
3.3. Serum and Feces Metabolomics Analysis
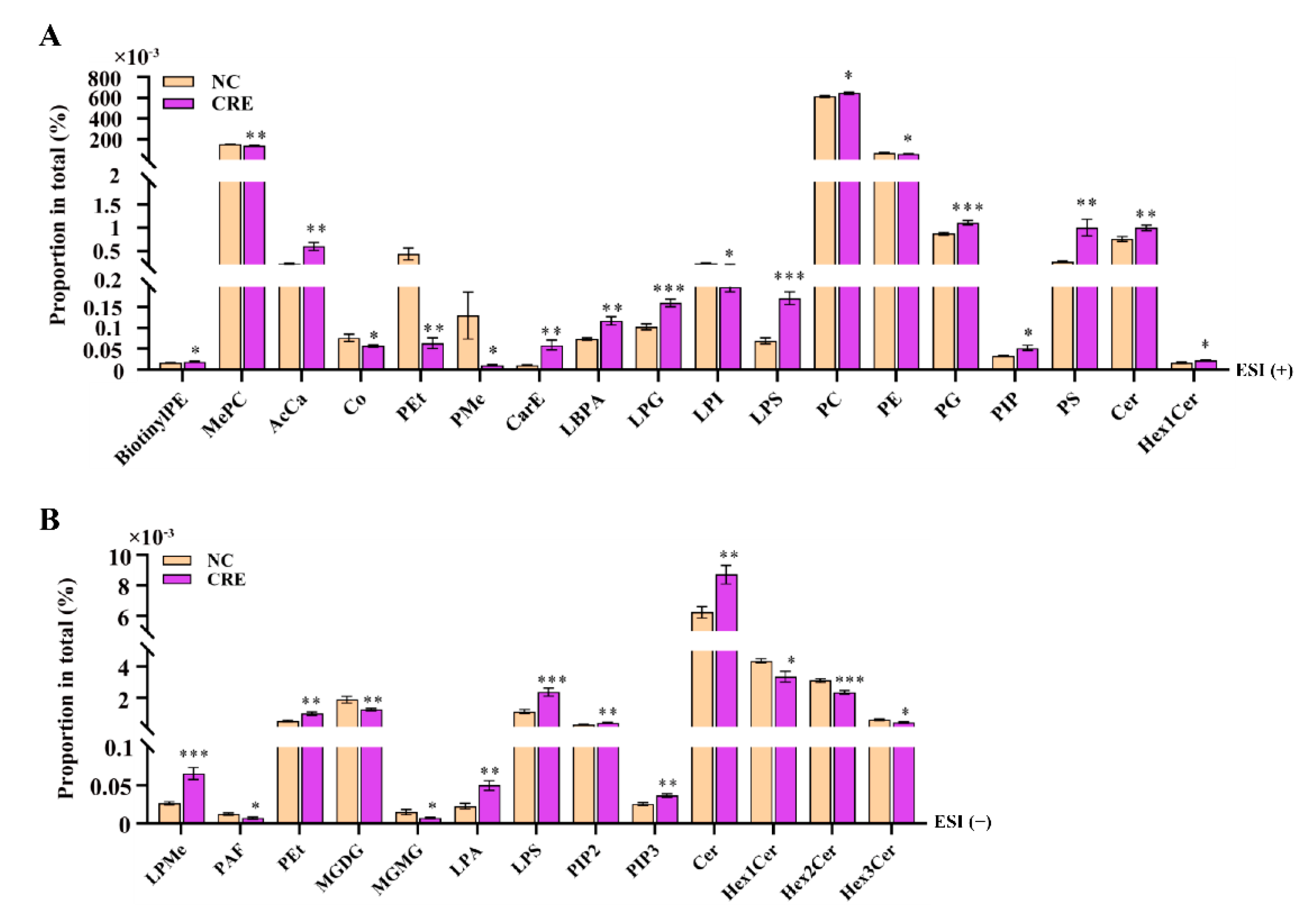
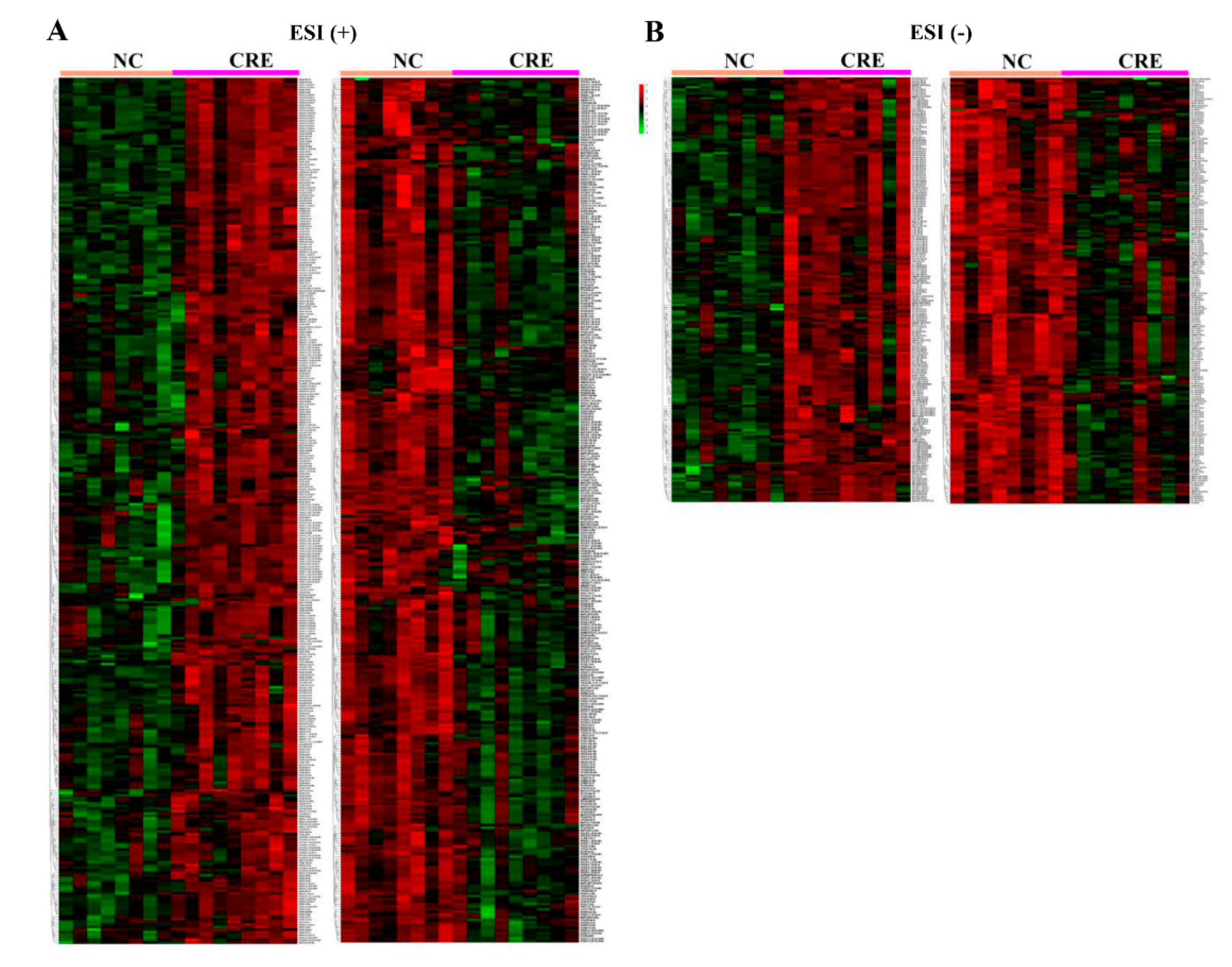
3.4. Hepatic Lipidomes Analysis
3.5. Association Analysis
3.6. Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, A.; Fahrbach, K.; Zhao, Q.; Lodise, T. Association between Carbapenem Resistance and Mortality Among Adult, Hospitalized Patients with Serious Infections Due to Enterobacteriaceae: Results of a Systematic Literature Review and Meta-analysis. Open Forum Infect. Dis. 2018, 5, ofy150. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.P.; Volling, C.; Green, K.; Uleryk, E.M.; Shah, P.S.; McGeer, A. Carbapenem Resistance, Initial Antibiotic Therapy, and Mortality in Klebsiella pneumoniae Bacteremia: A Systematic Review and Meta-Analysis. Infect. Control. Hosp. Epidemiol. 2017, 38, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.C.; Chang, Y.T.; Lin, S.Y.; Chen, Y.H.; Hsueh, P.R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.T.; Espinoza, H.V.; Espinoza, J.L. Emerging superbugs: The threat of Carbapenem Resistant Enterobacteriaceae. AIMS Microbiol 2020, 6, 176–182. [Google Scholar] [CrossRef]
- Tängdén, T.; Giske, C.G. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: Clinical perspectives on detection, treatment and infection control. J. Intern. Med. 2015, 277, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ni, Y.; Su, M.; Li, H.; Dong, F.; Chen, W.; Wei, R.; Zhang, L.; Guiraud, S.P.; Martin, F.P.; et al. High Throughput and Quantitative Measurement of Microbial Metabolome by Gas Chromatography/Mass Spectrometry Using Automated Alkyl Chloroformate Derivatization. Anal. Chem. 2017, 89, 5565–5577. [Google Scholar] [CrossRef] [PubMed]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels, L.M.J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Haak, B.W.; Prescott, H.C.; Wiersinga, W.J. Therapeutic Potential of the Gut Microbiota in the Prevention and Treatment of Sepsis. Front. Immunol. 2018, 9, 2042. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Oriach, C.S.; Robertson, R.C.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Food for thought: The role of nutrition in the microbiota-gut–brain axis. Clin. Nutr. Exp. 2016, 6, 25–38. [Google Scholar] [CrossRef]
- Byndloss, M.X.; Olsan, E.E.; Rivera-Chávez, F.; Tiffany, C.R.; Cevallos, S.A.; Lokken, K.L.; Torres, T.P.; Byndloss, A.J.; Faber, F.; Gao, Y.; et al. Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 2017, 357, 570–575. [Google Scholar] [CrossRef]
- Korach-Rechtman, H.; Hreish, M.; Fried, C.; Gerassy-Vainberg, S.; Azzam, Z.S.; Kashi, Y.; Berger, G. Intestinal Dysbiosis in Carriers of Carbapenem-Resistant Enterobacteriaceae. mSphere 2020, 5, e00173-20. [Google Scholar] [CrossRef]
- Hao, F.; Zhu, J.; Zhang, N.; He, P.; Miao, Q.; Liu, Y.; Gao, Y.; Liu, X.; Deng, G.; Zhang, Z.; et al. Association between gut microbiome and metabolome in mice suffering from acute carbapenem-resistant Escherichia coli infection. J. Pharm. Biomed. Anal. 2022, 215, 114770. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.P.R.; Slater, S.L.; O’Boyle, N.; Goldstone, R.J.; Crepin, V.F.; Ruano-Gallego, D.; Herzyk, P.; Smith, D.G.E.; Douce, G.R.; Frankel, G.; et al. Host-associated niche metabolism controls enteric infection through fine-tuning the regulation of type 3 secretion. Nat. Commun. 2018, 9, 4187. [Google Scholar] [CrossRef] [PubMed]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef]
- Hewelt-Belka, W.; Nakonieczna, J.; Belka, M.; Bączek, T.; Namieśnik, J.; Kot-Wasik, A. Untargeted Lipidomics Reveals Differences in the Lipid Pattern among Clinical Isolates of Staphylococcus aureus Resistant and Sensitive to Antibiotics. J. Proteome Res. 2016, 15, 914–922. [Google Scholar] [CrossRef]
- VanHook, A.M. Microbial metabolites shape lipid metabolism. Sci. Signal. 2020, 13, eabc1552. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Velagapudi, V.R.; Hezaveh, R.; Reigstad, C.S.; Gopalacharyulu, P.; Yetukuri, L.; Islam, S.; Felin, J.; Perkins, R.; Borén, J.; Oresic, M.; et al. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 2010, 51, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Chassaing, B.; Zhang, L.; San Yeoh, B.; Xiao, X.; Kumar, M.; Baker, M.T.; Cai, J.; Walker, R.; Borkowski, K.; et al. Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice. Cell Metab. 2015, 22, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kopczynski, D.; Coman, C.; Zahedi, R.P.; Lorenz, K.; Sickmann, A.; Ahrends, R. Multi-OMICS: A critical technical perspective on integrative lipidomics approaches. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 808–811. [Google Scholar] [CrossRef]
- Ritchie, M.D.; Holzinger, E.R.; Li, R.; Pendergrass, S.A.; Kim, D. Methods of integrating data to uncover genotype-phenotype interactions. Nat. Rev. Genet. 2015, 16, 85–97. [Google Scholar] [CrossRef]
- Altenbuchinger, M.; Zacharias, H.U.; Solbrig, S.; Schäfer, A.; Büyüközkan, M.; Schultheiß, U.T.; Kotsis, F.; Köttgen, A.; Spang, R.; Oefner, P.J.; et al. A multi-source data integration approach reveals novel associations between metabolites and renal outcomes in the German Chronic Kidney Disease study. Sci. Rep. 2019, 9, 13954. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, Y.; Yang, H.; Shang, D.; Zhang, C.; Zhang, Y.; Sun, Z.; Shi, X.; Feng, L.; Han, J.; et al. Global Prioritization of Disease Candidate Metabolites Based on a Multi-omics Composite Network. Sci. Rep. 2015, 5, 17201. [Google Scholar] [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00019–e00047. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, F.S.; Donkor, E.S. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e0217120. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infection. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Golden, A.R.; Zelenitsky, S.; Wiebe, K.; Lawrence, C.K.; Adam, H.J.; Idowu, T.; Domalaon, R.; Schweizer, F.; Zhanel, M.A.; et al. Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Drugs 2019, 79, 271–289. [Google Scholar] [CrossRef]
- Wang, J.; Jia, H. Metagenome-wide association studies: Fine-mining the microbiome. Nat. Rev. Microbiol. 2016, 14, 508–522. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Pace, N.R. A molecular view of microbial diversity and the biosphere. Science 1997, 276, 734–740. [Google Scholar] [CrossRef]
- Ju, F.; Zhang, T. 16S rRNA gene high-throughput sequencing data mining of microbial diversity and interactions. Appl. Microbiol. Biotechnol. 2015, 99, 4119–4129. [Google Scholar] [CrossRef]
- Alneberg, J.; Karlsson, C.M.G.; Divne, A.M.; Bergin, C.; Homa, F.; Lindh, M.V.; Hugerth, L.W.; Ettema, T.J.G.; Bertilsson, S.; Andersson, A.F.; et al. Genomes from uncultivated prokaryotes: A comparison of metagenome-assembled and single-amplified genomes. Microbiome 2018, 6, 173. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Almeida, M.; Juncker, A.S.; Rasmussen, S.; Li, J.; Sunagawa, S.; Plichta, D.R.; Gautier, L.; Pedersen, A.G.; Le Chatelier, E.; et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat. Biotechnol. 2014, 32, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.-U.; Zaura, E.; Buijs, M.J.; Keijser, B.J.F.; Crielaard, W.; Nord, C.E.; Weintraub, A. Determining the Long-term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin. Infect. Dis. 2015, 60, S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Fmark, S.L.; Edlund, C.; Jansson, J.K. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Buerger, M.; Stallmach, A.; Brun, T. Effects of Antibiotics on Gut Microbiota. Dig. Dis. 2016, 34, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zeng, M.Y.; Núñez, G. The interplay between host immune cells and gut microbiota in chronic inflammatory diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef]
- Kienesberger, S.; Cox, L.M.; Livanos, A.; Zhang, X.S.; Chung, J.; Perez-Perez, G.I.; Gorkiewicz, G.; Zechner, E.L.; Blaser, M.J. Gastric Helicobacter pylori Infection Affects Local and Distant Microbial Populations and Host Responses. Cell Rep. 2016, 14, 1395–1407. [Google Scholar] [CrossRef]
- Tong, L.T.; Xiao, T.; Wang, L.; Lu, C.; Liu, L.; Zhou, X.; Wang, A.; Qin, W.; Wang, F. Plant protein reduces serum cholesterol levels in hypercholesterolemia hamsters by modulating the compositions of gut microbiota and metabolites. iScience 2021, 24, 103435. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Zezina, E.; Namgaladze, D.; Gupta, S.; Angioni, C.; Geisslinger, G.; Lütjohann, D.; Brüne, B. A Novel Function for 15-Lipoxygenases in Cholesterol Homeostasis and CCL17 Production in Human Macrophages. Front. Immunol. 2018, 9, 1906. [Google Scholar] [CrossRef]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010, 501, 177–181. [Google Scholar] [CrossRef]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef]
- Toledo, A.; Benach, J.L. Hijacking and Use of Host Lipids by Intracellular Pathogens. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.K.; Gu, L.; Rowe, R.K.; Beatty, W.L. Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog. 2009, 5, e1000664. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.; Maamar, H.; Deb, C.; Sirakova, T.D.; Kolattukudy, P.E. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011, 7, e1002093. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Grassmé, H.; Jendrossek, V.; Riehle, A.; von Kürthy, G.; Berger, J.; Schwarz, H.; Weller, M.; Kolesnick, R.; Gulbins, E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 2003, 9, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, W.; Zheng, X.; Qi, L.; Wang, H.; Zhang, C.; Wan, X.; Zheng, Y.; Zhong, R.; Zhou, X.; et al. Targeting 7-Dehydrocholesterol Reductase Integrates Cholesterol Metabolism and IRF3 Activation to Eliminate Infection. Immunity 2020, 52, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y.A.; Scruel, O. Changes in the concentration and composition of plasma lipoproteins during the acute phase response. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 153–158. [Google Scholar] [CrossRef]
- Cirstea, M.; Walley, K.R.; Russell, J.A.; Brunham, L.R.; Genga, K.R.; Boyd, J.H. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J. Crit. Care 2017, 38, 289–294. [Google Scholar] [CrossRef]
- van Leeuwen, H.J.; Heezius, E.C.; Dallinga, G.M.; van Strijp, J.A.; Verhoef, J.; van Kessel, K.P. Lipoprotein metabolism in patients with severe sepsis. Crit. Care Med. 2003, 31, 1359–1366. [Google Scholar] [CrossRef]
- Maceyka, M.; Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 2014, 510, 58–67. [Google Scholar] [CrossRef]
- Henry, B.; Ziobro, R.; Becker, K.A.; Kolesnick, R.; Gulbins, E. Acid sphingomyelinase. Handb. Exp. Pharmacol. 2013, 25, 77–88. [Google Scholar] [CrossRef]
- Noh, S.K.; Koo, S.I. Egg sphingomyelin lowers the lymphatic absorption of cholesterol and alpha-tocopherol in rats. J. Nutr. 2003, 133, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.K.; Koo, S.I. Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J. Nutr. 2004, 134, 2611–2616. [Google Scholar] [CrossRef]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, J.C.; Zhou, H.; Brayfield, B.P.; Hontecillas, R.; Bassaganya-Riera, J.; Schmelz, E.M. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: Importance of peroxisome proliferator-activated receptor γ expression. J. Nutr. Biochem. 2011, 22, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Parham, K.A.; Zebol, J.R.; Tooley, K.L.; Sun, W.Y.; Moldenhauer, L.M.; Cockshell, M.P.; Gliddon, B.L.; Moretti, P.A.; Tigyi, G.; Pitson, S.M.; et al. Sphingosine 1-phosphate is a ligand for peroxisome proliferator-activated receptor-γ that regulates neoangiogenesis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 3638–3653. [Google Scholar] [CrossRef] [PubMed]
- Adams, F.G.; Trappetti, C.; Waters, J.K.; Zang, M.; Brazel, E.B.; Paton, J.C.; Snel, M.F.; Eijkelkamp, B.A. To Make or Take: Bacterial Lipid Homeostasis during Infection. mBio 2021, 12, e0092821. [Google Scholar] [CrossRef]
- Sperandeo, P.; Polissi, A.; De Fabiani, E. Fat Matters for Bugs: How Lipids and Lipid Modifications Make the Difference in Bacterial Life. Eur. J. Lipid Sci. Technol. 2019, 121, 1900204. [Google Scholar] [CrossRef]
- Hines, K.M.; Waalkes, A.; Penewit, K.; Holmes, E.A.; Salipante, S.J.; Werth, B.J.; Xu, L. Characterization of the Mechanisms of Daptomycin Resistance among Gram-Positive Bacterial Pathogens by Multidimensional Lipidomics. mSphere 2017, 2, e00492-17. [Google Scholar] [CrossRef]
- Meex, R.C.; Schrauwen, P.; Hesselink, M.K. Modulation of myocellular fat stores: Lipid droplet dynamics in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R913–R924. [Google Scholar] [CrossRef]
- Roberts, R.; Sciorra, V.A.; Morris, A.J. Human type 2 phosphatidic acid phosphohydrolases. Substrate specificity of the type 2a, 2b, and 2c enzymes and cell surface activity of the 2a isoform. J. Biol. Chem. 1998, 273, 22059–22067. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform 2011, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, R.J.; Jang, K.; Zhou, X.L.; Liu, Y.Z. Protective Effects of Pretreatment with Quercetin Against Lipopolysaccharide-Induced Apoptosis and the Inhibition of Osteoblast Differentiation via the MAPK and Wnt/β-Catenin Pathways in MC3T3-E1 Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 43, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- Grab, L.T.; Kearns, M.W.; Morris, A.J.; Daniel, L.W. Differential role for phospholipase D1 and phospholipase D2 in 12-O-tetradecanoyl-13-phorbol acetate-stimulated MAPK activation, Cox-2 and IL-8 expression. Biochim. Biophys. Acta 2004, 1636, 29–39. [Google Scholar] [CrossRef]
- Adler, D.H.; Cogan, J.D.; Phillips, J.A., 3rd; Schnetz-Boutaud, N.; Milne, G.L.; Iverson, T.; Stein, J.A.; Brenner, D.A.; Morrow, J.D.; Boutaud, O.; et al. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J. Clin. Investig. 2008, 118, 2121–2131. [Google Scholar] [CrossRef]
- Grassmé, H.; Becker, K.A. Bacterial infections and ceramide. Handb. Exp. Pharmacol. 2013, 216, 305–320. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, C.; Zhang, Q.; Bao, J.; Fan, Q.; Li, R.; Ishfaq, M.; Li, J. Arachidonic acid metabolism is elevated in Mycoplasma gallisepticum and Escherichia coli co-infection and induces LTC4 in serum as the biomarker for detecting poultry respiratory disease. Virulence 2020, 11, 730–738. [Google Scholar] [CrossRef]
- Caputa, G.; Matsushita, M.; Sanin, D.E.; Kabat, A.M.; Edwards-Hicks, J.; Grzes, K.M.; Pohlmeyer, R.; Stanczak, M.A.; Castoldi, A.; Cupovic, J.; et al. Intracellular infection and immune system cues rewire adipocytes to acquire immune function. Cell Metab. 2022, 34, 747.e6–760.e6. [Google Scholar] [CrossRef]



| NO. | Class | Species | MODE | Adduct | CalcMz | Formula | VIP | P | FDR | FC | Ery | Correlation (r, p) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TG | TC | HDL-c | LDL-c | ||||||||||||
| 1 | Sphingolipids | Cer (d17:1/16:0) | ESI (+) | [M+H-H2O]+ | 506.4932 | C33H64O2N | 1.48 | 1.45 × 10−3 | 2.3 × 10−3 | 1.87 | 0.93 *** | ns | 0.83 *** | 0.86 *** | 0.6 * |
| 2 | Sphingolipids | Cer (d18:1/16:0) | ESI (−) | [M+HCOO]− | 582.5103 | C35H68O5N | 1.81 | 2.87 × 10−5 | 2.52 × 10−4 | 2.04 | 0.9 *** | ns | −0.49 * | ns | ns |
| 3 | Sphingolipids | Cer (d18:1/16:0) | ESI (+) | [M+H-H2O]+ | 520.5088 | C34H66O2N | 1.79 | 1.49 × 10−5 | 1.74 × 10−4 | 2.19 | 0.87 ** | ns | 0.77 *** | 0.81 *** | ns |
| 4 | Sphingolipids | Cer (d18:1/17:0) | ESI (+) | [M+H-H2O]+ | 534.5245 | C35H68O2N | 1.62 | 2.72 × 10−4 | 8.77 × 10−4 | 2.41 | 0.85 ** | ns | 0.76 *** | 0.8 *** | ns |
| 5 | Sphingolipids | Cer (d18:2/16:0) | ESI (+) | [M+H]+ | 536.5037 | C34H66O3N | 1.57 | 6.1 × 10−4 | 1.08 × 10−3 | 2.04 | 0.83 ** | ns | 0.73 *** | 0.81 *** | 0.54 * |
| 6 | Sphingolipids | Cer (t17:0/16:0) | ESI (+) | [M+H-H2O]+ | 524.5037 | C33H66O3N | 1.63 | 2.76 × 10−4 | 8.77 × 10−4 | 2.16 | 0.88 ** | ns | 0.83 *** | 0.86 *** | 0.6 * |
| 7 | Sphingolipids | Cer (t18:1/16:0) | ESI (+) | [M+H-H2O]+ | 536.5037 | C34H66O3N | 1.58 | 5.56 × 10−4 | 1.08 × 10−3 | 2.06 | 0.83 ** | ns | 0.74 *** | 0.81 *** | 0.53 * |
| 8 | Sphingolipids | SM (d33:1) | ESI (+) | [M+H]+ | 689.5592 | C38H78O6N2P | 1.60 | 2.78 × 10−4 | 8.77 × 10−4 | 1.57 | 0.88 ** | ns | 0.69 ** | 0.73 *** | ns |
| 9 | Phospholipids | PG (40:8) | ESI (+) | [M+Na]+ | 841.499 | C46H75O10PNa | 1.41 | 2.03 × 10−3 | 2.54 × 10−3 | 1.62 | 0.82 ** | ns | 0.61 ** | 0.69 ** | ns |
| 10 | Phospholipids | PG (42:2) | ESI (+) | [M+NH4]+ | 876.6688 | C48H95O10NP | 1.78 | 1.2 × 10−5 | 1.74 × 10−4 | 2.21 | 0.9 *** | ns | 0.8 *** | 0.87 *** | 0.59 * |
| 11 | Phospholipids | PG (42:3) | ESI (+) | [M+NH4]+ | 874.6532 | C48H93O10NP | 1.61 | 3.01 × 10−4 | 8.77 × 10−4 | 2.38 | 0.92 *** | ns | 0.74 *** | 0.82 *** | ns |
| 12 | Phospholipids | PC (32:2) | ESI (+) | [M+H]+ | 730.5381 | C40H77O8NP | 1.60 | 4.48 × 10−4 | 1.04 × 10−3 | 0.29 | −0.82** | ns | −0.67 ** | −0.73 *** | ns |
| 13 | Phospholipids | PC (34:2e) | ESI (+) | [M+H]+ | 744.5902 | C42H83O7NP | 1.53 | 6.48 × 10−4 | 1.08 × 10−3 | 1.83 | 0.89 ** | ns | 0.73 *** | 0.73 *** | ns |
| 14 | Phospholipids | PC (36:3) | ESI (+) | [M+H]+ | 784.5851 | C44H83O8NP | 1.45 | 1.77 × 10−3 | 2.54 × 10−3 | 4.13 | 0.82 ** | ns | 0.59 * | 0.65 ** | 0.27 ns |
| 15 | Phospholipids | PC (42:4) | ESI (+) | [M+H]+ | 866.6633 | C50H93O8NP | 1.12 | 2.19 × 10−2 | 2.39 × 10−2 | 1.29 | 0.97 *** | ns | 0.5 * | 0.62 ** | ns |
| 16 | Phospholipids | LPC (24:2) | ESI (+) | [M+H]+ | 604.4337 | C32H63O7NP | 1.06 | 2.95 × 10−2 | 2.95 × 10−2 | 1.32 | 0.88 ** | ns | 0.62 ** | 0.55 * | ns |
| 17 | Phospholipids | MePC (36:7) | ESI (+) | [M+Na]+ | 812.5201 | C45H76O8NPNa | 1.42 | 3.91 × 10−3 | 4.56 × 10−3 | 0.77 | −0.88 ** | ns | −0.68 ** | −0.66 ** | ns |
| 18 | Phospholipids | PG (18:0/18:2) | ESI (+) | [M+H]+ | 775.5484 | C42H80O10P | 1.37 | 3.62 × 10−3 | 4.37 × 10−3 | 1.94 | 0.85 ** | ns | 0.7 ** | 0.72 ** | ns |
| 19 | Phospholipids | PG (18:1/18:2) | ESI (+) | [M+H]+ | 773.5327 | C42H78O10P | 1.71 | 5.52 × 10−5 | 3.82 × 10−4 | 2.49 | 0.85 ** | ns | 0.75 *** | 0.82 *** | ns |
| 20 | Phospholipids | PG (38:5) | ESI (+) | [M+NH4]+ | 814.5593 | C44H81O10NP | 1.43 | 1.91 × 10−3 | 2.54 × 10−3 | 1.76 | 0.93 *** | ns | 0.75 *** | 0.78 *** | 0.51 * |
| 21 | Phospholipids | LPG (18:2) | ESI (+) | [M+Na]+ | 531.2693 | C24H45O9PNa | 1.37 | 7.03 × 10−3 | 7.93 × 10−3 | 2.08 | 0.87 ** | ns | 0.77 *** | 0.86 *** | 0.55 * |
| 22 | Phospholipids | PS (42:0) | ESI (+) | [M+H]+ | 876.6688 | C48H95O10NP | 1.57 | 4.13 × 10−4 | 1.03 × 10−3 | 2.27 | 0.9 *** | ns | −0.52 * | ns | ns |
| 23 | Phospholipids | MLCL (62:1) | ESI (−) | [M-2H]− | 653.4657 | C71H136O16P2 | 1.40 | 2.01 × 10−3 | 2.54 × 10−3 | 1.38 | 0.85 ** | −0.49* | −0.5 * | −0.37 ns | ns |
| 24 | Phospholipids | PIP (52:3) | ESI (+) | [M+Na]+ | 1187.747 | C61H114O16P2Na | 1.14 | 2.56 × 10−2 | 2.64 × 10−2 | 1.57 | 0.82 ** | ns | 0.54 * | 0.63 ** | ns |
| 25 | Phospholipids | PIP2 (18:1/20:4) | ESI (−) | [M-H]− | 1043.467 | C47H82O19P3 | 1.65 | 3.28 × 10−4 | 8.84 × 10−4 | 2.12 | 0.87 ** | ns | 0.8 *** | 0.87 *** | 0.58 * |
| 26 | Glycerolipids | DG (20:2) | ESI (+) | [M+NH4]+ | 414.3214 | C23H44O5N | 1.94 | 2.42 × 10−7 | 8.45 × 10−6 | 10.69 | 0.92 *** | ns | 0.77 *** | 0.82 *** | 0.55 * |
| 27 | Glycerolipids | DG (34:1e) | ESI (+) | [M+Na]+ | 603.5323 | C37H72O4Na | 1.70 | 8.05 × 10−5 | 3.82 × 10−4 | 2.01 | 0.82 ** | ns | 0.66 ** | 0.7 ** | ns |
| 28 | Glycerolipids | DG (36:4e) | ESI (+) | [M+H]+ | 603.5347 | C39H71O4 | 1.70 | 8.05 × 10−5 | 3.82 × 10−4 | 2.01 | 0.82 ** | ns | 0.66 ** | 0.7 ** | ns |
| 29 | Glycerolipids | DG (38:6e) | ESI (+) | [M+H]+ | 627.5347 | C41H71O4 | 1.57 | 6.54 × 10−4 | 1.08 × 10−3 | 2.24 | 0.87 ** | ns | 0.66 ** | 0.78 *** | ns |
| 30 | Glycerolipids | TG (17:0/11:2/11:2) | ESI (+) | [M+NH4]+ | 690.5667 | C42H76O6N | 1.53 | 5.94 × 10−4 | 1.08 × 10−3 | 1.54 | 0.83 ** | ns | 0.72 ** | 0.72 ** | ns |
| 31 | Glycerolipids | TG (22:6/12:4/14:4) | ESI (+) | [M+Na]+ | 801.5065 | C51H70O6Na | 1.68 | 8.72 × 10−5 | 3.82 × 10−4 | 2.44 | 0.83 ** | ns | 0.82 *** | 0.83 *** | 0.56 * |
| 32 | Fatty acyl and others | AEA (18:2) | ESI (+) | [M+H]+ | 324.2897 | C20H38O2N | 1.14 | 2.3 × 10−2 | 2.44 × 10−2 | 2.77 | 0.82 ** | ns | 0.92 *** | 0.91 *** | 0.69 ** |
| 33 | Fatty acyl and othes | AEA (20:3) | ESI (+) | [M+H]+ | 350.3054 | C22H40O2N | 1.47 | 1.96 × 10−3 | 2.54 × 10−3 | 1.56 | 0.87 ** | 0.52* | 0.89 *** | 0.82 *** | 0.71 ** |
| 34 | Fatty acyl and others | AcCa (22:1) | ESI (+) | [M+H]+ | 482.4204 | C29H56O4N | 1.54 | 5.2 × 10−4 | 1.08 × 10−3 | 2.37 | 0.83 ** | ns | 0.64 ** | 0.72 ** | ns |
| 35 | Fatty acyl and others | PEt (18:1/22:6) | ESI (−) | [M-H]− | 773.5127 | C45H74O8P | 1.50 | 1.78 × 10−3 | 2.54 × 10−3 | 1.89 | 0.9 *** | −0.49* | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Peng, Y.; Zhao, L.; He, P.; Zhu, J.; Liu, Y.; Liu, X.; Liu, X.; Deng, G.; Zhang, Z.; et al. Integrated Analysis of Gut Microbiome and Lipid Metabolism in Mice Infected with Carbapenem-Resistant Enterobacteriaceae. Metabolites 2022, 12, 892. https://doi.org/10.3390/metabo12100892
Zhang N, Peng Y, Zhao L, He P, Zhu J, Liu Y, Liu X, Liu X, Deng G, Zhang Z, et al. Integrated Analysis of Gut Microbiome and Lipid Metabolism in Mice Infected with Carbapenem-Resistant Enterobacteriaceae. Metabolites. 2022; 12(10):892. https://doi.org/10.3390/metabo12100892
Chicago/Turabian StyleZhang, Ning, Yuanyuan Peng, Linjing Zhao, Peng He, Jiamin Zhu, Yumin Liu, Xijian Liu, Xiaohui Liu, Guoying Deng, Zhong Zhang, and et al. 2022. "Integrated Analysis of Gut Microbiome and Lipid Metabolism in Mice Infected with Carbapenem-Resistant Enterobacteriaceae" Metabolites 12, no. 10: 892. https://doi.org/10.3390/metabo12100892
APA StyleZhang, N., Peng, Y., Zhao, L., He, P., Zhu, J., Liu, Y., Liu, X., Liu, X., Deng, G., Zhang, Z., & Feng, M. (2022). Integrated Analysis of Gut Microbiome and Lipid Metabolism in Mice Infected with Carbapenem-Resistant Enterobacteriaceae. Metabolites, 12(10), 892. https://doi.org/10.3390/metabo12100892








