Copper/Zinc Ratio in Childhood and Adolescence: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
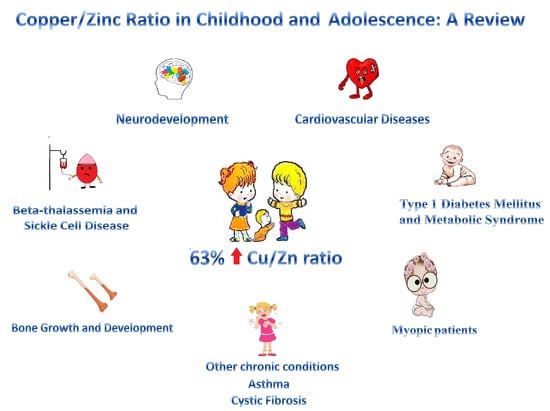
4.1. Zinc and Copper Levels and Copper to Zinc Ratio
4.2. Copper to Zinc Ratio in Acute Infections
4.3. Cardiovascular Diseases
4.4. Type 1 Diabetes Mellitus and Metabolic Syndrome
4.5. Myopic Patients
4.6. Other Chronic Conditions
4.7. Bone Growth and Development
4.8. Beta-Thalassemia and Sickle Cell Disease
4.9. Neurodevelopment
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic Fibrosis |
| CVD | Cardiovascular Disease |
| DM | Diabetes Mellitus |
| MetS | Metabolic Syndrome |
| Cu | Copper |
| Zn | Zinc |
| OHS | Occipital Horn Syndrome |
| ASD | Autism Spectrum Disorder |
| CSS | Cross-Sectional Study |
| CCS | Case-Control Study |
| RTS | Randomized Trial Study |
| NHANES | National Health and Nutrition Examination Survey |
| CP | Ceruloplasmin |
| CRP | C-Reactive Protein |
| OS | Oxidative Stress |
| SOD | Superoxide Dismutase |
| HTN | Hypertension |
| CHD | Congenital Heart Disease |
| BMI | Body Mass Index |
| ROS | Reactive Oxygen Species |
| T1DM | Type 1 Diabetes Mellitus |
| A1c % | Glycosylated Hemoglobin |
| TC | Total Cholesterol |
| Hb | Hemoglobin |
| Fe | Iron |
| β-TM | Beta-Thalassemia Major |
| TDT | Transfusion-Dependent Thalassemia |
| SCD | Sickle Cell Disease |
| SCA | Sickle Cell Anemia |
| NDR | Neurodevelopmental Regression |
| ADHD | Attention Deficit Hyperactivity Disorder |
References
- Zhang, H.; Man, Q.; Song, P.; Li, S.; Liu, X.; Wang, L.; Li, Y.; Hu, Y.; Yang, L. Association of whole blood copper, magnesium and zinc levels with metabolic syndrome components in 6-12-year-old rural Chinese children: 2010–2012 China National Nutrition and Health Survey. Nutr. Metab. 2021, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 3578. [Google Scholar] [CrossRef] [PubMed]
- Escobedo-Monge, M.F.; Barrado, E.; Alonso, C.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M.; Redondo del Río, M.P. Cu and Copper/Zn Ratio in a Series of Cystic Fibrosis Patients. Nutrients 2020, 12, 3344. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Pontiroli, A.E. Prevention of Diabetes and Cardiovascular Disease in Obesity. Int. J. Mol. Sci. 2020, 21, 8178. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warnakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, C.; Bu, J. Relationship between Selected Serum Metallic Elements and Obesity in Children and Adolescent in the U.S. Nutrients 2017, 9, 104. [Google Scholar] [CrossRef]
- Freitas, E.P.; Cunha, A.T.; Aquino, S.L.; Pedrosa, L.F.; Lima, S.C.; Lima, J.G.; Almeida, M.G.; Sena-Evangelista, K.C.M. Zinc Status Biomarkers and Cardiometabolic Risk Factors in Metabolic Syndrome: A Case Control Study. Nutrients 2017, 9, 175. [Google Scholar] [CrossRef]
- Tarnacka, B.; Flaga, A.; Adamczyk, A. Cu Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar]
- Genoud, S.; Senior, A.M.; Hare, D.J.; Double, K.L. Meta-analysis of Cu and iron in Parkinson’s disease brain and biofuids. Mov. Disord. 2020, 35, 662–671. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Shi, H.; Peng, Y.; Fan, X.; Li, C. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflug. Arch-Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef]
- Zhang, M.; Li, W.; Wang, Y.; Wang, T.; Ma, M.; Tian, C. Association between the change of serum Cu and ischemic stroke: A systematic review and meta-analysis. J. Mol. Neurosci. 2020, 70, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.J.; Hahn, S.H. The genetics of Wilson disease. Handb. Clin. Neurol. 2017, 142, 19–34. [Google Scholar]
- Kaler, S.G.; DiStasio, A.T. ATP7A-Related Copper Transport Disorders; GeneReviews®; Adam, M.P., Everman, D.B., Mirzaa, G.M., Eds.; University of Washington: Seattle, WA, USA, 1993–2022. [Google Scholar]
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper deficiency anemia: Review article. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Kaslow, J.E. Copper/Zn Imbalance. Medical Board of California. Available online: http://www.mbc.ca.gov (accessed on 2 November 2022).
- Escobedo Monge, M.F.; Barrado, E.; Alonso Vicente, C.; Redondo del Río, M.P.; Manuel Marugán de Miguelsanz, J. Zinc Nutritional Status in Patients with Cystic Fibrosis. Nutrients 2019, 11, 150. [Google Scholar] [CrossRef]
- Hofstee, P.; McKeating, D.R.; Perkins, A.V.; Cuffe, J.S.M. Placental adaptations to micronutrient dysregulation in the programming of chronic disease. Clin. Exp. Pharmacol. Physiol. 2018, 45, 871–884. [Google Scholar] [CrossRef]
- El-Meshad, G.M.; El-Nabi, S.S.A.; Moharam, N.M.; El-Khair, M.S.A. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Menoufia Med. J. 2017, 30, 727–733. [Google Scholar]
- Grieger, J.A. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609. [Google Scholar] [CrossRef]
- Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Grzeskowiak, L.E.; Dekker, G.A.; Roberts, C.T. Early pregnancy maternal trace mineral status and the association with adverse pregnancy outcome in a cohort of Australian women. J. Trace Elem. Med. Biol. 2018, 46, 103–109. [Google Scholar] [CrossRef]
- Wisniewska, M.; Cremer, M.; Wiehe, L.; Becker, N.-P.; Rijntjes, E.; Martitz, J.; Renko, K.; Bührer, C.; Schomburg, L. Copper to Zinc Ratio as Disease Biomarker in Neonates with Early-Onset Congenital Infections. Nutrients 2017, 9, 343. [Google Scholar] [CrossRef]
- Agbonlahor, O.J.; Emokpae, M.A.; Osuntade, O.P. Cord Serum Copper-to-Zinc Ratio Correlates with Birth Weight among Neonates in Benin City, Nigeria. J. Med. Lab. Sci. 2021, 31, 47–57. [Google Scholar]
- Zang, X.; Huang, H.; Zhuang, Z.; Chen, R.; Xie, Z.; Xu, C.; Mo, X. The association between serum copper concentrations and cardiovascular disease risk factors in children and adolescents in NHANES. Environ. Sci. Pollut. Res. 2018, 25, 16951–16958. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, K.; Birdi, A.; Tomo, S.; Sreenivasulu, K.; Charan, J.; Yadav, D.; Purohit, P.; Sharma, P. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Indian J. Clin. Biochem. 2021, 36, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, C. Review of Copper Provision in the Parenteral Nutrition of Adults. Nutr. Clin. Pract. 2017, 32, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Rehmani, N.; Zafar, A.; Arif, H.; Hadi, S.M.; Wani, A.A. Copper-mediated DNA damage by the neurotransmitter dopamine and L-DOPA: A pro-oxidant mechanism. Toxicol. Vitr. 2017, 40, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Cruces-Sande, A.; Méndez-Alvarez, E.; Soto-Otero, R. Copper increases the ability of 6-hydroxydopamine to generate oxidative stress and the ability of ascorbate and glutathione to potentiate this effect: Potential implications in Parkinson’s disease. J. Neurochem. 2017, 141, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Focarelli, F.; Giachino, A.; Waldron, K.J. Copper microenvironments in the human body define patterns of copper adaptation in pathogenic bacteria. PLOS Pathog. 2022, 18, e1010617. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Nayak, S.; Sahu, S.; Patra, S.; John, J. Assessment of Copper and Zinc Levels in Hair and Urine of Children With Attention Deficit Hyperactivity Disorder: A Case-Control Study in Eastern India. Cureus 2021, 13, e20692. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Witt, B.; Schaumlöffel, D.; Schwerdtle, T. Subcellular localization of copper—Cellular bioimaging with focus on neurological disorders. Int. J. Mol. Sci. 2020, 21, 2341. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M.; Kaplan, J.H. Copper transporters and copper chaperones: Roles in cardiovascular physiology and disease. Am. J. Physiol. Cell Physiol. 2018, 315, C186–C201. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.T.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Serum copper-to-zinc-ratio and risk of incident infection in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Eur. J. Epidemiol. 2020, 35, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 3 November 2022).
- Oldenburg, C.E.; Guerin, P.J.; Berthé, F.; Grais, R.F.; Isanaka, S. Malaria and Nutritional Status Among Children With Severe Acute Malnutrition in Niger: A Prospective Cohort Study. Clin. Infect. Dis. 2018, 67, 1027–1034. [Google Scholar] [CrossRef]
- Jimoh, B.O.; Fadipe, M.T.; Emokpae, M.A. Serum copper, zinc, and copper−zinc ratio in children with malaria. Saudi. J. Health Sci. 2022, 11, 119–124. [Google Scholar]
- Vasquez, M.; Zuniga, M.; Rodriguez, A. Oxidative Stress and Pathogenesis in Malaria. Front. Cell. Infect. Microbiol. 2021, 11, 768182. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X.; et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef] [PubMed]
- Grandis, D.J.; Nah, G.; Whitman, I.R.; Vittinghoff, E.; Dewland, T.A.; Olgin, J.E.; Marcus, G.M. Wilson’s Disease and Cardiac Myopathy. Am. J. Cardiol. 2017, 120, 2056–2060. [Google Scholar] [CrossRef]
- Mc Namara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Yalçin, S.S.; Dönmez, Y.; Aypar, E.; Yalçin, S. Element profiles in blood and teeth samples of children with congenital heart diseases in comparison with healthy ones. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2021, 63, 126662. [Google Scholar] [CrossRef]
- Candelino, M.; Tagi, V.M.; Chiarelli, F. Cardiovascular risk in children: A burden for future generations. Ital. J. Pediatr. 2022, 48, 57. [Google Scholar] [CrossRef]
- Development Initiatives. Global Nutrition Report: Shining a Light to Spur Action on Nutrition. Bristol: Development Initiatives Poverty Research. 2022. Available online: https://globalnutritionreport.org/ (accessed on 3 November 2022).
- Liu, C.; Liao, Y.; Zhu, Z.; Yang, L.; Zhang, Q.; Li, L. The association between serum copper concentrations and elevated blood pressure in US children and adolescents: National Health and Nutrition Examination Survey 2011–2016. BMC Cardiovasc. Disord. 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Vivek, S.M.; Dayal, D.; Khaiwal, R.; Bharti, B.; Bhalla, A.; Singh, S.; Kaur, H.; Attri, S.V. Low serum copper and zinc concentrations in North Indian children with overweight and obesity. Pediatr Endocrinol. Diabetes Metab. 2020, 26, 79–83. [Google Scholar] [PubMed]
- Güneş, H.; Alkan Baylan, F.; Güneş, H.; Temiz, F. Can Nesfatin-1 Predict Hypertension in Obese Children? J. Clin. Res. Pediatr Endocrinol. 2020, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zabłocka-Słowińska, K.; Prescha, A.; Płaczkowska, S.; Porębska, I.; Kosacka, M.; Pawełczyk, K. Serum and Whole Blood Cu and Zn Status in Predicting Mortality in Lung Cancer Patients. Nutrients 2020, 13, 60. [Google Scholar] [CrossRef]
- Amer, O.E.; Sabico, S.; Khattak, M.N.K.; Alnaami, A.M.; Aljohani, N.J.; Alfawaz, H.; AlHameidi, A.; Al-Daghri, N.M. Increasing Prevalence of Pediatric Metabolic Syndrome and Its Components among Arab Youth: A Time-Series Study from 2010–2019. Children (Basel) 2021, 8, 1129. [Google Scholar] [CrossRef]
- Alghobashy, A.A.; Alkholy, U.M.; Talat, M.A.; Abdalmonem, N.; Zaki, A.; Ahmed, I.A.; Mohamed, R.H. Trace elements and oxidative stress in children with type 1 diabetes mellitus. Diabetes Metab. Syndr. Obes. 2018, 11, 85–92. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Stefanowicz, F.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Total plasma magnesium, zinc, copper and selenium concentrations in type-I and type-II diabetes. BioMetals 2019, 32, 123–138. [Google Scholar] [CrossRef]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2018, 168, 397–405. [Google Scholar] [CrossRef]
- Carrier, A. Metabolic Syndrome and Oxidative Stress: A Complex Relationship. Antioxid. Redox Signal. 2017, 26, 429–431. [Google Scholar] [CrossRef]
- Yin, J.; Wang, X.; Li, S.; Zhu, Y.; Chen, S.; Li, P.; Luo, C.; Huang, Y.; Li, X.; Hu, X.; et al. Interactions between plasma Cu concentrations and SOD1 gene polymorphism for impaired glucose regulation and type 2 diabetes. Redox Biol. 2019, 24, 101172. [Google Scholar] [CrossRef]
- Barber, R.G.; Grenier, Z.A.; Burkhead, J.L. Copper Toxicity Is Not Just Oxidative Damage: Zinc Systems and Insight from Wilson Disease. Biomedicines 2021, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Fedor, M.; Socha, K.; Urban, B.; Soroczyńska, J.; Matyskiela, M.; Borawska, M.H.; Bakunowicz-Łazarczyk, A. Serum Concentration of Zinc, Copper, Selenium, Manganese, and Cu/Zn Ratio in Children and Adolescents with Myopia. Biol. Trace Elem. Res. 2017, 176, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Gładysiak, A.; Ślęzak, D. Early Intervention and Nonpharmacological Therapy of Myopia in Young Adults. J. Ophthalmol. 2018, 2018, 4680603. [Google Scholar] [CrossRef]
- Mérida, S.; Villar, V.M.; Navea, A.; Desco, C.; Sancho-Tello, M.; Peris, C.; Bosch-Morell, F. Imbalance Between Oxidative Stress and Growth Factors in Human High Myopia. Front. Physiol. 2020, 11, 463. [Google Scholar] [CrossRef]
- Fedor, M.; Urban, B.; Socha, K.; Soroczyńska, J.; Krętowska, M.; Borawska, M.H.; Bakunowicz-Łazarczyk, A. Concentration of Zinc, Copper, Selenium, Manganese, and Cu/Zn Ratio in Hair of Children and Adolescents with Myopia. J. Ophthalmol. 2019, 2019, 56438. [Google Scholar] [CrossRef]
- Murrison, L.B.; Brandt, E.B.; Myers, J.B.; Hershey, G.K.K. Environmental exposures and mechanisms in allergy and asthma development. J. Clin. Investig. 2019, 129, 1504–1515. [Google Scholar] [CrossRef]
- Yalçın, S.S.; Emiralioğlu, N.; Yalçın, S. Evaluation of blood and tooth element status in asthma cases: A preliminary case–control study. BMC Pulm. Med. 2021, 21, 201. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, X.; Hui, Y.; Xiang, H.; Wang, Q.; Xu, S.; Li, L. Sex diference between trace elements and pulmonary functions in children. Biol. Trace Elem. Res. 2020, 197, 405–410. [Google Scholar] [CrossRef]
- Mao, S.; Wu, L.; Shi, W. Association between trace elements levels and asthma susceptibility. Respir. Med. 2018, 145, 110–119. [Google Scholar] [CrossRef]
- Alsharnoubi, J.; Alkharbotly, A.; Waheed, H.; Elkhayat, Z.; Hussein, D.Y. Could we diagnose childhood asthma by LIBS technique? Lasers Med. Sci. 2020, 35, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Bahi, G.A.; Boyvin, L.; Méité, S.; M’Boh, G.M.; Yeo, K.; N’Guessan, K.R.; Bidié, A.D.; Djaman, A.J. Assessments of serum copper and zinc concentration, and the Cu/Zn ratio determination in patients with multidrug resistant pulmonary tuberculosis (MDR-TB) in Côte d’Ivoire. BMC Infect Dis. 2017, 17, 257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Man, Q.; Li, Y.; Lu, J.; Yang, L. Study on Reference Range of Zinc, Copper and Copper/Zinc Ratio in Childbearing Women of China. Nutrients 2021, 13, 946. [Google Scholar] [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Yao, Q. Copper-based biomaterials for bone and cartilage tissue engineering. J. Orthop. Transl. 2021, 29, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yuan, Y.; Tian, J.; Long, F.; Luo, W. The associations between serum trace elements and bone mineral density in children under 3 years of age. Sci. Rep. 2021, 11, 1890. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Bachrach, L.K. Osteoporosis in Children with Chronic Illnesses: Diagnosis, Monitoring, and Treatment. Curr. Osteoporos. Rep. 2017, 15, 271–282. [Google Scholar] [CrossRef]
- Schryver, E.; Klein, G.L.; Herndon, D.N.; Suman, O.E.; Branski, L.K.; Sousse, L.E. Bone metabolism in pediatric burned patients: A review. Burns 2018, 44, 1863–1869. [Google Scholar] [CrossRef]
- Mericq, V.N.; Gajardo, H.C.; Eggers, M.; Avila, A.; Cassorla, F. Effects of treatment with GH alone or in combination with LHRH analog on bone mineral density in pubertal GH-deficient patients. J. Clin. Endocrinol. Metab. 2020, 87, 84–89. [Google Scholar] [CrossRef]
- Fan, Y.; Ni, S.; Zhang, H. Associations of Cu Intake with Bone Mineral Density and Osteoporosis in Adults: Data from the National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2022, 200, 2062–2068. [Google Scholar] [CrossRef]
- Takahashi, A. Role of Zinc and Copper in Erythropoiesis in Patients on Hemodialysis. J. Ren. Nutr. 2022, 32, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Wahidiyat, P.A.; Sastroasmoro, S.; Fucharoen, S.; Setianingsih, I.; Putriasih, S.A. Applicability of a clinical scoring criteria for disease severity of ß-thalassemia/hemoglobin E in Indonesia. Med. J. Indones. 2018, 27, 26–32. [Google Scholar] [CrossRef]
- Alhillawi, Z.H.; Al-Hakeim, H.K.; Moustafa, S.R.; Maes, M. Increased zinc and albumin but lowered Cu in children with transfusion-dependent thalassemia. J. Trace Elem. Med. Biol. 2021, 65, 126713. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers. 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed]
- Hasanato, R. Alterations in serum levels of copper, zinc, and selenium among children with sickle cell anemia. Turk. J. Med. Sci. 2019, 49, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Yousif, O.O.; Hassan, M.K.; Al-Naama, L.M. Red Blood Cell and Serum Magnesium Levels Among Children and Adolescents with Sickle Cell Anemia. Biol. Trace Elem. Res. 2018, 186, 295–304. [Google Scholar] [CrossRef]
- Emokpae, M.A.; Fatimehin, E.B. Copper-To-Zinc Ratio as an Inflammatory Marker in Patients with Sickle Cell Disease. Science 2020, 2, 89. [Google Scholar] [CrossRef]
- Curtin, P.; Austin, C.; Curtin, A.; Gennings, C.; Arora, M. (for the Emergent Dynamical Systems Group); Tammimies, K.; Willfors, C.; Berggren, S.; Siper, P.; Rai, D.; et al. Dynamical features in fetal and postnatal zinc-copper metabolic cycles predict the emergence of autism spectrum disorder. Sci. Adv. 2018, 4, eaat1293. [Google Scholar] [CrossRef]
- Bölte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef]
- Ramaekers, V.T.; Sequeira, J.M.; Thöny, B.; Quadros, E.V. Oxidative Stress, Folate Receptor Autoimmunity, and CSF Findings in Severe Infantile Autism. Autism Res. Treat. 2020, 2020, 9095284. [Google Scholar] [CrossRef]
- Frye, R.E.; Cakir, J.; Rose, S.; Delhey, L.; Bennuri, S.C.; Tippett, M.; Palmer, R.F.; Austin, C.; Curtin, P.; Arora, M. Early life metal exposure dysregulates cellular bioenergetics in children with regressive autism spectrum disorder. Transl. Psychiatry 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Omotosho, I.O.; Akinade, A.O.; Lagunju, I.A.; Yakubu, M.A. Oxidative stress indices in ASD children in Sub-Sahara Africa. J. Neurodev. Disord. 2021, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Higazi, A.; Kamek, H.M.; Abdek-Naeenm, E.A.; Abdykkagm, N.M.; Magriysm, D.M.; Osman, A.M. Expression analysis of selected genes involved in tryptophan metabolic pathways in Egyptian children with Autism Spectrum Disorder and learning disabilities. Sci. Rep. 2021, 11, 6931. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-L.; Mao, S.-S.; Lin, X.; Yang, R.-W.; Zhu, Z.-W. Evaluation of Whole Blood Trace Element Levels in Chinese Children with Autism Spectrum Disorder. Biol. Trace Elem. Res. 2019, 191, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Robberecht, H.; Verlaet, A.; Breynaert, A.; De Bruyne, T.; Hermans, N. Magnesium, Iron, Zinc, Copper and Selenium Status in Attention-Deficit/Hyperactivity Disorder (ADHD). Molecules 2020, 25, 4440. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Notova, S.V.; Chernova, L.N.; Skalny, A.A.; Burtseva, T.I.; Tinkov, A.A. Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD). J. Trace Elements Med. Biol. 2020, 61, 126539. [Google Scholar] [CrossRef]
- Tippairote, T.; Temviriyanukul, P.; Benjapong, W.; Trachootham, D. Hair Zinc and Severity of Symptoms Are Increased in Children with Attention Deficit and Hyperactivity Disorder: A Hair Multi-Element Profile Study. Biol. Trace Element Res. 2017, 179, 185–194. [Google Scholar] [CrossRef]
- Elbaz, F.; Zahra, S.; Hanafy, H. Magnesium, zinc and copper estimation in children with attention deficit hyperactivity disorder (ADHD). Egypt. J. Med. Hum. Genet. 2017, 18, 153–163. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chao, J.C.; Chernova, L.N.; Shakieva, R.A.; Kopylov, P.Y.; Skalny, A.A.; et al. Serum zinc, copper, zinc-to-copper ratio, and other essential elements and minerals in children with attention deficit/hyperactivity disorder (ADHD). J. Trace Elements Med. Biol. 2020, 58, 126445. [Google Scholar] [CrossRef]
- Rucklidge, J.J.; Eggleston, M.J.F.; Darling, K.A.; Stevens, A.J.; Kennedy, M.A.; Frampton, C.M. Can we predict treatment response in children with ADHD to a vitamin-mineral supplement? An investigation into pre-treatment nutrient serum levels, MTHFR status, clinical correlates and demographic variables. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 181–192. [Google Scholar] [CrossRef]
- Ivanova, I.D.; Pal, A.; Simonelli, I.; Atanasova, B.; Ventriglia, M.; Rongioletti, M.; Squitti, R. Evaluation of zinc, copper, and Cu:Zn ratio in serum, and their implications in the course of COVID-19. J. Trace Elem. Med. Biol. 2022, 71, 126944. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Baker, Z.N.; Cobine, P.A.; Leary, S.C. The mitochondrion: A central architect of copper homeostasis. Metallomics 2017, 9, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Borchard, S.; Bork, F.; Rieder, T.; Eberhagen, C.; Popper, B.; Lichtmannegger, J.; Schmitt, S.; Adamski, J.; Klingenspor, M.; Weiss, K.-H.; et al. The exceptional sensitivity of brain mitochondria to copper. Toxicol. Vitr. 2018, 51, 11–22. [Google Scholar] [CrossRef] [PubMed]
| Chronic Conditions | Study Design | Year | N° | Age Years | Serum Cu μg/dL | Serum Zinc μg/dL | Serum Cu/Zinc Ratio | Serum Zinc/Cu Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Control | Cases | Control | Cases | Control | Cases | Control | |||||
| Children | ||||||||||||
| Infected term/preterm [17] (plasma) | CCS | 2017 | 21 | neonates | 52.3 | - | 164.2 ± 43.8 | - | 0.48 * | 0.28 | - | - |
| Small for gestational age (cord blood) [18] | CCS | 2021 | 41 | neonates | 32.6 ± 14.0 | 30.3 ± 10.3 | 61.8 ± 2.4 * | 75.6 ± 1.3 | 0.59 ± 0.061 * | 0.43 ± 0.06 | - | - |
| Malaria-infected children [19] | CSS | 2018 | 200 | 0.5–11 | 104.2 ± 2.8 ** | 95.7 ± 2.8 | 93.4 ± 2.8 ** | 105.6 ± 2.7 | 1.16 ± 0.02 ** | 0.90 ± 0.02 | - | - |
| Autism spectrum disorder [20] ‡ | CCS | 2019 | 113 | 2–5 | 19.9 ± 5.1 | 20.9 ± 4.4 | 80.2 ± 13.4 | 84.9 ± 12.1 | - | - | 8.10 ± 2.71 | 8.68 ± 2.74 |
| Transfusion-dependent thalassemia [21] | CCS | 2021 | 60 | 3–12 | 69.3 ± 3.5 * | 80.8 ± 4.9 | 57.3 ± 4.2 ** | 78.2 ± 3.0 | −0.28 ± 0.12 ¥,** | 0.55 ± 0.17 ¥ | - | - |
| ADHD [22] ∞ | CCS | 2020 | 68 | 4–9 | 1.22 ± 0.2 | 1.18 ± 0.2 | 0.93 ± 0.10 * | 1.007 ± 0.17 * | 1.32 ± 0.27 ** | 1.19 ± 0.22 | - | - |
| Autism spectrum disorder [23] (plasma) | CCS | 2021 | 25 | 5.9 ± 1.4 | 55.3 ± 22 * | 92.3 ± 44.6 | 222.3 ± 63.8 ** | 438.5 ± 185.5 | 2.34 | - | 4.32 ± 1.02 * | 4.88 ± 0.94 |
| Metabolic syndrome [1] (whole blood) # | CSS | 2021 | 911 | 6–12 | 1.01 ± 0.13 | - | 5.21 ± 1.07 | - | 0.20 ± 0.05 | - | - | - |
| Asthma [24] (blood) | CCS | 2021 | 17 | 6–12 | 113 ± 2 | 105 ± 2 | 504 ± 106 * | 586 ± 116 | - | - | 4.54 ± 0.92 * | 5.64 ± 1.03 |
| Autism spectrum disorder [25] ‽ | CCS | 2017 | 20 | 6.6 ± 2.9 | 151.6 ± 54.6 * | 105.4 ± 16.1 | 68.7 ± 26.4 * | 94.7 ± 11.9 | - | - | 0.62 ± 0.2 * | 0.9 ± 0.09 |
| ADHD pre/post DEN treatment [26] ‡ | RTS | 2019 | 71 | 7–12 | 16.0 ± 3.4 | 15.5 ± 3.2 | 12.5 ± 1.8 | 12.6 ± 1.6 | 1.3 ± 0.3 | 1.2 ± 0.2 | - | - |
| Severe infantile autism [27] | CCS | 2020 | 38 | 7.25 ± 3.9 | 113 ± 3 * | 99 ± 25 | 84 ± 16 | 84 ± 11 | 1.34 ± 0.35 * | 1.17 ± 0.23 | - | - |
| Congenital heart disease [28] (blood) # | CCS | 2021 | 39 | 8.2 ± 1.8 | 1.1 ± 0.2 | 1.1 ± 0.1 | 5.7 ± 1.2 | 5.9 ± 1.3 | - | - | 5.26 ± 0.73 | 5.28 ± 0.99 |
| Sickle cell anemia [29] ° | CSS | 2019 | 33 | 8.5 ± 4.1 | 130 ** | 88 | 61 ** | 94 | 1.92 | 0.98 | - | - |
| Overweight-obese/healthy children [30] | CSS | 2020 | 69 | 10.9 ± 1.9 | 109.9 ± 47.9 ** | 206.4 ± 100.7 | 85.2 ± 40.6 ** | 152.9 ± 79.7 | 1.32 ± 0.33 | 1.51 ± 0.85 | - | - |
| Children and adolescents | ||||||||||||
| A series of chronic diseases [2] | CSS | 2021 | 78 | 1–19 | 118 ± 29 | - | 87 ± 12 | - | 1.4 ± 0.4 | - | 0.8 ± 0.6 | - |
| Obese/eutrophic patients | 119 ± 23 | 122 ± 3 | 87 ± 12 | 88 ± 13 | 1.4 ± 0.2 | 1.4 ± 0.4 | 0.7 ± 1.4 | 0.8 ± 0.2 | ||||
| Undernutrition/eutrophic patients | 114 ± 35 | 122 ± 3 | 85 ± 13 | 88 ± 13 | 1.4 ± 0.2 | 1.4 ± 0.4 | 0.9 ± 0.9 | 0.8 ± 0.2 | ||||
| Sickle cell anemia [31] | CCS | 2019 | 100 | 4–20 | 112.1 ± 2.4 ** | 102.6 ± 1.6 | 40.5 ± 1.8 ** | 54.6 ± 1.2 | 3.35 ± 0.16 ** | 1.93 ± 0.05 | - | - |
| In steady clinical state | 74 | 105.8 ± 2.5 | 102.6 ± 1.6 | 46.3 ± 1.9 ** | 54.6 ± 1.2 | 2.57 ± 0.107 ** | 1.94 ± 0.052 | - | - | |||
| In painful crisis/in steady clinical state | 26 | 131.1 ± 4.3 ** | 105.8 ± 2.5 | 24.1 ± 0.9 ** | 46.3 ± 1.9 | 5.59 ± 0.249 ** | 2.57 ± 0.107 | - | - | |||
| Adolescents | ||||||||||||
| Myopic patients [32] | CCS | 2017 | 83 | 14.36 ± 2.49 | 95.6 ± 28.2 | 92.6 ± 18.3 | 86.5 ± 22 ** | 105.4 ± 17.4 | 0.99 ± 0.20 ** | 1.196 ± 0.45 | - | - |
| Cystic fibrosis [3] | CSS | 2020 | 17 | 14.8 ± 8 | 113 ± 23.5 | - | 87.2 ± 16.7 | - | 1.32 ± 0.28 | - | 0.79 ± 0.18 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper/Zinc Ratio in Childhood and Adolescence: A Review. Metabolites 2023, 13, 82. https://doi.org/10.3390/metabo13010082
Escobedo-Monge MF, Barrado E, Parodi-Román J, Escobedo-Monge MA, Torres-Hinojal MC, Marugán-Miguelsanz JM. Copper/Zinc Ratio in Childhood and Adolescence: A Review. Metabolites. 2023; 13(1):82. https://doi.org/10.3390/metabo13010082
Chicago/Turabian StyleEscobedo-Monge, Marlene Fabiola, Enrique Barrado, Joaquín Parodi-Román, María Antonieta Escobedo-Monge, María Carmen Torres-Hinojal, and José Manuel Marugán-Miguelsanz. 2023. "Copper/Zinc Ratio in Childhood and Adolescence: A Review" Metabolites 13, no. 1: 82. https://doi.org/10.3390/metabo13010082
APA StyleEscobedo-Monge, M. F., Barrado, E., Parodi-Román, J., Escobedo-Monge, M. A., Torres-Hinojal, M. C., & Marugán-Miguelsanz, J. M. (2023). Copper/Zinc Ratio in Childhood and Adolescence: A Review. Metabolites, 13(1), 82. https://doi.org/10.3390/metabo13010082