Metabolic Profiling during Acute Myeloid Leukemia Progression Using Paired Clinical Bone Marrow Serum Samples
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
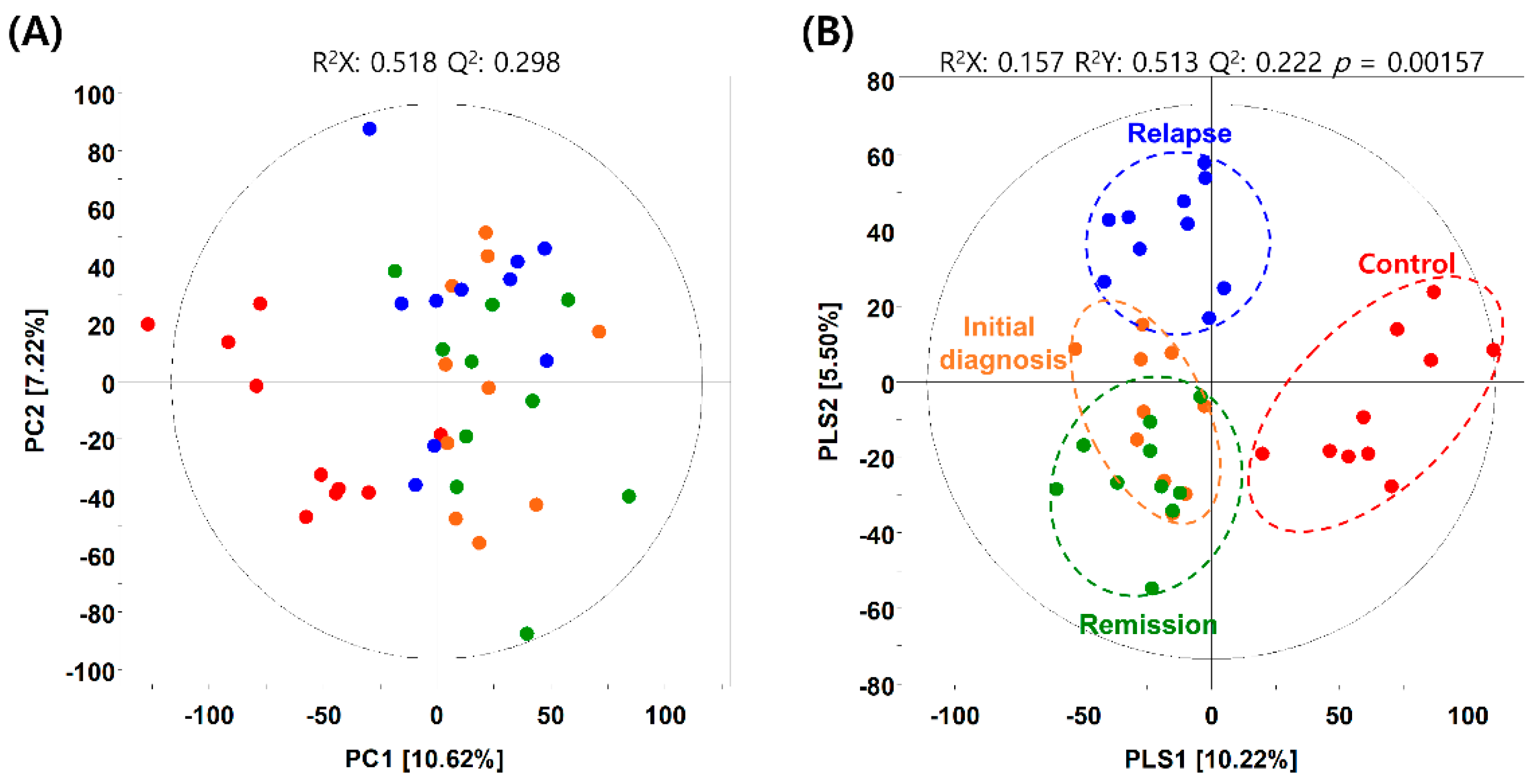
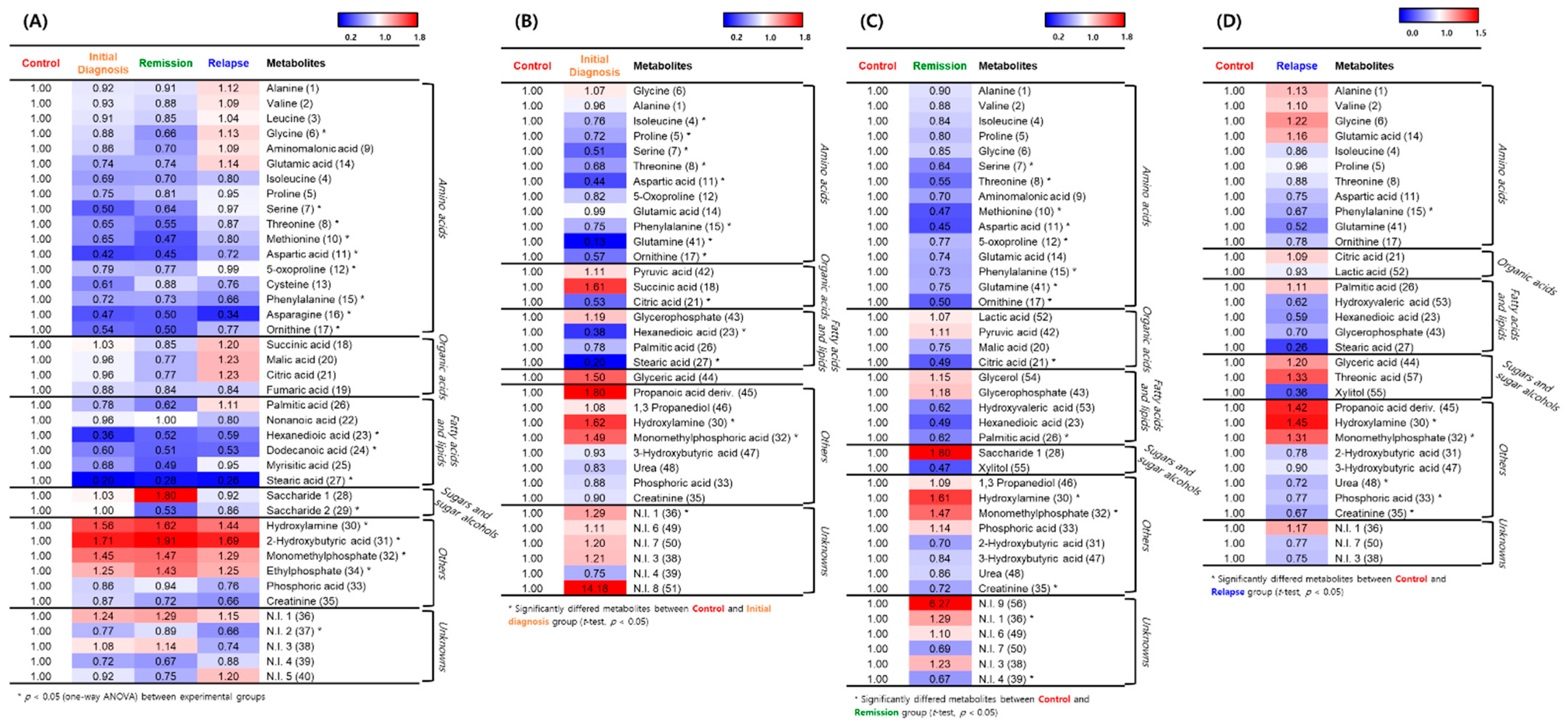
2.2. Metabolic Differences in BM Microenvironment between Patients with AML and Healthy Individuals
2.3. Hierarchical Clustering of Metabolites among the AML Initial Diagnosis, Relapse, and Remission Groups
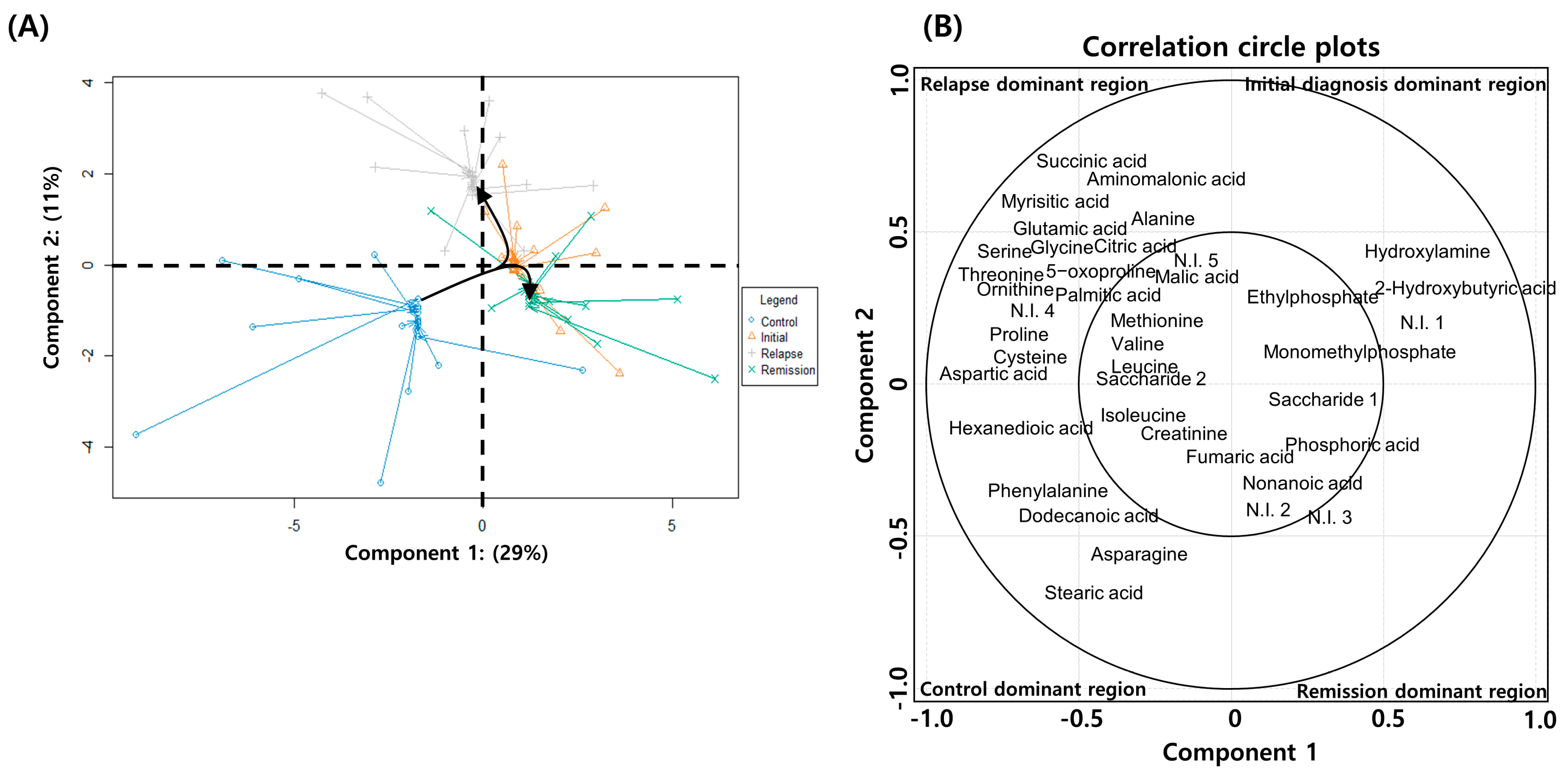
2.4. Serial Metabolic Changes from AML Diagnosis to Relapse
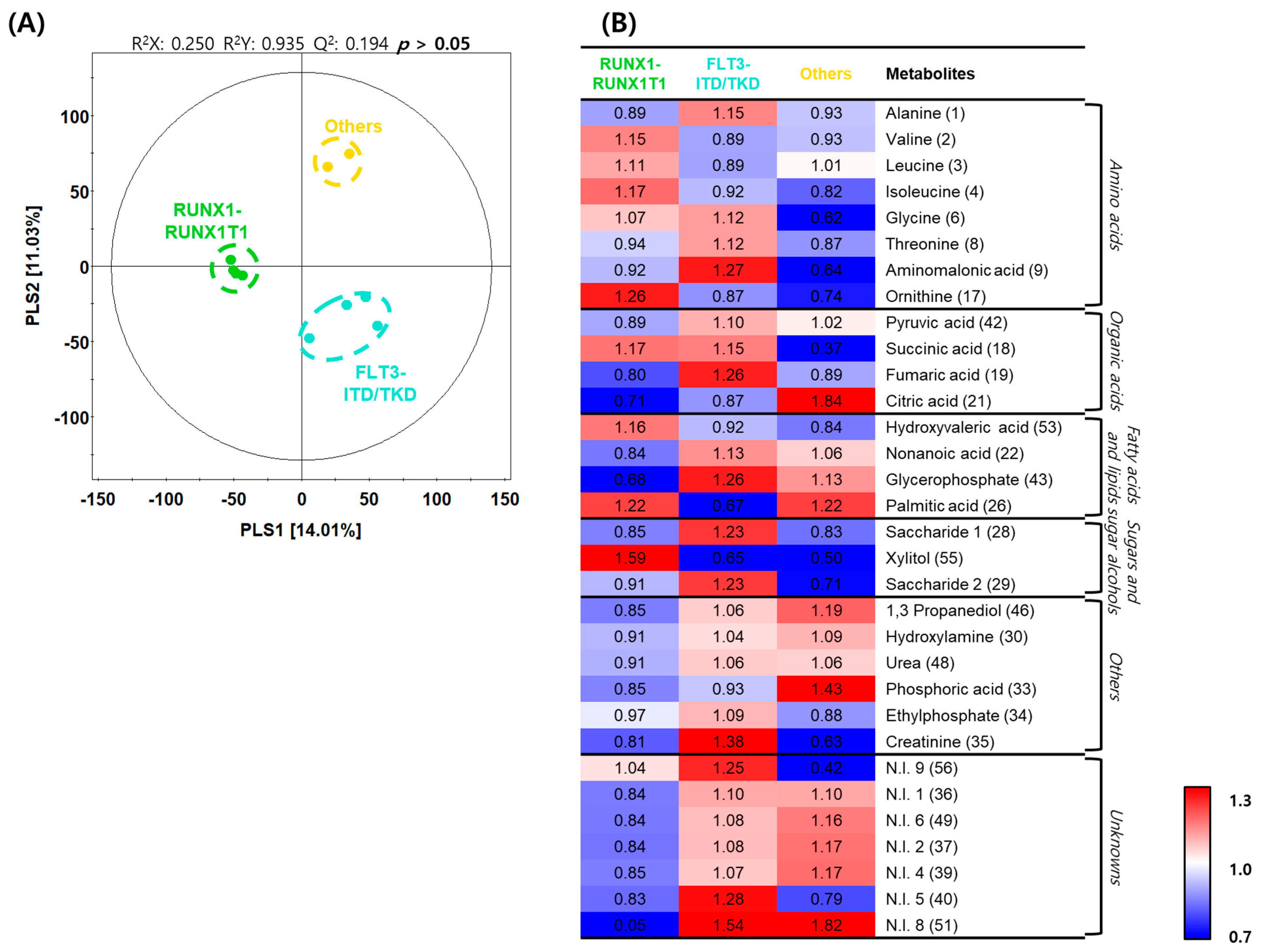
2.5. Differences in Metabolite Profiles with Genetic Variations in AML
3. Discussion
4. Materials and Methods
4.1. Patient Sample Collection
4.2. Sample Preparation for Metabolite Analysis
4.3. GC–TOF–MS Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Genome Atlas Research Network; Ley, T.J.; Miller, C.; Ding, L.; Raphael, B.J.; Mungall, A.J.; Robertson, A.; Hoadley, K.; Triche, T.J.; Laird, P.W.; et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Druker, B.J.; Guilhot, F.; O’Brien, S.G.; Gathmann, I.; Kantarjian, H.; Gattermann, N.; Deininger, M.W.; Silver, R.T.; Goldman, J.M.; Stone, R.M.; et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N. Engl. J. Med. 2006, 355, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Castro, I.; Sampaio-Marques, B.; Ludovico, P. Targeting metabolic reprogramming in acute myeloid leukemia. Cells 2019, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Mueckler, M.M.; Usher, P.; Lodish, H.F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science 1987, 235, 1492–1495. [Google Scholar] [CrossRef]
- Hay, N. Reprogramming glucose metabolism in cancer: Can it be exploited for cancer therapy? Nat. Rev. Cancer 2016, 16, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative phosphorylation as an emerging target in cancer therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef]
- Song, B.H.; Son, S.Y.; Kim, H.K.; Ha, T.W.; Im, J.S.; Ryu, A.; Jeon, H.; Chung, H.Y.; Oh, J.S.; Lee, C.H.; et al. Profiling of metabolic differences between hematopoietic stem cells and acute/chronic myeloid leukemia. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Morrish, F.; Noonan, J.; Perez-Olsen, C.; Gafken, P.R.; Fitzgibbon, M.; Kelleher, J.; VanGilst, M.; Hockenbery, D. Myc-dependent mitochondrial generation of acetyl-CoA contributes to fatty acid biosynthesis and histone acetylation during cell cycle entry. J. Biol. Chem. 2010, 285, 36267–36274. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- Son, J.; Lyssiotis, C.A.; Ying, H.; Wang, X.; Hua, S.; Ligorio, M.; Perera, R.M.; Ferrone, C.R.; Mullarky, E.; Shyh-Chang, N.; et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013, 496, 101–105. [Google Scholar] [CrossRef]
- Ye, H.; Adane, B.; Khan, N.; Alexeev, E.; Nusbacher, N.; Minhajuddin, M.; Stevens, B.M.; Winters, A.C.; Lin, X.; Ashton, J.M.; et al. Subversion of systemic glucose metabolism as a mechanism to support the growth of leukemia cells. Cancer Cell 2018, 34, 659–673.e6. [Google Scholar] [CrossRef]
- Rashkovan, M.; Ferrando, A. Metabolic dependencies and vulnerabilities in leukemia. Genes Dev. 2019, 33, 1460–1474. [Google Scholar] [CrossRef]
- Höpken, U.E.; Rehm, A. Targeting the tumor microenvironment of leukemia and lymphoma. Trends Cancer 2019, 5, 351–364. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Z.M.; Ma, P.; Ji, H.C.; Lu, H.M. GC-MS profiling of leukemia cells: An optimized preparation protocol for the intracellular metabolome. Anal. Methods 2018, 10, 1266–1274. [Google Scholar] [CrossRef]
- Lê Cao, K.A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef]
- Hemmati, P.G.; Terwey, T.H.; Na, I.K.; le Coutre, P.; Jehn, C.F.; Vuong, L.G.; Dörken, B.; Arnold, R. Impact of early remission by induction therapy on allogeneic stem cell transplantation for acute myeloid leukemia with an intermediate-risk karyotype in first complete remission. Eur. J. Haematol. 2015, 94, 431–438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lo Presti, C.; Fauvelle, F.; Jacob, M.C.; Mondet, J.; Mossuz, P. The metabolic reprogramming in acute myeloid leukemia patients depends on their genotype and is a prognostic marker. Blood Adv. 2021, 5, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.A.; Chen, C.L.; Huang, Y.H.; Evans, E.E.; Cheng, C.C.; Chuang, Y.J.; Zhang, C.; Le, A. Inhibition of glutaminolysis in combination with other therapies to improve cancer treatment. Curr. Opin. Chem. Biol. 2021, 62, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Son, S.Y.; Lee, A.Y.; Park, H.G.; Lee, W.L.; Lee, C.H. Metabolite profiling revealed that a gardening activity program improves cognitive ability correlated with BDNF levels and serotonin metabolism in the elderly. Int. J. Environ. Res. Public Health 2020, 17, 541. [Google Scholar] [CrossRef] [PubMed]
| No. | Ret (min) a | VIP1 | VIP2 | Metabolites b | Unique Mass (m/z) | MS Fragment Pattern (m/z) | ID c |
|---|---|---|---|---|---|---|---|
| Amino Acids | |||||||
| 1 | 5.48 | 0.26 | 1.54 | Alanine | 116 | 116, 73, 147, 117, 59, 100, 103, 190, 79, 148 | STD/MS |
| 2 | 6.65 | 0.30 | 1.17 | Valine | 218 | 73, 144, 218, 147, 100, 145, 59, 146, 219 | STD/MS |
| 3 | 7.19 | 0.49 | 1.08 | Leucine | 158 | 73, 158, 147, 117, 103, 205, 75, 59, 133 | STD/MS |
| 4 | 7.41 | 1.41 | 1.10 | Isoleucine | 158 | 73, 158, 142, 75, 147, 218, 100, 74, 159, 59 | STD/MS |
| 5 | 7.46 | 1.12 | 1.26 | Proline | 142 | 142, 73, 147, 75, 59, 70, 74, 66, 144, 216 | STD/MS |
| 6 | 7.54 | 0.59 | 1.70 | Glycine | 174 | 73, 174, 86, 147, 100, 59, 248, 133, 176 | STD/MS |
| 7 | 8.04 | 1.30 | 1.65 | Serine | 204 | 73, 204, 218, 100, 147, 205, 116, 188, 219 | STD/MS |
| 8 | 8.30 | 1.79 | 1.89 | Threonine | 219 | 73, 117, 101, 219, 218, 147, 75, 100, 129 | STD/MS |
| 9 | 9.06 | 0.55 | 1.29 | Aminomalonic acid | 218 | 73, 147, 218, 86, 133, 59, 174, 100, 320, 148 | STD/MS |
| 10 | 9.48 | 1.36 | 1.33 | Methionine | 176 | 73, 156, 147, 176, 128, 75, 100, 157, 218 | STD/MS |
| 11 | 9.49 | 1.75 | 1.54 | Aspartic acid | 232 | 73, 156, 232, 147, 75, 100, 157, 176, 218 | STD/MS |
| 12 | 9.53 | 1.08 | 1.51 | 5-oxoproline | 156 | 156, 73, 147, 157, 75, 230, 258, 59, 84, 158 | STD/MS |
| 13 | 9.80 | 1.13 | 0.90 | Cysteine | 220 | 73, 115, 147, 100, 143, 220, 218, 116, 171 | STD/MS |
| 14 | 10.37 | 0.59 | 1.49 | Glutamic acid | 246 | 73, 246, 128, 84, 147, 156, 247, 100, 230, 129 | STD/MS |
| 15 | 10.42 | 2.10 | 1.68 | Phenylalanine | 192 | 73, 218, 192, 100, 147, 219, 193, 220, 130, 120 | STD/MS |
| 16 | 10.84 | 1.62 | 1.33 | Asparagine | 116 | 73, 103, 116, 147, 132, 217, 119, 117, 231, 188 | STD/MS |
| 17 | 12.17 | 1.84 | 1.66 | Ornithine | 142 | 73, 142, 174, 86, 59, 143, 100, 200, 175, 128 | STD/MS |
| Organic Acids | |||||||
| 18 | 7.56 | 0.01 | 1.61 | Succinic acid | 133 | 147, 73, 174, 148, 79, 55, 86, 149, 247, 133 | STD/MS |
| 19 | 7.85 | 1.04 | 0.77 | Fumaric acid | 245 | 147, 73, 245, 148, 99, 52, 149, 133, 143, 241, 117 | STD/MS |
| 20 | 9.21 | 0.16 | 1.33 | Malic acid | 233 | 73, 147, 133, 55, 233, 163, 207, 148, 101 | STD/MS |
| 21 | 12.28 | 1.85 | 1.51 | Citric acid | 273 | 73, 147, 273, 117, 129, 133, 211, 148 | STD/MS |
| Fatty Acids and Lipids | |||||||
| 22 | 7.97 | 0.47 | 1.46 | Nonanoic acid | 215 | 73, 117, 204, 215, 55, 132, 129, 131, 218, 147 | MS |
| 23 | 9.30 | 1.73 | 1.28 | Hexanedioic acid | 115 | 73, 100, 115, 147, 75, 117, 128, 111, 243 | MS |
| 24 | 10.55 | 1.90 | 1.41 | Dodecanoic acid | 257 | 73, 117, 129, 132, 257, 131, 145, 211 | STD/MS |
| 25 | 12.32 | 1.60 | 1.28 | Myrisitic acid | 117 | 73, 117, 147, 129, 132, 285, 133, 211 | STD/MS |
| 26 | 14.64 | 0.22 | 1.20 | Palmitic acid | 313 | 75, 117, 73, 55, 132, 129, 145, 57, 69, 313 | STD/MS |
| 27 | 17.26 | 2.12 | 1.56 | Stearic acid | 341 | 117, 73, 75, 132, 55, 129, 145, 131, 341, 133 | STD/MS |
| Sugars and Sugar Alcohols | |||||||
| 28 | 9.42 | 0.50 | 1.05 | Saccharide 1 | 217 | 73, 147, 217, 103, 117, 205, 133, 189, 129 | MS |
| 29 | 12.55 | 1.85 | 1.36 | Saccharide 2 | 217 | 73, 147, 217, 191, 129, 103, 218, 133, 117, 101 | MS |
| Others | |||||||
| 30 | 5.61 | 1.92 | 1.46 | Hydroxylamine | 146 | 73, 133, 146, 119, 59, 147, 86, 79, 88, 74, 155 | STD/MS |
| 31 | 5.74 | 2.16 | 1.60 | 2-Hydroxybutyric acid | 133 | 73, 131, 147, 75, 66, 74, 148, 133, 132, 81, 149 | MS |
| 32 | 6.30 | 1.61 | 1.27 | Monomethylphosphate | 241 | 241, 79, 73, 163, 133, 242, 211, 135, 243 | MS |
| 33 | 7.29 | 0.76 | 1.12 | Phosphoric acid | 211 | 73, 299, 133, 74, 300, 75, 193, 207, 59, 314, 211 | STD/MS |
| 34 | 9.60 | 1.60 | 1.26 | Ethylphosphate | 299 | 73, 147, 156, 79, 84, 299, 211, 133, 155, 315, 343 | MS |
| 35 | 9.82 | 1.04 | 0.93 | Creatinine | 115 | 115, 73, 143, 100, 147, 116, 171, 329, 114, 144 | STD/MS |
| Unknowns | |||||||
| 36 | 4.82 | 1.21 | 1.03 | N.I. 1 | 152 | 73, 207, 79, 208, 123, 152, 93, 50, 295, 209 | ‒ d |
| 37 | 4.91 | 1.21 | 1.62 | N.I. 2 | 138 | 79, 50, 52, 78, 73, 69, 51, 140, 147, 77, 80, 110 | ‒ |
| 38 | 6.59 | 0.10 | 1.64 | N.I. 3 | 228 | 73, 144, 228, 110, 69, 77, 58, 134, 184, 74, 147 | ‒ |
| 39 | 8.93 | 1.31 | 1.28 | N.I. 4 | 128 | 73, 147, 128, 75, 59, 100, 115, 350, 129, 133 | ‒ |
| 40 | 9.08 | 0.27 | 1.38 | N.I. 5 | 232 | 73, 147, 232, 100, 59, 133, 148, 233, 131, 155 | ‒ |
| No. | Ret (min) a | VIP1 | VIP2 | Metabolites b | Unique Mass (m/z) | MS Fragment Pattern (m/z) | ID c | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental Group d | RUNXvsFLT e | RUNX vs Others f | FLT vs Others g | ||||||||
| Amino Acids | |||||||||||
| 1 | 5.48 | 1.22 | 1.09 | Alanine | 116 | 116, 73, 147, 117, 59, 100, 103, 190, 79, 148 | STD/MS | 0.373 | 0.137 | 0.889 | 0.347 |
| 2 | 6.65 | 1.70 | 1.24 | Valine | 218 | 73, 144, 218, 147, 100, 145, 59, 146, 219 | STD/MS | 0.238 | 0.135 | 0.294 | 0.836 |
| 3 | 7.19 | 1.16 | 0.90 | Leucine | 158 | 73, 158, 147, 117, 103, 205, 75, 59, 133 | STD/MS | 0.513 | 0.255 | 0.654 | 0.652 |
| 4 | 7.41 | 1.11 | 0.86 | Isoleucine | 158 | 73, 158, 142, 75, 147, 218, 100, 74, 159, 59 | STD/MS | 0.526 | 0.421 | 0.308 | 0.774 |
| 6 | 7.54 | 0.37 | 1.61 | Glycine | 174 | 73, 174, 86, 147, 100, 59, 248, 133, 176 | STD/MS | 0.050 | 0.746 | 0.020 * | 0.070 |
| 8 | 8.30 | 0.86 | 1.14 | Threonine | 219 | 73, 117, 101, 219, 218, 147, 75, 100, 129 | STD/MS | 0.351 | 0.244 | 0.722 | 0.248 |
| 9 | 9.06 | 0.75 | 1.30 | Aminomalonic acid | 218 | 73, 147, 218, 86, 133, 59, 174, 100, 320, 148 | STD/MS | 0.196 | 0.264 | 0.177 | 0.183 |
| 17 | 12.17 | 1.90 | 1.45 | Ornithine | 142 | 73, 142, 174, 86, 59, 143, 100, 200, 175, 128 | STD/MS | 0.101 | 0.102 | 0.133 | 0.443 |
| Organic Acids | |||||||||||
| 42 | 5.89 | 1.72 | 1.27 | Pyruvic acid | 220 | 73, 147, 133, 59, 100, 86, 89, 220, 148, 103, 235 | STD/MS | 0.221 | 0.121 | 0.361 | 0.564 |
| 18 | 7.56 | 0.53 | 1.28 | Succinic acid | 247 | 147, 73, 174, 148, 79, 55, 86, 149, 247, 133 | STD/MS | 0.196 | 0.970 | 0.139 | 0.096 |
| 19 | 7.85 | 1.65 | 1.43 | Fumaric acid | 245 | 147, 73, 245, 148, 99, 52, 149, 133, 143, 241, 117 | STD/MS | 0.144 | 0.087 | 0.644 | 0.260 |
| 21 | 12.28 | 0.75 | 1.19 | Citric acid | 273 | 73, 147, 273, 117, 129, 133, 211, 148 | STD/MS | 0.244 | 0.571 | 0.211 | 0.287 |
| Fatty Acids and Lipids | |||||||||||
| 53 | 6.13 | 1.31 | 1.00 | Hydroxyvaleric acid | 145 | 73, 79, 145, 147, 130, 128, 148, 146, 133, 131 | MS | 0.412 | 0.328 | 0.361 | 0.634 |
| 22 | 7.97 | 1.58 | 1.16 | Nonanoic acid | 215 | 73, 117, 204, 215, 55, 132, 129, 131, 218, 147 | MS | 0.303 | 0.177 | 0.429 | 0.639 |
| 43 | 11.86 | 1.75 | 1.28 | Glycerophosphate | 299 | 73, 299, 147, 357, 101, 103, 133, 129, 211 | MS | 0.214 | 0.088 | 0.232 | 0.779 |
| 26 | 14.64 | 0.10 | 1.03 | Palmitic acid | 313 | 75, 117, 73, 55, 132, 129, 145, 57, 69, 313 | STD/MS | 0.452 | 0.286 | 0.996 | 0.078 |
| Sugars and Sugar Alcohols | |||||||||||
| 28 | 9.42 | 1.10 | 1.13 | Saccharide 1 | 217 | 73, 147, 217, 103, 117, 205, 133, 189, 129 | MS | 0.361 | 0.245 | 0.949 | 0.344 |
| 55 | 11.39 | 1.03 | 0.76 | Xylitol | 103 | 73, 103, 217, 147, 117, 129, 205, 218, 133, 243 | STD/MS | 0.613 | 0.445 | 0.559 | 0.502 |
| 29 | 12.55 | 1.95 | 1.46 | Saccharide 2 | 217 | 73, 147, 217, 191, 129, 103, 218, 133, 117, 101 | MS | 0.374 | 0.365 | 0.669 | 0.069 |
| Others | |||||||||||
| 46 | 4.98 | 1.35 | 1.09 | 1,3 Propanediol | 115 | 147, 73, 115, 130, 66, 59, 79, 148, 177, 131, 103 | MS | 0.335 | 0.326 | 0.155 | 0.620 |
| 30 | 5.61 | 1.14 | 0.88 | Hydroxylamine | 146 | 73, 133, 146, 119, 59, 147, 86, 79, 88, 74, 155 | STD/MS | 0.512 | 0.407 | 0.336 | 0.764 |
| 48 | 6.97 | 1.22 | 0.89 | Urea | 189 | 147, 189, 73, 171, 66, 74, 148, 99, 75, 59, 87, 100 | STD/MS | 0.509 | 0.298 | 0.508 | 0.961 |
| 33 | 7.29 | 0.85 | 1.42 | Phosphoric acid | 211 | 73, 299, 133, 74, 300, 75, 193, 207, 59, 314, 211 | STD/MS | 0.117 | 0.724 | 0.124 | 0.034 * |
| 34 | 9.60 | 0.59 | 1.09 | Ethylphosphate | 299 | 73, 147, 156, 79, 84, 299, 211, 133, 155, 315, 343 | MS | 0.351 | 0.355 | 0.552 | 0.181 |
| 35 | 9.82 | 0.95 | 1.14 | Creatinine | 115 | 115, 73, 143, 100, 147, 116, 171, 329, 114, 144 | STD/MS | 0.326 | 0.268 | 0.067 | 0.345 |
| Unknowns | |||||||||||
| 56 | 4.45 | 0.07 | 1.30 | N.I. 9 | 117 | 73, 117, 147, 66, 118, 59, 148, 81, 133, 149 | ‒ h | 0.188 | 0.573 | 0.150 | 0.110 |
| 36 | 4.82 | 1.73 | 1.26 | N.I. 1 | 152 | 73, 207, 79, 208, 123, 152, 93, 50, 295, 209 | ‒ | 0.218 | 0.148 | 0.175 | 0.998 |
| 49 | 4.87 | 1.47 | 1.13 | N.I. 6 | 123 | 123, 93, 55, 73, 125, 95, 79, 103, 75, 124 | ‒ | 0.309 | 0.257 | 0.188 | 0.734 |
| 37 | 4.91 | 1.50 | 1.15 | N.I. 2 | 138 | 79, 50, 52, 78, 73, 69, 51, 140, 147, 77, 80, 110 | ‒ | 0.285 | 0.248 | 0.165 | 0.703 |
| 39 | 8.93 | 1.09 | 0.84 | N.I. 4 | 128 | 73, 147, 128, 75, 59, 100, 115, 350, 129, 133 | ‒ | 0.531 | 0.252 | 0.445 | 0.769 |
| 40 | 9.08 | 1.56 | 1.60 | N.I. 5 | 232 | 73, 147, 232, 100, 59, 133, 148, 233, 131, 155 | ‒ | 0.067 | 0.045 * | 0.793 | 0.156 |
| 51 | 9.96 | 1.13 | 0.85 | N.I. 8 | 247 | 73, 147, 129, 75, 247, 157, 203, 299, 349 | ‒ | 0.545 | 0.351 | 0.177 | 0.916 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.K.; Son, S.Y.; Oh, J.S.; Song, Y.N.; Byun, J.M.; Koh, Y.; Hong, J.; Yoon, S.-S.; Lee, C.H.; Shin, D.-Y.; et al. Metabolic Profiling during Acute Myeloid Leukemia Progression Using Paired Clinical Bone Marrow Serum Samples. Metabolites 2021, 11, 586. https://doi.org/10.3390/metabo11090586
Kim HK, Son SY, Oh JS, Song YN, Byun JM, Koh Y, Hong J, Yoon S-S, Lee CH, Shin D-Y, et al. Metabolic Profiling during Acute Myeloid Leukemia Progression Using Paired Clinical Bone Marrow Serum Samples. Metabolites. 2021; 11(9):586. https://doi.org/10.3390/metabo11090586
Chicago/Turabian StyleKim, Hyun Kyu, Su Young Son, Jae Sang Oh, Ye Na Song, Ja Min Byun, Youngil Koh, Junshik Hong, Sung-Soo Yoon, Choong Hwan Lee, Dong-Yeop Shin, and et al. 2021. "Metabolic Profiling during Acute Myeloid Leukemia Progression Using Paired Clinical Bone Marrow Serum Samples" Metabolites 11, no. 9: 586. https://doi.org/10.3390/metabo11090586
APA StyleKim, H. K., Son, S. Y., Oh, J. S., Song, Y. N., Byun, J. M., Koh, Y., Hong, J., Yoon, S.-S., Lee, C. H., Shin, D.-Y., & Lee, M. R. (2021). Metabolic Profiling during Acute Myeloid Leukemia Progression Using Paired Clinical Bone Marrow Serum Samples. Metabolites, 11(9), 586. https://doi.org/10.3390/metabo11090586







