Effects of Soaking Tempe in Vinegar on Metabolome and Sensory Profiles
Abstract
:1. Introduction
2. Results and Discussion
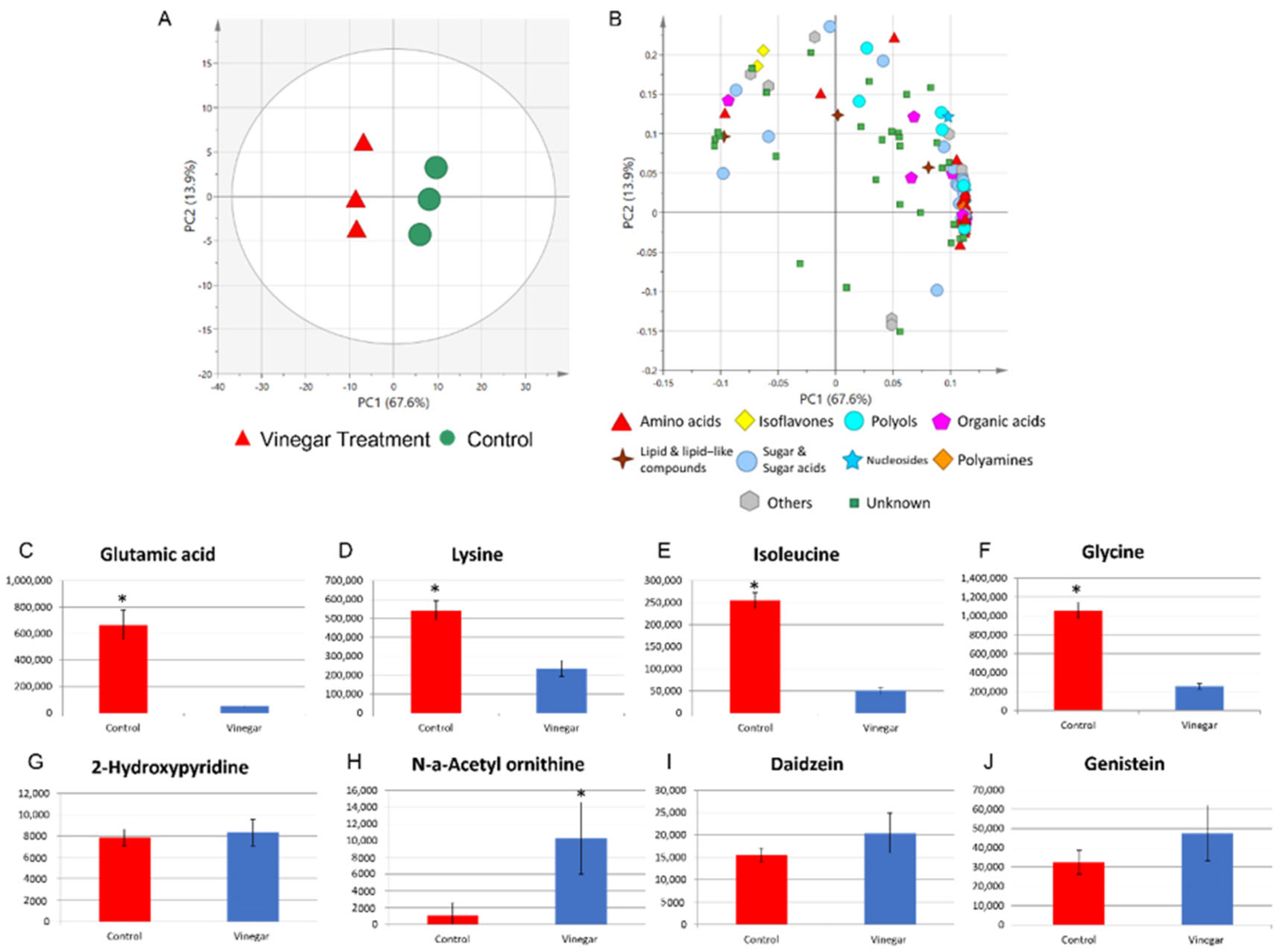
2.1. Metabolite Profiles of Tempe with and without Soaking in Vinegar over Time
2.2. Sensory Differences between Tempe Fermented with and without Vinegar Assessed Using Triangle Tests
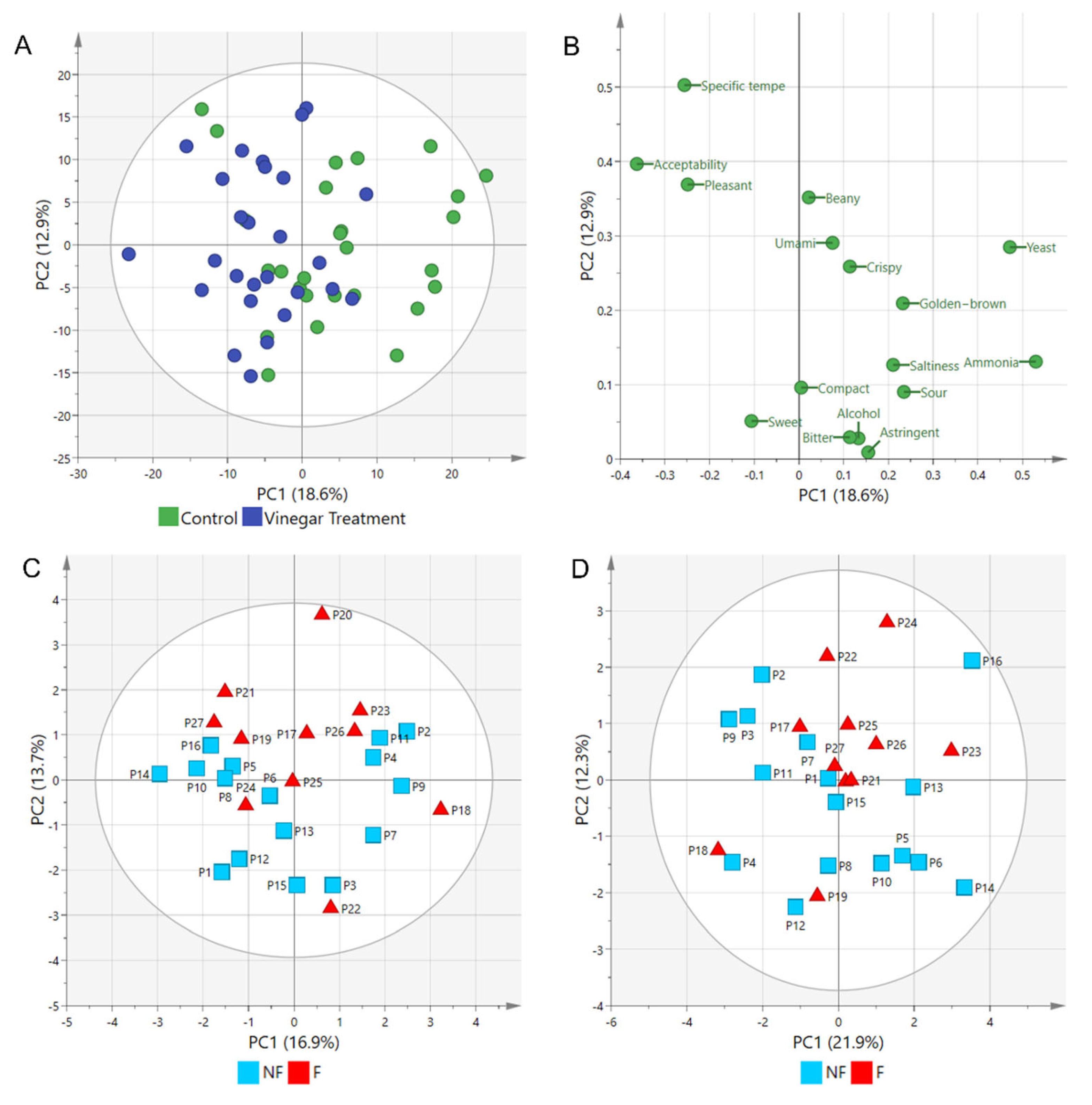
2.3. Sensory Attributes of Tempe Fermented with and without Vinegar Using Rate-All-That-Apply (RATA) Tests
2.4. Metabolite Profile of Control Tempe and Vinegar-Treated Tempe
3. Materials and Methods
3.1. Sample Preparation
3.2. Metabolite Extraction and Derivatization
3.3. GC/MS Analysis
3.4. Metabolome Data Analysis
3.5. Cooking and Serving Method for Triangle and Rate-All-That-Apply (RATA) Tests
3.6. Triangle Test
3.7. Rate-All-That-Apply (RATA) Tests
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A semicentennial review on its health benefits, fermentation, safety, processing, sustainability, and affordability. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1717–1767. [Google Scholar] [CrossRef] [PubMed]
- Kadar, A.D.; Aditiawati, P.; Astawan, M.; Putri, S.P.; Fukusaki, E. Gas chromatography coupled with mass spectrometry-based metabolomics for the classification of tempe from different regions and production processes in Indonesia. J. Biosci. Bioeng. 2018, 126, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Nout, M.; Kiers, J. Tempe fermentation, innovation and functionality: Update into the third millenium. J. Appl. Microbiol. 2005, 98, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Astuti, M.; Andreanyta, M.; Dalais, F.S.; Wahlqvist, M.L. Tempe, a nutritious and healthy food from Indonesia. Asia Pac. J. Clin. Nutr. 2000, 9, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Kementerian Perindustrian Republik Indonesia. Industri Tempe-Tahu Pangkas Separuh Volume Produksi. Available online: https://kemenperin.go.id/artikel/7222/profil/71/profil/71/rencana-strategis-kementerian-perindustrian (accessed on 9 December 2021).
- Astawan, M.; Wresdiyati, T.; Maknun, L. Tempe Sumber Zat Gizi Dan Komponen Bioaktif Untuk Kesehatan; PT Penerbit IPB Press: Bogor, Indonesia, 2017. [Google Scholar]
- Pan, Z.; Tangratanavalee, W. Characteristics of soybeans as affected by soaking conditions. LWT Food Sci. Technol. 2003, 36, 143–151. [Google Scholar] [CrossRef]
- Mulyowidarso, R.K.; Fleet, G.H.; Buckle, K.A. The microbial ecology of soybean soaking for tempe production. Int. J. Food Microbiol. 1989, 8, 35–46. [Google Scholar] [CrossRef]
- Starzynska-Janiszewska, A.; Stodolak, B.; Mickowska, B. Effect of controlled lactic acid fermentation on selected bio-active and nutritional parameters of tempeh obtained from unhulled common bean (Phaseolus vulgaris) seeds. J. Sci. Food Agric. 2014, 94, 359–366. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. A special report on the history of traditional fermented soyfoods. In History of Soybeans and Soyfoods, 1100 B.C. to the 1980s; Shurtleff, W., Aoyagi, A., Eds.; Soyinfo Center: Lafayette, CA, USA, 2007. [Google Scholar]
- History of Tempeh and Tempeh Products (1815–2020): 4673 References in Chronological Order. In History of Tempeh and Tempeh Products (1815–2020): Bibliography and Sourcebook; Shurtleff, W.; Aoyagi, A. (Eds.) Soyinfo Center: Lafayette, CA, USA, 2020; pp. 149–162. [Google Scholar]
- German, J.B.; Hammock, B.D.; Watkins, S.M. Metabolomics: Building on a century of biochemistry to guide human health. Metabolomics 2005, 1, 3–9. [Google Scholar] [CrossRef]
- Jumhawan, U.; Putri, S.P.; Yusianto; Marwani, E.; Bamba, T.; Fukusaki, E. Selection of discriminant markers for authentication of Asian palm civet coffee (kopi luwak): A metabolomics approach. J. Agric. Food Chem. 2013, 61, 7994–8001. [Google Scholar] [CrossRef]
- Putri, S.P.; Irifune, T.; Yusianto; Fukusaki, E. GC/MS based metabolite profiling of Indonesian specialty coffee from different species and geographical origin. Metabolomics 2019, 15, 126. [Google Scholar] [CrossRef]
- Rahmawati, D.; Astawan, M.; Putri, S.P.; Fukusaki, E. Gas chromatography-mass spectrometry-based metabolite profiling and sensory profile of Indonesian fermented food (tempe) from various legumes. J. Biosci. Bioeng. 2021, 132, 487–495. [Google Scholar] [CrossRef]
- Cao, Z.-H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.-Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef]
- Kadar, A.D.; Astawan, M.; Putri, S.P.; Fukusaki, E. Metabolomics-based study of the effect of raw materials to the end product of tempe—an Indonesian fermented soybean. Metabolites 2020, 10, 367. [Google Scholar] [CrossRef]
- Kameda, T.; Aoki, H.; Yanaka, N.; Kumrungsee, T.; Kato, N. Production of isoflavone aglycone-enriched tempeh with rhizopus stolonifer. Food Sci. Technol. Res. 2018, 24, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Denardi-Souza, T.; Massarolo, K.C.; Tralamazza, S.M.; Badiale-Furlong, E. Monitoring of fungal biomass changed by Rhizopus oryzae in relation to amino acid and essential fatty acids profile in soybean meal, wheat and rice. CyTA-J. Food 2017, 16, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Rehms, H.; Barz, W. Degradation of stachyose, raffinose, melibiose and sucrose by different tempe-producing rhizopus fungi. Appl. Microbiol. Biotechnol. 1995, 44, 47–52. [Google Scholar] [CrossRef]
- Sinkinson, C. Chapter 7—Triangle Test. In Discrimination Testing in Sensory Science; Rogers, L., Ed.; Woodhead Publishing: Duxford, UK, 2017; pp. 153–170. [Google Scholar]
- Appendix 2—Statistical tables. In Discrimination Testing in Sensory Science; Rogers, L. (Ed.) Woodhead Publishing: Duxford, UK, 2017; pp. 363–487. [Google Scholar]
- Allen, M.; Poggiali, D.; Whitaker, K.; Marshall, T.R.; van Langen, J.; Kievit, R.A. Raincloud plots: A multi-platform tool for robust data visualization. Wellcome Open Res. 2021, 4, 63. [Google Scholar] [CrossRef]
- Ruijter, G.J.G.; Visser, J.; Rinzema, A. Polyol accumulation by Aspergillus oryzae at low water activity in solid-state fermentation. Microbiology 2004, 150, 1095–1101. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Chang, S.K.; Zhang, Y. Innovative soaking and grinding methods and cooking affect the retention of isoflavones, antioxidant and antiproliferative properties in soymilk prepared from black soybean. J. Food Sci. 2016, 81, H1016–H1023. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K. Science of umami taste: Adaptation to gastronomic culture. Flavour 2015, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Schindler, A.; Dunkel, A.; Stähler, F.; Backes, M.; Ley, J.; Meyerhof, W.; Hofmann, T. Discovery of salt taste enhancing arginyl dipeptides in protein digests and fermented fish sauces by means of a sensomics approach. J. Agric. Food Chem. 2011, 59, 12578–12588. [Google Scholar] [CrossRef] [PubMed]
- Hemmler, D.; Roullier-Gall, C.; Marshall, J.; Rychlik, M.; Taylor, A.J.; Schmitt-Kopplin, P. Insights into the chemistry of non-enzymatic browning reactions in different ribose-amino acid model systems. Sci. Rep. 2018, 8, 16879. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How maillard reaction influences sensorial properties (color, flavor and texture) of food products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Maga, J.A. Pyridines in Foods. J. Agric. Food Chem. 1981, 29, 895–898. [Google Scholar] [CrossRef]
- Sawano, S.; Oza, K.; Murakami, T.; Nakamura, M.; Tatsumi, R.; Mizunoya, W. Effect of gender, rearing, and cooking on the metabolomic profile of porcine muscles. Metabolites 2020, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Roland, W.S.; Vincken, J.-P.; Gouka, R.J.; van Buren, L.; Gruppen, H.; Smit, G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors htas2r14 and htas2r39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, V.; Holik, A.-K.; Hölz, K.; Dingjan, T.; Hans, J.; Ley, J.P.; Krammer, G.E.; Niv, M.Y.; Somoza, M.M.; Somoza, V. Bitter-tasting amino acids l-arginine and l-isoleucine differentially regulate proton secretion via t2r1 signaling in human parietal cells in culture. J. Agric. Food Chem. 2020, 68, 3434–3444. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Kustyawati, M.E. Profile of aroma compounds and acceptability of modified tempeh. Int. Food Res. J. 2017, 24, 734–740. [Google Scholar]
- CXS 313R-2013; Regional Standard for Tempe. Codex Alimentarius: Rome, Italy, 2017.
- ISO 5492:2008; Sensory analysis—Vocabulary. ISO: Geneva, Switzerland, 2008.
- Pujchakarn, T.; Suwonsichon, S.; Suwonsichon, T. Development of a sensory lexicon for a specific subcategory of soy sauce: Seasoning soy sauce. J. Sens. Stud. 2016, 31, 443–452. [Google Scholar] [CrossRef]
- Lee, J.; Chambers, D.H. A lexicon for flavor descriptive analysis of green tea. J. Sens. Stud. 2007, 22, 256–272. [Google Scholar] [CrossRef]
- Meyners, M.; Jaeger, S.R.; Ares, G. On the analysis of rate-all-that-apply (RATA) Data. Food Qual. Prefer. 2016, 49, 1–10. [Google Scholar] [CrossRef]
- JASP Team. Jasp (Version 0.15). Available online: https://jasp-stats.org (accessed on 23 December 2021).



| Number of panelists | 25 (13 male, 12 female) |
| Sample | 2 × Control tempe (48 h) 1 × Vinegar-treated tempe (72 h) |
| Number of panelists successful distinguishing vinegar-treated tempe | 15 |
| Alpha risk study (Differentiation test) | Significantly different (α = 0.01) |
| Sensory Attributes | Tempe Types | Median | Mean | Std. Deviation | p-Value of Shapiro–Wilk | p-Value of Mann–Whitney U |
|---|---|---|---|---|---|---|
| Golden-brown | Control | 3 | 3.37 | 0.839 | 0.004 | 0.009 |
| Vinegar | 3 | 2.741 | 0.764 | 0.001 | ||
| Compact | Control | 3 | 3.148 | 0.907 | <0.001 | 0.503 |
| Vinegar | 3 | 3.296 | 0.869 | 0.003 | ||
| Crispy | Control | 3 | 2.481 | 0.975 | 0.006 | 0.207 |
| Vinegar | 2 | 2.148 | 0.864 | 0.002 | ||
| Beany | Control | 3 | 3.037 | 1.16 | 0.032 | 0.666 |
| Vinegar | 3 | 3.148 | 0.949 | 0.009 | ||
| Specific tempe | Control | 3 | 3.222 | 1.086 | 0.027 | 0.359 |
| Vinegar | 4 | 3.481 | 0.975 | 0.006 | ||
| Alcohol | Control | 2 | 1.815 | 0.921 | <0.001 | 0.316 |
| Vinegar | 1 | 1.556 | 0.751 | <0.001 | ||
| Pleasant | Control | 2 | 2.148 | 0.989 | <0.001 | 0.003 |
| Vinegar | 3 | 3.000 | 1.109 | 0.036 | ||
| Yeast | Control | 3 | 2.963 | 1.315 | 0.024 | 0.228 |
| Vinegar | 3 | 2.519 | 1.189 | 0.012 | ||
| Ammonia | Control | 2 | 2.593 | 1.526 | <0.001 | 0.041 |
| Vinegar | 1 | 1.741 | 1.023 | <0.001 | ||
| Sweet | Control | 2 | 1.852 | 0.77 | <0.001 | 0.227 |
| Vinegar | 2 | 2.333 | 1.271 | 0.002 | ||
| Sour | Control | 2 | 2.185 | 1.145 | <0.001 | 0.063 |
| Vinegar | 1 | 1.593 | 0.694 | <0.001 | ||
| Saltiness | Control | 3 | 2.741 | 1.023 | 0.002 | 0.006 |
| Vinegar | 2 | 1.926 | 0.958 | <0.001 | ||
| Bitter | Control | 2 | 2.296 | 1.031 | 0.004 | 0.392 |
| Vinegar | 2 | 2.074 | 1.072 | <0.001 | ||
| Umami | Control | 3 | 2.963 | 0.98 | 0.024 | 0.453 |
| Vinegar | 3 | 2.704 | 1.068 | 0.002 | ||
| Astringent | Control | 2 | 2.259 | 1.13 | 0.001 | 0.231 |
| Vinegar | 2 | 1.926 | 1.072 | <0.001 | ||
| Acceptability | Control | 3 | 2.778 | 0.934 | 0.017 | 0.026 |
| Vinegar | 4 | 3.407 | 1.083 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahlan, H.A.; Nambu, Y.; Putri, S.P.; Fukusaki, E. Effects of Soaking Tempe in Vinegar on Metabolome and Sensory Profiles. Metabolites 2022, 12, 30. https://doi.org/10.3390/metabo12010030
Dahlan HA, Nambu Y, Putri SP, Fukusaki E. Effects of Soaking Tempe in Vinegar on Metabolome and Sensory Profiles. Metabolites. 2022; 12(1):30. https://doi.org/10.3390/metabo12010030
Chicago/Turabian StyleDahlan, Hadi Akbar, Yosuke Nambu, Sastia Prama Putri, and Eiichiro Fukusaki. 2022. "Effects of Soaking Tempe in Vinegar on Metabolome and Sensory Profiles" Metabolites 12, no. 1: 30. https://doi.org/10.3390/metabo12010030
APA StyleDahlan, H. A., Nambu, Y., Putri, S. P., & Fukusaki, E. (2022). Effects of Soaking Tempe in Vinegar on Metabolome and Sensory Profiles. Metabolites, 12(1), 30. https://doi.org/10.3390/metabo12010030