Aerobic Glycolysis: A DeOxymoron of (Neuro)Biology
Abstract
1. Introduction
2. Functional Brain Energy Metabolism
3. ‘Aerobic Glycolysis’ Is a Paradox
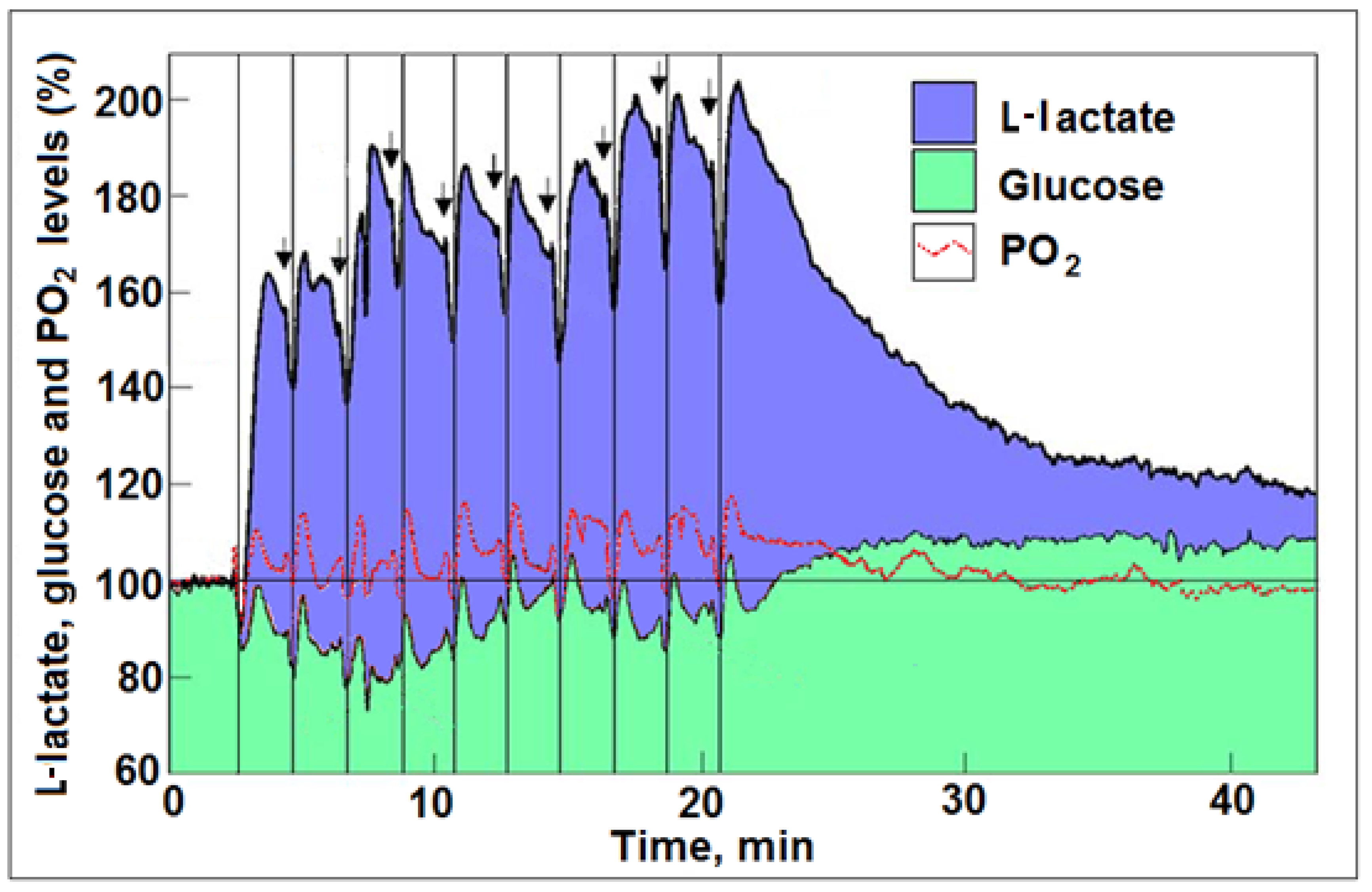
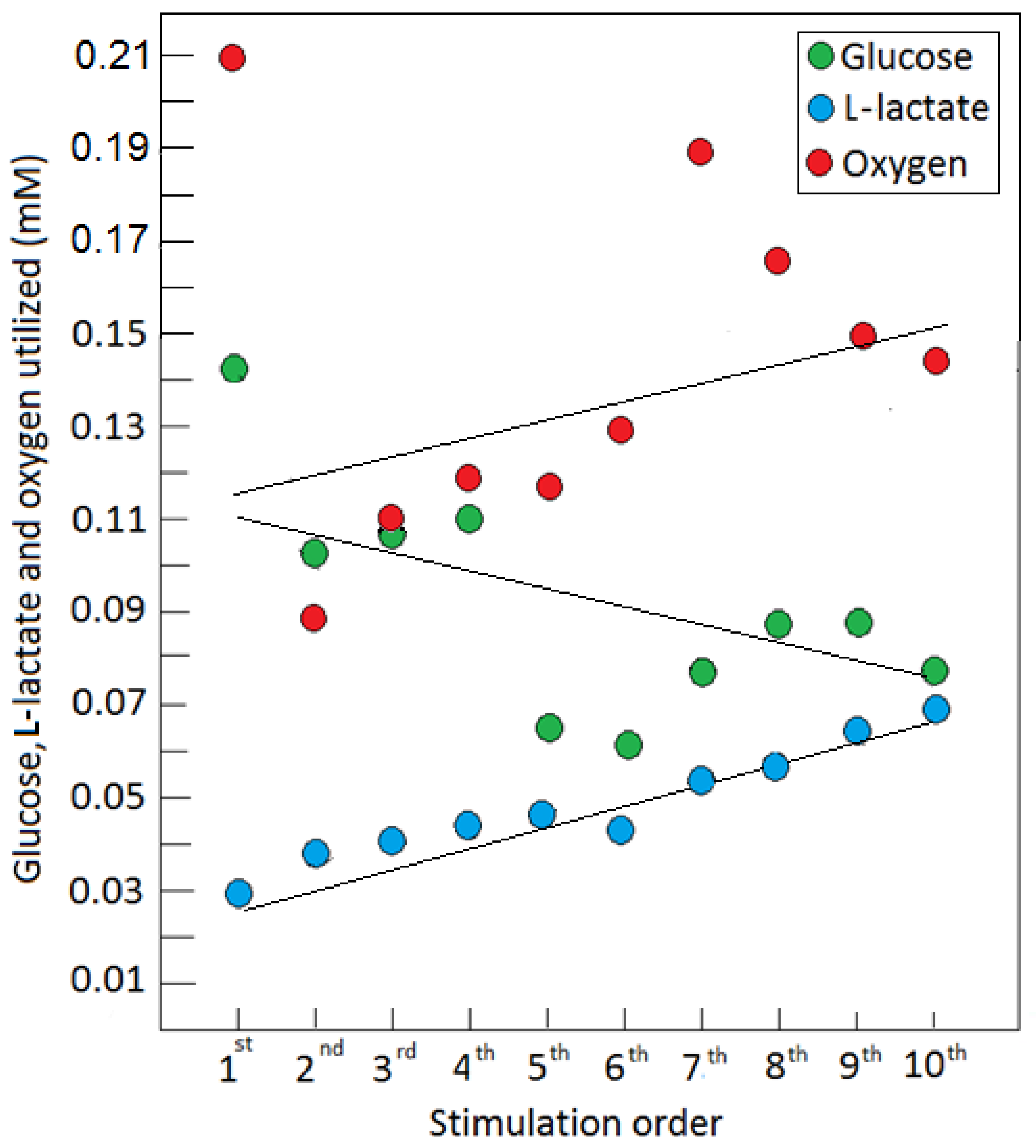
4. Astroglial Glycolysis and Neuronal Oxphos—The Lactate Shuttle at Work
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Warburg, O. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.T.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tsukamoto, H.; Takenaka, S.; Olesen, N.D.; Petersen, L.G.; Sørensen, H.; Nielsen, H.B.; Secher, N.H.; Ogoh, S. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 2018, 32, 1417–1427. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tsukamoto, H.; Ando, S.; Ogoh, S. Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites 2021, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Theriault, J.E.; Shaffer, C.; Dienel, G.A.; Sander, C.Y.; Hooker, J.M.; Rosen, B.R.; Dickerson, B.C.; Whitfield-Gabrieli, S.; Feldman Barrett, L.; Quigley, K.S. Aerobic glycolysis, the efficiency tradeoff hypothesis, and the biological basis of neuroimaging: A solution to a metabolic mystery at the heart of neuroscience. PsyArXiv 2021. [Google Scholar] [CrossRef]
- Hill, L.; Nabarro, D.N. On the exchange of blood-gases in brain and muscle during states of rest and activity. J. Physiol. 1895, 18, 218–229. [Google Scholar] [CrossRef][Green Version]
- Tashiro, S. Carbon dioxide production from nerve fibres when resting and when stimulated; a contribution to the chemical basis of irritability. Am. J. Physiol. 1913, 32, 107–136. [Google Scholar] [CrossRef]
- Sokoloff, L.; Reivich, M.; Kennedy, C.; Des Rosiers, M.H.; Patlak, C.S.; Pettigrew, K.D.; Sakurada, O.; Shinohara, M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977, 28, 897–916. [Google Scholar] [CrossRef]
- Sokoloff, L. Localization of Functional Activity in the Central Nervous System by Measurement of Glucose Utilization with Radioactive Deoxyglucose. J. Cereb. Blood Flow Metab. 1981, 1, 7–36. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.L.; Hasselbalch, S.G.; Hagemann, L.P.; Olsen, K.S.; Bulow, J.; Holm, S.; Wildschiliodtz, G.; Paulson, O.B.; Lassen, N.A. Persistent Resetting of the Cerebral Oxygen/Glucose Uptake Ratio by Brain Activation: Evidence Obtained with the Kety-Schmidt Technique. J. Cereb. Blood Flow Metab. 1995, 15, 485–491. [Google Scholar] [CrossRef]
- Madsen, P.L.; Linde, R.; Hasselbalch, S.G.; Paulson, O.B.; Lassent, N.A. Activation-Induced Resetting of Cerebral Oxygen and Glucose Uptake in the Rat. J. Cereb. Blood Flow Metab. 1998, 18, 742–748. [Google Scholar] [CrossRef]
- Madsen, P.L.; Cruz, N.F.; Sokoloff, L.; Dienel, G.A. Cerebral Oxygen/Glucose Ratio is Low during Sensory Stimulation and Rises above Normal during Recovery: Excess Glucose Consumption during Stimulation is Not Accounted for by Lactate Efflux from or Accumulation in Brain Tissue. J. Cereb. Blood Flow Metab. 1999, 19, 393–400. [Google Scholar] [CrossRef]
- Lowry, J.P.; Fillenz, M. Evidence for uncoupling of oxygen and glucose utilization during neuronal activation in rat striatum. J. Physiol. 1997, 498, 497–501. [Google Scholar] [CrossRef]
- Vlassenko, A.G.; Rundle, M.M.; Mintun, M.A. Human brain glucose metabolism may evolve during activation: Findings from a modified FDG PET paradigm. Neuroimage 2006, 33, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garcia, C.M.; Mongeon, R.; Lahmann, C.; Koveal, D.; Zucker, H.; Yellen, G. Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 2017, 26, 361–374. [Google Scholar] [CrossRef]
- Lipton, P.; Robacker, K. Glycolysis and brain function: [K+]o stimulation of protein synthesis and K+ uptake require glycolysis. Fed. Proceed. 1983, 42, 2875–2880. [Google Scholar]
- Balaban, R.S.; Bader, J.P. Studies on the relationship between glycolysis and (Na++K+)-ATPase in cultured cells. Biochim. Biophys. Acta 1984, 17, 419–426. [Google Scholar] [CrossRef]
- Malonek, D.; Grinvald, A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: Implications for functional brain mapping. Science 1996, 272, 551–554. [Google Scholar] [CrossRef]
- Buxton, R.B.; Frank, L.R. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J. Cereb. Blood Flow Metab. 1997, 17, 64–72. [Google Scholar] [CrossRef]
- Hu, Y.; Wilson, G.S. A temporary local energy pool coupled to neuronal activity: Fluctuation of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef]
- Hyder, F.; Rothman, D.L.; Mason, G.F.; Rangarajan, A.; Behar, K.L.; Shulman, R.G. Oxidative glucose metabolism in rat brain during single forepaw stimulation: A spatially localized 1H[13C]nuclear magnetic resonance study. J. Cereb. Blood Flow Metab. 1997, 17, 1040–1047. [Google Scholar] [CrossRef]
- Vanzetta, I.; Grinvald, A. Increased cortical oxidative metabolism due to sensory stimulation: Implication for functional brain imaging. Science 1999, 286, 1555–1558. [Google Scholar] [CrossRef]
- Gjedde, A.; Marrett, S.; Vaface, M. Oxidative and nonoxidative metabolism of excited neurons and astrocytes. J. Cereb. Blood Flow Metab. 2002, 22, 1–14. [Google Scholar] [CrossRef]
- Thompson, J.K.; Peterson, M.R.; Freemean, R.D. Single-neuron activity and tissue oxygenation in cerebral cortex. Science 2003, 299, 1070–1072. [Google Scholar] [CrossRef]
- Giove, F.; Mangia, S.; Bianciardi, M.; Garreffa, G.; Di Salle, F.; Morrone, R.; Maraviglia, B. The physiology and metabolism of neuronal activation: In vivo studies by NMR and other methods. Magn. Reson. Imaging 2003, 21, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.G.; Rothman, D.L.; Behar, K.L.; Hyder, F. Energetic basis of brain activity: Implications for neuroimaging. Trends Neurosci. 2004, 27, 491–495. [Google Scholar] [CrossRef]
- Buxton, R.B.; Wong, E.C.; Frank, L.R. Dynamics of blood flow and oxygenation changes during brain activation: The balloon model. Magn. Reson. Med. 1998, 39, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Hyder, F.; Shulman, R.G.; Rothman, D.L. Regulation of Cerebral Oxygen Delivery. In Oxygen Transport to Tissue XXI; Eke, A., Delpy, D.T., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; Volume 471, pp. 99–110. [Google Scholar] [CrossRef]
- Obata, T.; Liu, T.T.; Miller, K.L.; Luh, W.-M.; Wong, E.C.; Frank, L.R.; Buxton, R.B. Discrepancies between BOLD and flow dynamics in primary and supplementary motor areas: Application of the balloon model to the interpretation of BOLD transients. NeuroImage 2004, 21, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Gozal, E. Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress. Front. Pharmacol. 2012, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. Glycolysis Paradigm Shift Dictates a Reevaluation of Glucose and Oxygen Metabolic Rates of Activated Neural Tissue. Front. Neurosci. 2018, 12, 700. [Google Scholar] [CrossRef] [PubMed]
- Choeiri, C.; Staines, W.; Miki, T.; Seino, S.; Messier, C. Glucose transporter plasticity during memory processing. Neuroscience 2005, 130, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. Lactate: The ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006, 26, 142–152. [Google Scholar] [CrossRef]
- Schurr, A.; Payne, R.S. Lactate, not pyruvate, is neuronal aerobic glycolysis end product: An in vitro electrophysiological study. Neuroscience 2007, 147, 613–619. [Google Scholar] [CrossRef]
- Schurr, A. Cerebral glycolysis: A century of persistent misunderstanding and misconception. Front. Neurosci. 2014, 8, 1–18. [Google Scholar] [CrossRef]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, B.L. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Schurr, A.; Portincasa, P. Mitochondrial Transport in Glycolysis and Gluconeogenesis: Achievements and Perspectives. Int. J. Mol. Sci. 2021, 22, 12620. [Google Scholar] [CrossRef]
- Hyder, F.; Shulman, R.G.; Rothman, D.L. A model for the regulation of cerebral oxygen delivery. J. Appl. Physiol. 1998, 85, 554–564. [Google Scholar] [CrossRef]
- Prichard, J.; Rothman, D.; Novotny, E.; Pertroff, O.; Kuwabara, T.; Avisom, M.; Howseman, A.; Hanstock, C.; Shulman, R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc. Natl. Acad. Sci. USA 1991, 88, 5829–5831. [Google Scholar] [CrossRef]
- Sappey-Marinier, D.; Calabrese, G.; Fein, G.; Hugg, J.W.; Biggins, C.; Weiner, M.W. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 1992, 12, 584–592. [Google Scholar] [CrossRef]
- Schurr, A.; Miller, J.J.; Payne, R.S.; Rigor, B.M. An increase in lactate output by brain tissue serves to meet energy needs of glutamate-activated neurons. J. Neurosci. 1999, 19, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Hagberg, A.; Haraldseth, O.; Unsgard, G.; Sonnewald, U. 13C MR Spectoscopy study of lactate as substrate for rat brain. Dev. Neurosci. 2000, 22, 429–436. [Google Scholar] [CrossRef]
- Dalsgaard, M.K.; Quistorff, B.; Danielsen, E.R.; Selmer, C.; Vogelsang, T.; Secher, N.H. A reduced cerebral metabolic ration in exercise reflects metabolism and not accumulation of lactate within the human brain. J. Physiol. 2003, 554, 571–578. [Google Scholar] [CrossRef]
- Urrila, A.S.; Hakkarainen, A.; Heikkinen, S.; Vuori, K.; Stenberg, D.; Hakkinen, A.-M.; Lundbom, N.; Porkka-Heiskanen, T. Metabolic imaging of human cognition: An fMRI/1H-MRS study of brain lactate response to silent word generation. J. Cereb. Blood Flow Metab. 2003, 23, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Pernet, A.; Hallett, W.A.; Bingham, E.; Marsden, P.K.; Amiel, S.A. Lactate: A preferred fuel for human brain metabolism in vivo. J. Cereb. Blood Flow Metab. 2003, 23, 658–664. [Google Scholar] [CrossRef]
- Medina, J.M.; Tabernero, A. Lactate utilization by brain cells and its role in CNS development. J. Neurosci. Res. 2005, 79, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hussien, R.; Cho, H.-S.; Kaufer, D.; Brooks, G.A. Evidence for the mitochondrial lactate oxidation complex in rat neurons: Demonstration of an essential component of brain lactate shuttles. PLoS ONE 2008, 3, e2915. [Google Scholar] [CrossRef]
- Passarella, S.L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Atlante, A. Mitochondria and l-lactate metabolism. FEBS Lett. 2008, 582, 3576. [Google Scholar] [CrossRef]
- Quistorff, B.; Secher, N.H.; Van Lieshout, J.J. Lactate fuels the human brain during exercise. FASEB J. 2008, 22, 3443–3449. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Jolivet, R.; Buck, A.; Magistretti, P.J.; Weber, B. In vivo evidence for lactate as a neuronal energy source. J. Neurosci. 2011, 31, 7477–7485. [Google Scholar] [CrossRef]
- Dienel, A. Lactate muscles its way into consciousness: Fueling brain activation. Am. J. Physiol. 2004, 287, R519–R521. [Google Scholar] [CrossRef]
- Rosenthal, M.; Sick, T.J. Glycolytic and oxidative metabolic contributions to potassium ion transport in rat cerebral cortex. Can. J. Physiol. Pharmacol. 1992, 70, S165–S169. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- De Bari, L.; Chieppa, G.; Marra, E.; Passarella, S. L-lactate metabolism can occur in normal and cancer prostate cells via the novel mitochondrial L-lactate dehydrogenase. Int. J. Oncol. 2010, 37, 1607–1620. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pizzuto, R.; Paventi, G.; Porcile, C.; Sarnataro, D.; Daniele, A.; Passarella, S. l-Lactate metabolism in HEP G2 cell mitochondria due to the l-lactate dehydrogenase determines the occurrence of the lactate/pyruvate shuttle and the appearance of oxaloacetate, malate and citrate outside mitochondria. Biochim. Biophys. Acta 2012, 1817, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Schurr, A. l-Lactate Transport and Metabolism in Mitochondria of Hep G2 Cells—The Cori Cycle Revisited. Front. Oncol. 2018, 8, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B. Lactate metabolism: A new paradigm for the new millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef]
- Bonvento, G.; Herard, A.-S.; Voutsinos-Porche, B. The astrocyte–neuron lactate shuttle: A debated but still valuable hypothesis for brain imaging. J. Cereb. Blood Flow Metab. 2005, 25, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, A.; Gallopin, T.; Lacroix, A.; Plaisier, F.; Piquet, J.; Geoffroy, H.; Hepp, R.; Naudé, J.; Le Gac, B.; Egger, R.; et al. Lactate is an energy substrate for rodent cortical neurons and enhances their firing activity. eLife 2021, 10, e71424. [Google Scholar] [CrossRef]
- Béland-Millar, A.; Larcher, J.; Courtemanche, J.; Yuan, T.; Messier, C. Effects of Systemic Metabolic Fuels on Glucose and Lactate Levels in the Brain Extracellular Compartment of the Mouse. Front. Neurosci. 2017, 11, 7. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate: Glycolytic End Product and Oxidative Substrate During Sustained Exercise in Mammals—The “Lactate Shuttle”. In Circulation, Respiration, and Metabolism; Gilles, R., Ed.; Proceedings in Life Sciences; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate production under fully aerobic conditions: The lactate shuttle during rest and exercise. Fed. Proc. 1986, 45, 2924–2929. [Google Scholar]
- Brooks, G.A. Role of the Heart in Lactate Shuttling. Front. Nutr. 2021, 8, 663560. [Google Scholar] [CrossRef]
- Bittner, C.X.; Valdebenito, R.; Ruminot, I.; Loaiza, A.; Larenas, V.; Sotelo-Hitschfeld, T.; Moldenhauer, H.; San Martín, A.; Gutie’rrez, R.; Zambrano, M.; et al. Fast and Reversible Stimulation of Astrocytic Glycolysis by K_ and a Delayed and Persistent Effect of Glutamate. J. Neurosci. 2011, 31, 4709–4713. [Google Scholar] [CrossRef] [PubMed]
- Perge, J.A.; Koch, K.; Miller, R.; Sterling, P.; Balasubramanian, V. How the Optic Nerve Allocates Space, Energy Capacity, and Information. J. Neurosci. 2009, 29, 7917–7928. [Google Scholar] [CrossRef] [PubMed]
- Perge, J.A.; Niven, J.E.; Mugnaini, E.; Balasubramanian, V.; Sterling, P. Why Do Axons Differ in Caliber? J. Neurosci. 2012, 32, 626–638. [Google Scholar] [CrossRef]
- Smith, G.M.; Gallo, G. The role of mitochondria in axon development and regeneration. Dev. Neurobiol. 2018, 78, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Rawson, R.L.; Yam, L.; Weimer, R.M.; Bend, E.G.; Hartwieg, E.; Horvitz, H.R.; Clark, S.G.; Jorgensen, E.M. Axons Degenerate in the Absence of Mitochondria in C. elegans. Curr. Biol. 2014, 24, 760–765. [Google Scholar] [CrossRef]
- Cairns, C.B.; Walther, J.; Harken, A.H.; Banerjea, E. Mitochondrial oxidative phosphorylation thermodynamic efficiencies reflect physiological organ roles. Dev. Physiol. 1998, 274, R1376–R1383. [Google Scholar] [CrossRef]
- Margolis, H. Paradigms and Barriers: How Habits of Mind Govern Scientific Beliefs; The University of Chicago Press, Ltd.: London, UK, 1993. [Google Scholar]
- John, H. “Heavy Metals”—A Meaningless Term. Chem. Int. Newsmag. IUPAC 2001, 23, 163–167. [Google Scholar] [CrossRef]
- Pasquinelli, E. Neuromyths: Why Do They Exist and Persist? Mind Brain Educ. 2012, 6, 89–96. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schurr, A.; Passarella, S. Aerobic Glycolysis: A DeOxymoron of (Neuro)Biology. Metabolites 2022, 12, 72. https://doi.org/10.3390/metabo12010072
Schurr A, Passarella S. Aerobic Glycolysis: A DeOxymoron of (Neuro)Biology. Metabolites. 2022; 12(1):72. https://doi.org/10.3390/metabo12010072
Chicago/Turabian StyleSchurr, Avital, and Salvatore Passarella. 2022. "Aerobic Glycolysis: A DeOxymoron of (Neuro)Biology" Metabolites 12, no. 1: 72. https://doi.org/10.3390/metabo12010072
APA StyleSchurr, A., & Passarella, S. (2022). Aerobic Glycolysis: A DeOxymoron of (Neuro)Biology. Metabolites, 12(1), 72. https://doi.org/10.3390/metabo12010072







