Radial Flow Perfusion Enables Real-Time Profiling of Cellular Metabolism at Low Oxygen Levels with Hyperpolarized 13C NMR Spectroscopy
Abstract
1. Introduction
2. Results
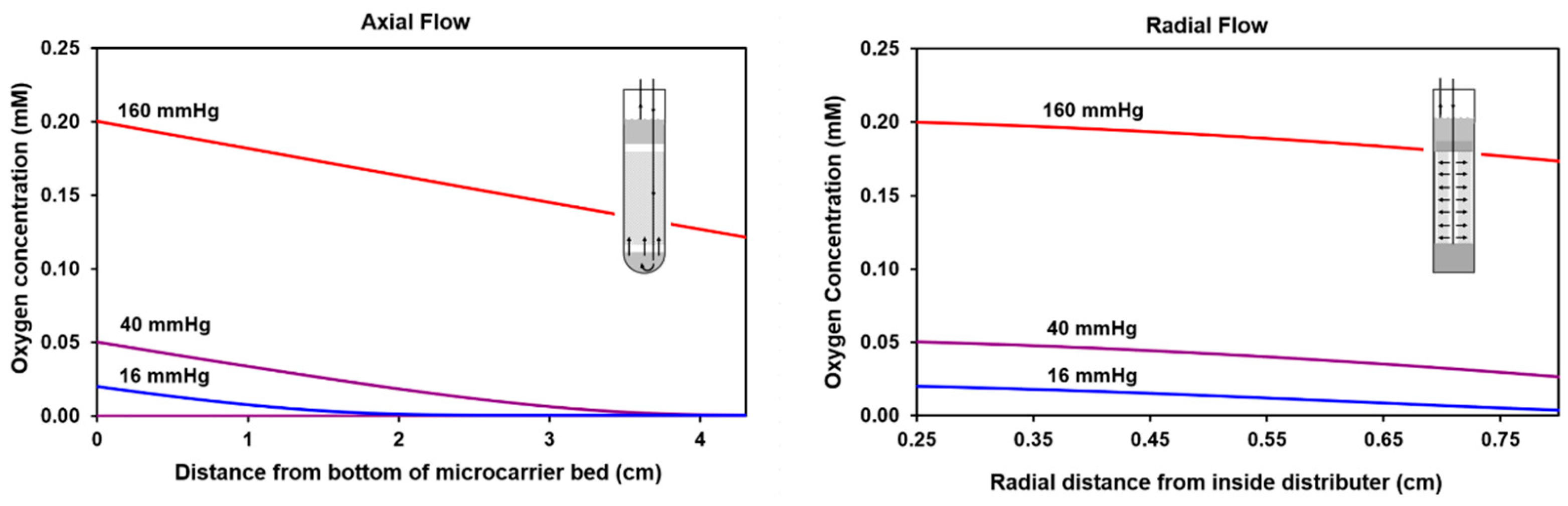
2.1. Calculated Oxygen Profiles for Axial and Radial Flow
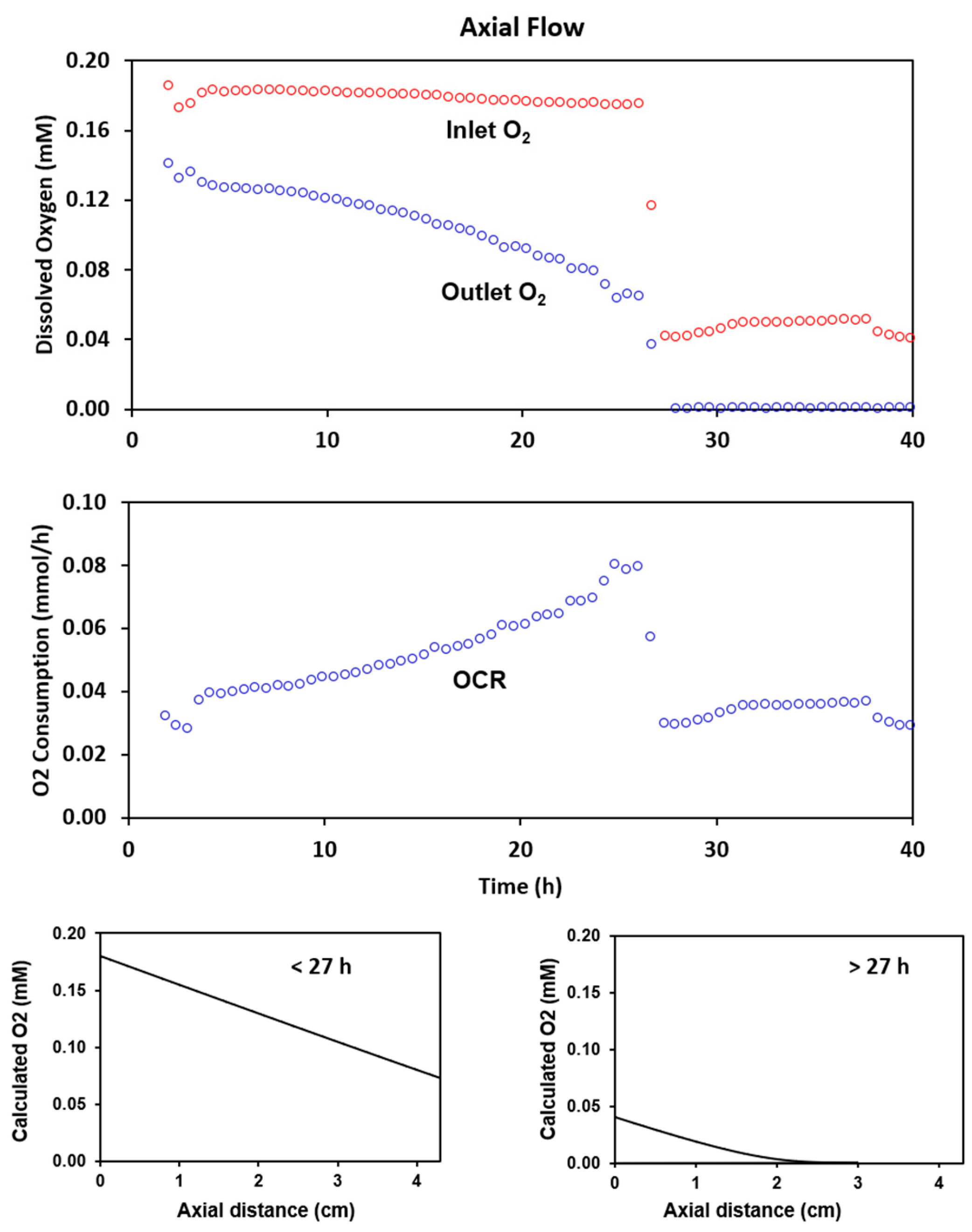
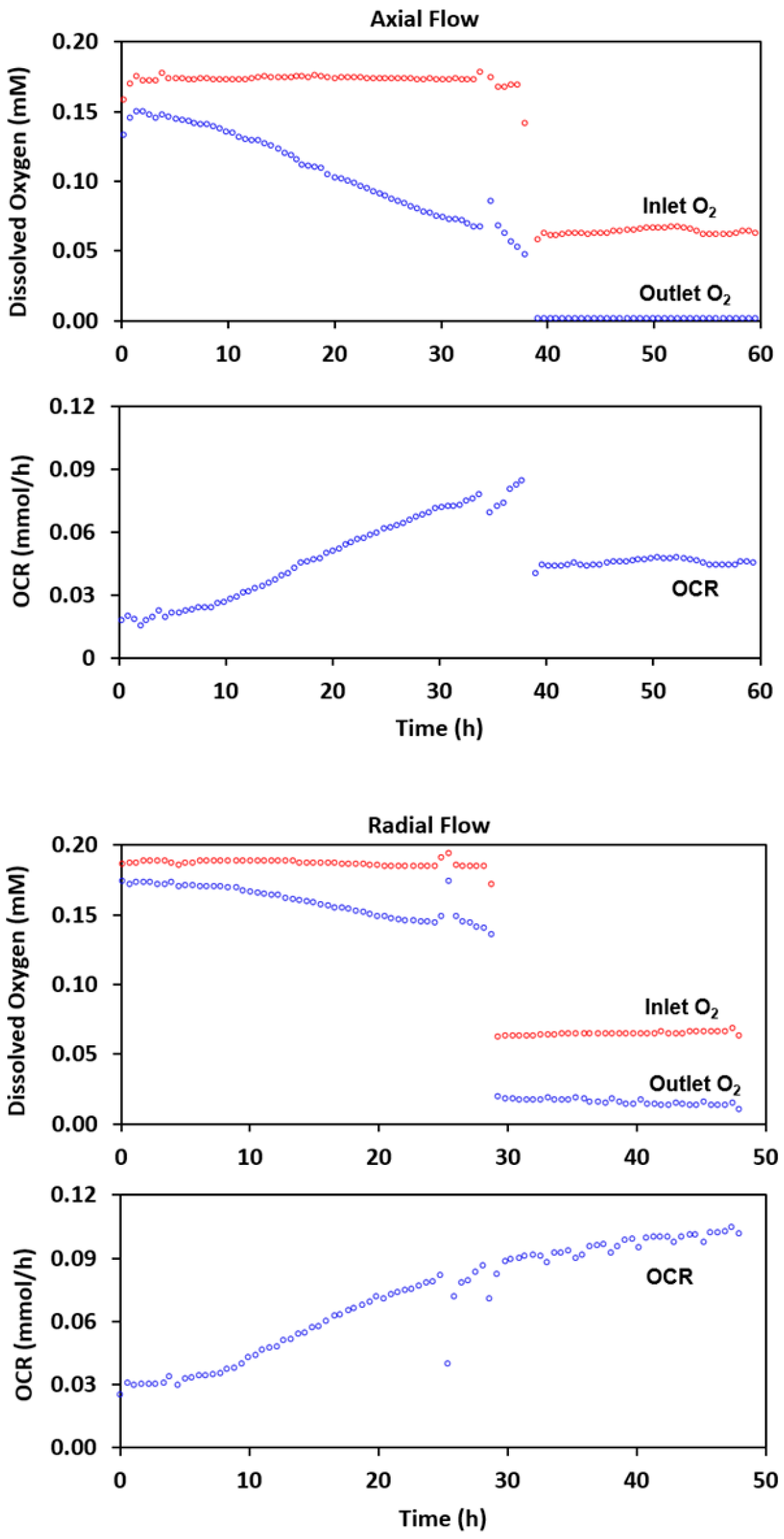
2.2. Experimental Results with Axial Flow
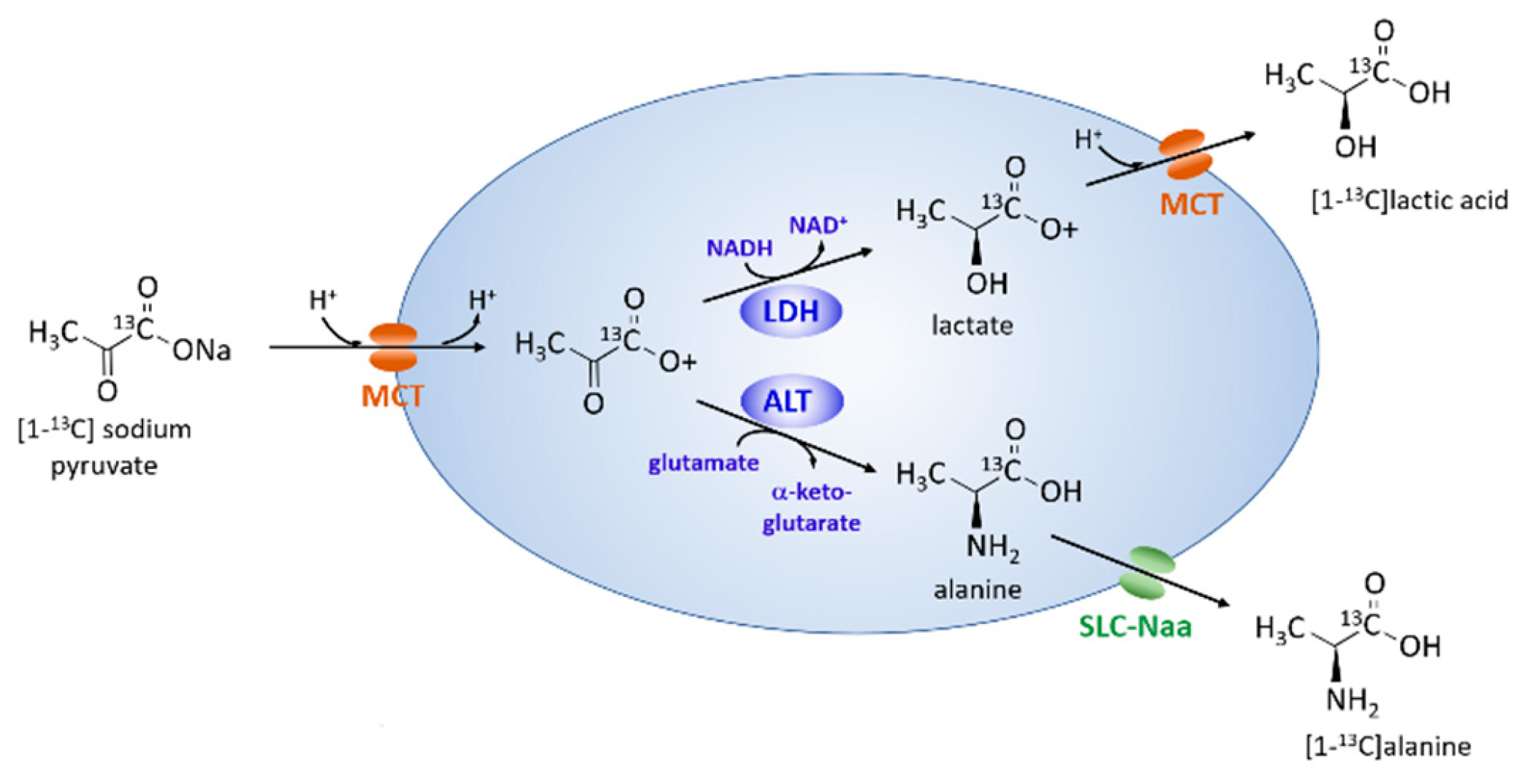
2.3. Metabolism of Hyperpolarized [1-13C] Pyruvate with Axial Flow
2.4. Radial Flow Improves Oxygen Delivery
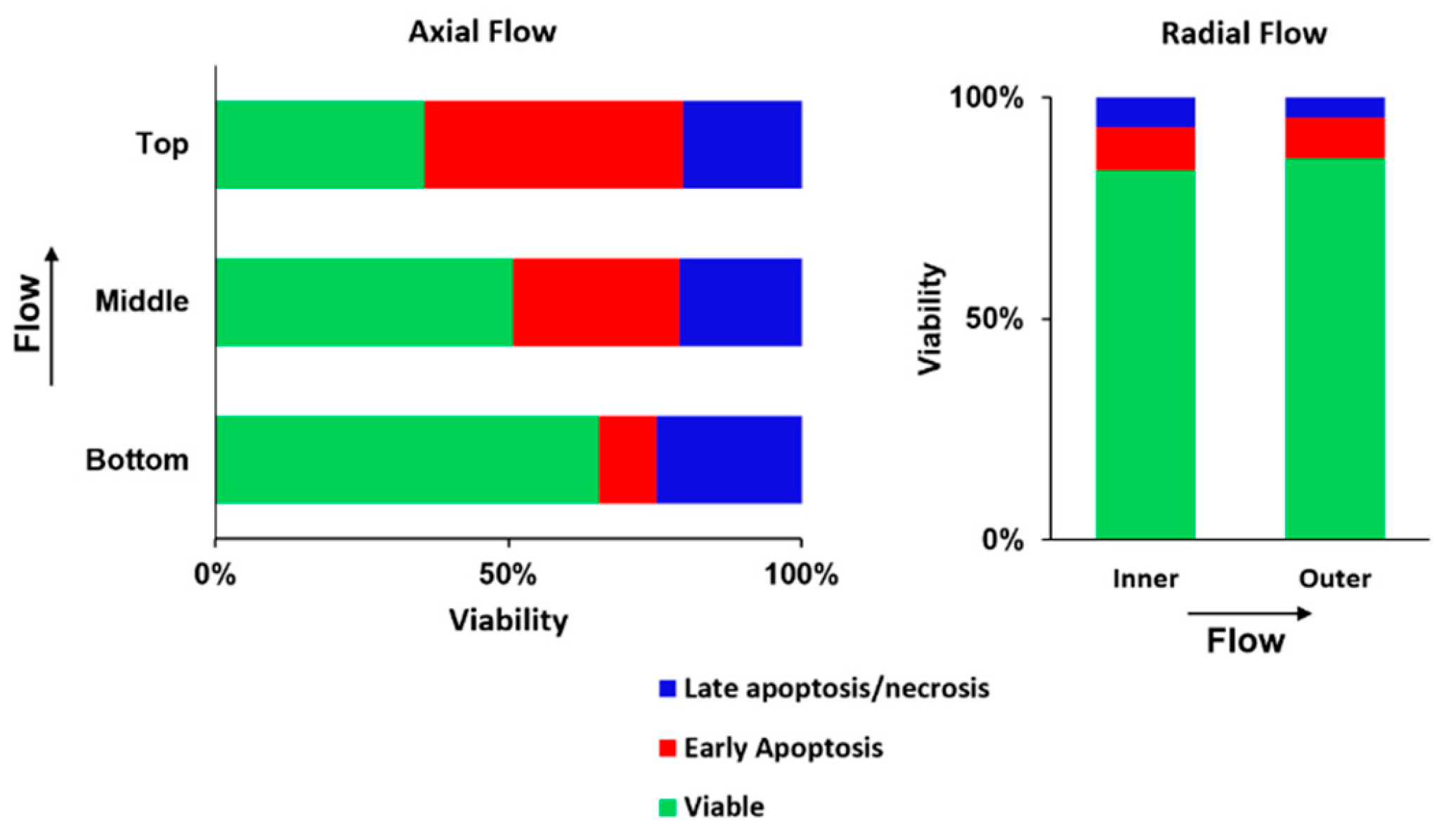
2.5. Radial Flow Improves Viability
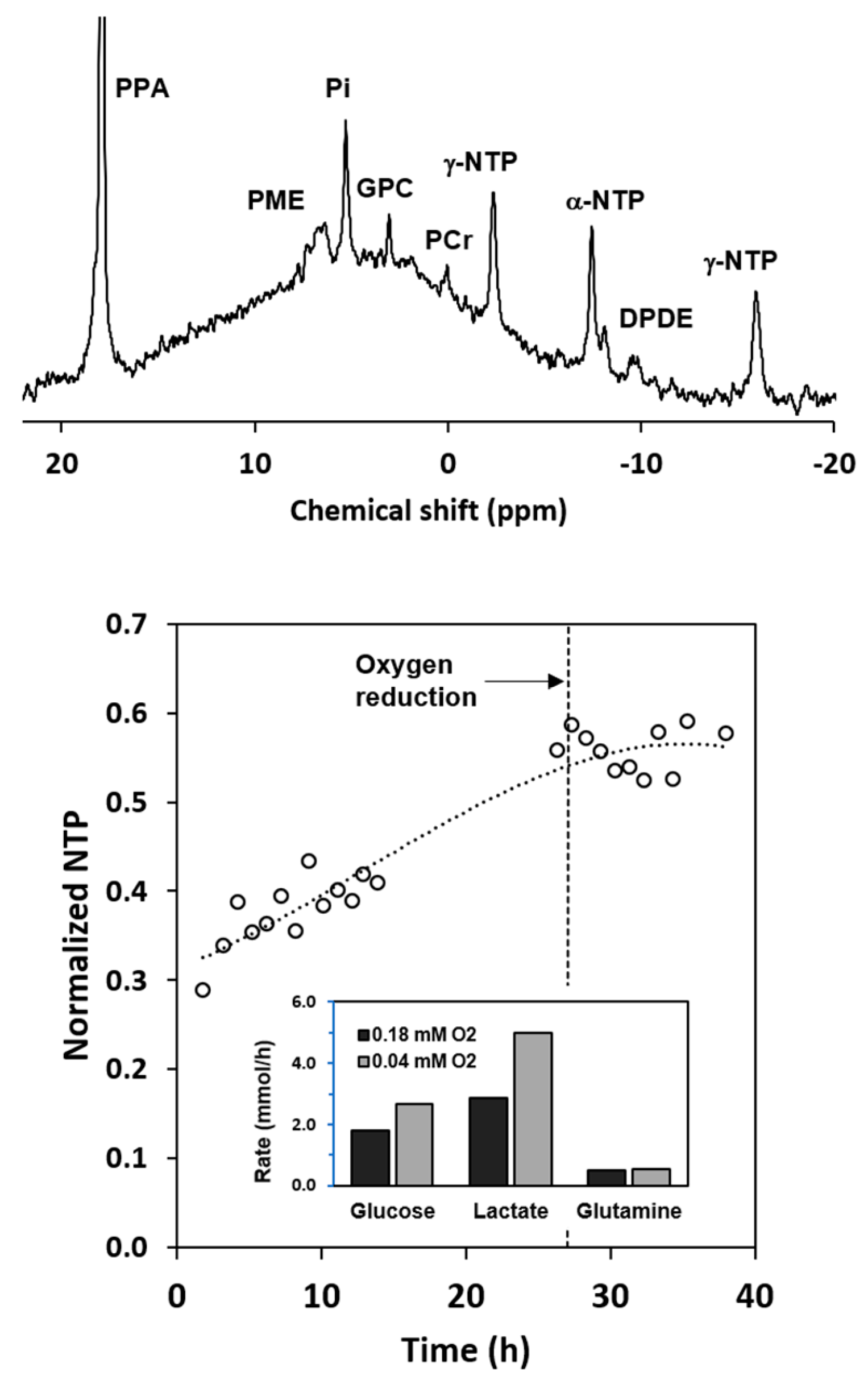
2.6. Metabolism of Hyperpolarized [1-13C] Pyruvate
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Microcarrier Preparation
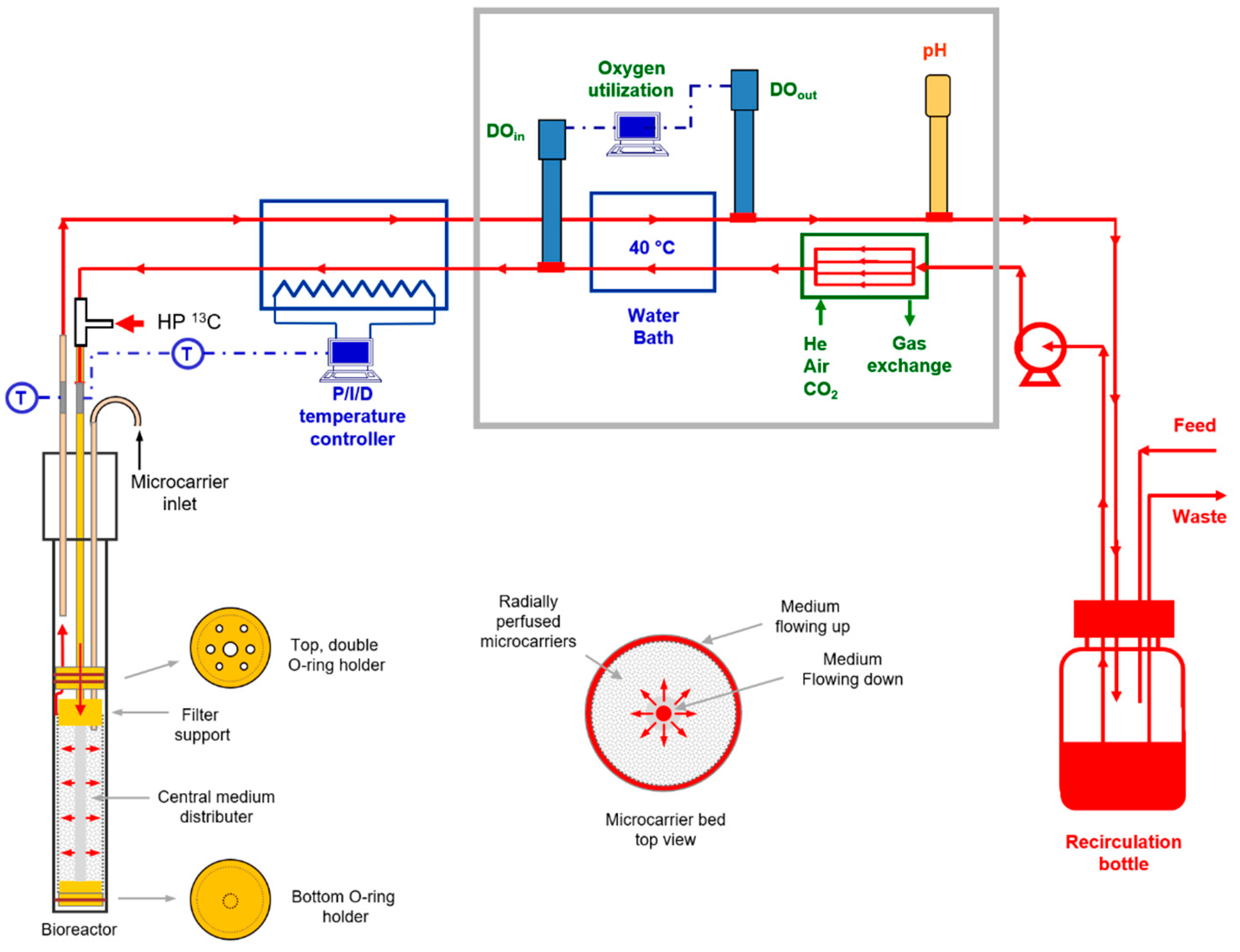
4.3. Cell Perfusion
4.4. NMR Spectroscopy
4.5. NMR Flow Cell Designs
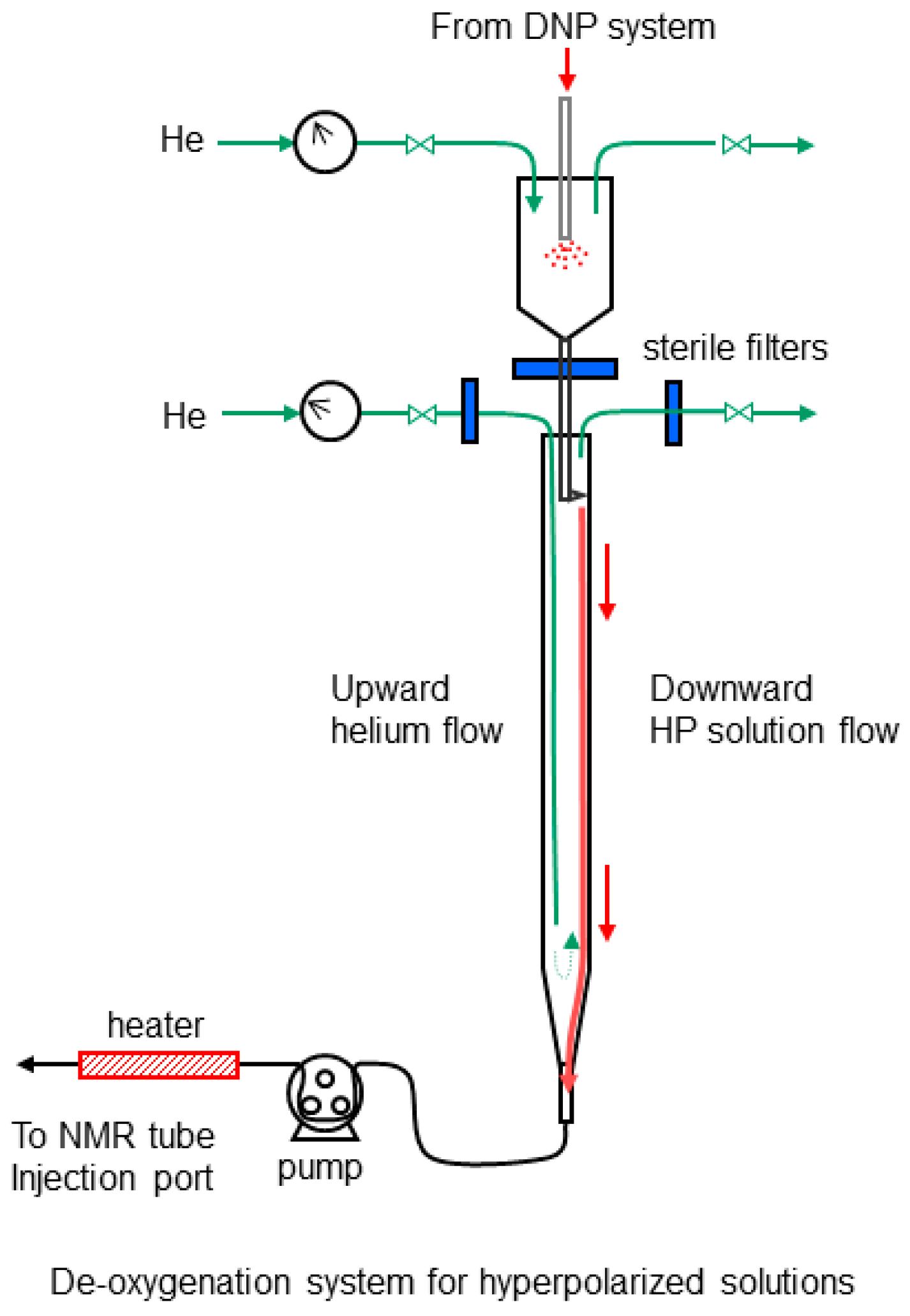
4.6. Hyperpolarization and Deoxygenation of [1-13C] Pyruvic Acid
4.7. Small Metabolite Analysis
4.8. Flow Cytometry
4.9. Western Analysis
4.10. Spectral Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and Surveillance for Hepatocellular Carcinoma: New Trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Sarveazad, A.; Agah, S.; Babahajian, A.; Amini, N.; Bahardoust, M. Predictors of 5 Year Survival Rate in Hepatocellular Carcinoma Patients. J. Res. Med. Sci. 2019, 24, 86. [Google Scholar] [PubMed]
- Kim, E.; Viatour, P. Hepatocellular Carcinoma: Old Friends and New Tricks. Exp. Mol. Med. 2020, 52, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, J.; Wu, L.; Yu, Q.; Ji, J.; Wu, J.; Dai, W. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 126. [Google Scholar] [CrossRef]
- Tenen, D.G.; Chai, L.; Tan, J.L. Metabolic alterations and vulnerabilities in hepatocellular carcinoma. Gastroenterol. Rep. 2021, 9, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.; Tu, K.; Wang, Y.; Yao, B.; Li, Q.; Wang, L.; Dou, C.; Liu, Q.; Zheng, X. Hypoxia Accelerates Aggressiveness of Hepatocellular Carcinoma Cells Involving Oxidative Stress, Epithelial-Mesenchymal Transition and Non-Canonical Hedgehog Signaling. Cell. Physiol. Biochem. 2017, 44, 1856–1868. [Google Scholar] [CrossRef]
- Lin, C.-A.; Chang, L.-L.; Zhu, H.; He, Q.-J.; Yang, B. Hypoxic Microenvironment and Hepatocellular Carcinoma Treatment. Hepatoma Res. 2018, 4, 26. [Google Scholar] [CrossRef]
- Peng, H.-S.; Liao, M.-B.; Zhang, M.-Y.; Xie, Y.; Xu, L.; Zhang, Y.-J.; Zheng, X.F.S.; Wang, H.-Y.; Chen, Y.-F. Synergistic Inhibitory Effect of Hyperbaric Oxygen Combined with So-rafenib on Hepatoma Cells. PLoS ONE 2014, 9, e100814. [Google Scholar]
- Juengpanich, S.; Topatana, W.; Lu, C.; Staiculescu, D.; Li, S.; Cao, J.; Lin, J.; Hu, J.; Chen, M.; Chen, J.; et al. Role of Cellular, Molecular and Tumor Microenvironment in Hepatocel-lular Carcinoma: Possible Targets and Future Directions in the Regorafenib Era. Int. J. Cancer 2020, 147, 1778–1792. [Google Scholar] [CrossRef]
- Xiong, X.X.; Qiu, X.Y.; Hu, D.X.; Chen, X.Q. Advances in Hypoxia-Mediated Mechanisms in Hepatocellular Carcinoma. Mol. Pharmacol. 2017, 92, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; Zandt, R.I.; Lerche, M.; Pehrson, R.; Ardenkjaer-Larsen, J.H. Metabolic Imaging by Hyperpolarized 13C Magnetic Resonance Imaging for in Vivo Tumor Diagnosis. Cancer Res. 2006, 66, 10855–10860. [Google Scholar] [CrossRef] [PubMed]
- Day, S.E.; Kettunen, M.I.; Gallagher, F.A.; Hu, D.-E.; Lerche, M.; Wolber, J.; Golman, K.; Ardenkjaer-Larsen, J.H.; Brindle, K.M. Detecting Tumor Response to Treatment Using Hyperpolarized 13C Magnetic Resonance Imaging and Spectroscopy. Nat. Med. 2007, 13, 1382–1387. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Larson, P.E.Z.; Woods, S.M.; Cai, L.; Eriksson, P.; Robinson, A.E.; Lupo, J.M.; Vigneron, D.B.; Nelson, S.J.; Pieper, R.O.; et al. Hyperpolarized [1-13C] Glutamate: A Metabolic Imaging Biomarker of IDH1 Mutational Status in Glioma. Cancer Res. 2014, 74, 4247–4257. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C] Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Aggarwal, R.; Bok, R.A.; Ohliger, M.A.; Zhu, Z.; Lee, P.; Gordon, J.W.; van Criekinge, M.; Carvajal, L.; Slater, J.B.; et al. Hyperpolarized 13C-Pyruvate MRI Detects Real-Time Metabolic Flux in Prostate Cancer Metastases to Bone and Liver: A Clinical Feasibility Study. Prostate Cancer Prostatic Dis. 2020, 23, 269–276. [Google Scholar] [CrossRef]
- Miloushev, V.Z.; Granlund, K.L.; Boltyanskiy, R.; Lyashchenko, S.K.; DeAngelis, L.M.; Mellinghoff, I.K.; Brennan, C.W.; Tabar, V.; Yang, T.J.; Holodny, A.I.; et al. Metabolic Imaging of the Human Brain with Hyperpolarized 13C Pyruvate Demonstrates 13C Lactate Pro-duction in Brain Tumor Patients. Cancer Res. 2018, 78, 3755–3760. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Najac, C.; Ronen, S.M. Studies of Metabolism Using 13C MRS of Hyperpolarized Probes. Methods Enzymol. 2015, 561, 1–71. [Google Scholar] [PubMed]
- Witney, T.H.; Kettunen, M.I.; Hu, D.; Gallagher, F.A.; Bohndiek, S.E.; Napolitano, R.; Brindle, K.M. Detecting Treatment Response in a Model of Human Breast Adenocarcinoma Using Hyperpolarised [1-13C] Pyruvate and [1,4-13C2] Fumarate. Br. J. Cancer 2010, 103, 1400–1406. [Google Scholar] [CrossRef]
- Mancuso, A.; Glickson, J. Applications of NMR Spectroscopy and Imaging to the Study of Immobilised Cell Physiology. In Fundamentals of Cell Immobilisation Biotechnology; Nedovic, V., Willaert, R., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 439–467. [Google Scholar]
- Ugurbil, K.; Guernsey, D.L.; Brown, T.R.; Glynn, P.; Tobkes, N.; Edelman, I.S. 31P NMR Studies of Intact Anchorage-Dependent Mouse Embryo Fibroblasts. Proc. Natl. Acad. Sci. USA 1981, 78, 4843–4847. [Google Scholar] [CrossRef]
- Neeman, M.; Rushkin, E.; Kadouri, A.; Degani, H. Adaptation of Culture Methods for NMR Studies of Anchorage-Dependent Cells. Magn. Reson. Med. 1988, 7, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Pilatus, U.; Shim, H.; Artemov, D.; Davis, D.; van Zijl, P.C.; Glickson, J.D. Intracellular Volume and Apparent Diffusion Constants of Perfused Cancer Cell Cultures, as Measured by NMR. Magn. Reson. Med. 1997, 37, 825–832. [Google Scholar] [CrossRef]
- Pilatus, U.; Aboagye, E.; Artemov, D.; Mori, N.; Ackerstaff, E.; Bhujwalla, Z.M. Real-Time Measurements of Cellular Oxygen Consumption, PH, and Energy Metabolism Using Nuclear Magnetic Resonance Spectroscopy. Magn. Reson. Med. 2001, 45, 749–755. [Google Scholar] [CrossRef]
- Mancuso, A.; Beardsley, N.J.; Wehrli, S.; Pickup, S.; Matschinsky, F.M.; Glickson, J.D. Real-Time Detection of 13C NMR Labeling Kinetics in Perfused EMT6 Mouse Mammary Tumor Cells and BetaHC9 Mouse Insulinomas. Biotechnol. Bioeng. 2004, 87, 835–848. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining Normoxia, Physoxia and Hypoxia in Tumors-Implications for Treatment Response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial Pressure of Oxygen in the Human Body: A General Review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar]
- Hsu, C.-C.; Wu, L.-C.; Hsia, C.-Y.; Yin, P.-H.; Chi, C.-W.; Yeh, T.-S.; Lee, H.-C. Energy Metabolism Determines the Sensitivity of Human Hepatocellular Carcinoma Cells to Miochondrial Inhibitors and Biguanide Drugs. Oncol. Rep. 2015, 34, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zuo, X.; Zhang, Y.; Han, G.; Zhang, L.; Wu, J.; Wang, X. MiR-3662 Suppresses Hepatocellular Carcinoma Growth through Inhibition of HIF-1α-Mediated Warburg Effect. Cell Death Dis. 2018, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond Aerobic Glycolysis: Transformed Cells Can Engage in Glutamine Metabolism That Exceeds the Requirement for Protein and Nucleotide Synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef] [PubMed]
- Shestov, A.A.; Mancuso, A.; Lee, S.-C.; Guo, L.; Nelson, D.S.; Roman, J.C.; Henry, P.-G.; Leeper, D.B.; Blair, I.A.; Glickson, J.D. Bonded Cumomer Analysis of Human Melanoma Metabolism Monitored by 13C NMR Spectroscopy of Perfused Tumor Cells. J. Biol. Chem. 2016, 291, 5157–5171. [Google Scholar] [CrossRef]
- Mancuso, A.; Fernandez, E.J.; Blanch, H.W.; Clark, D.S. A Nuclear Magnetic Resonance Technique for Determining Hybridoma Cell Concentration in Hollow Fiber Bioreactors. Bio/Technology 1990, 8, 1282–1285. [Google Scholar]
- Cima, L.G.; Blanch, H.W.; Wilke, C.R. A Theoretical and Experimental Evaluation of a Novel Radial-Flow Hollow Fiber Reactor for Mammalian Cell Culture. Bioprocess Eng. 1990, 5, 19–30. [Google Scholar] [CrossRef]
- Søgaard, L.V.; Schilling, F.; Janich, M.A.; Menzel, M.I.; Ardenkjaer-Larsen, J.H. In Vi-vo Measurement of Apparent Diffusion Coefficients of Hyperpolarized 13C-Labeled Metab-olites. NMR Biomed. 2014, 27, 561–569. [Google Scholar] [CrossRef]
- Carr, E.J. Characteristic Time Scales for Diffusion Processes through Layers and across Interfaces. Phys. Rev. E 2018, 97, 042115. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.J.; Mancuso, A.; Murphy, M.K.; Blanch, H.W.; Clark, D.S. Nuclear Magnetic Resonance Methods for Observing the Intra-cellular Environment of Mammalian Cells. Ann. N. Y. Acad. Sci. 1990, 589, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Ackerstaff, E.; Artemov, D.; Gillies, R.J.; Bhujwalla, Z.M. Hypoxia and the Presence of Human Vascular Endothelial Cells Affect Prostate Cancer Cell Invasion and Metabolism. Neoplasia 2007, 9, 1138–1151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, T.; Eliyahu, G.; Frydman, L.; Degani, H. Kinetics of Hyperpolarized 13C1-Pyruvate Transport and Metabolism in Living Human Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18131–18136. [Google Scholar] [CrossRef] [PubMed]
- Thièry, J.P.; Blazsek, I.; Legras, S.; Marion, S.; Reynes, M.; Anjo, A.; Adam, R.; Misset, J.L. Hepatocellular Carcinoma Cell Lines from Diethylnitrosamine Phenobarbital-Treated Rats. Characterization and Sensitivity to Endothall, a Protein Serine/Threonine Phospha-tase-2A Inhibitor. Hepatology 1999, 29, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Monson, L.; Moon, S.I.; Extrand, C.W. Permeation Resistance of Poly(Ether Ether Ketone) to Hydrogen, Nitrogen, and Oxygen Gases. J. Appl. Polym. Sci. 2013, 127, 1637–1642. [Google Scholar] [CrossRef]
- Mancuso, A.; Sharfstein, S.T.; Tucker, S.N.; Clark, D.S.; Blanch, H.W. Examination of Primary Metabolic Pathways in a Murine Hybridoma with Carbon-13 Nuclear Magnetic Resonance Spectroscopy. Biotechnol. Bioeng. 1994, 44, 563–585. [Google Scholar] [CrossRef]
- Ledezma, G.A.; Folch, A.; Bhatia, S.N.; Balis, U.J.; Yarmush, M.L.; Toner, M. Numerical Model of Fluid Flow and Oxygen Transport in a Radial-Flow Microchannel Containing Hepatocytes. J. Biomech. Eng. 1999, 121, 58–64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delgado, J.M.P.Q. A Critical Review of Dispersion in Packed Beds. Heat Mass Transf. 2006, 42, 279–310. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kirkpatrick, J.P.; Brizel, D.M.; Dewhirst, M.W. A Mathematical Model of Tumor Oxygen and Glucose Mass Transport and Metabolism with Complex Reaction Kinetics. Radiat. Res. 2003, 159, 336–344. [Google Scholar] [CrossRef]


| Axial | Radial | ||||
|---|---|---|---|---|---|
| Initial | Post O2 Reduction | Initial | Post O2 Reduction | ||
| Inlet O2 (mM) | 0.168 ± 0.012 | 0.061 ± 0.001 | Inlet O2 (mM) | 0.183 ± 0.001 | 0.065 ± 0.003 |
| Outlet O2 (mM) | 0.051 ± 0.000 | <0.0002 | Outlet O2 (mM) | 0.139 ± 0.000 | 0.014 ± 0.000 |
| OCR (mmol/h) | 0.083 ± 0.000 | 0.044 ± 0.001 | OCR (mmol/h) | 0.086 ± 0.003 | 0.097 ± 0.001 |
| NTP (normalized) | 0.14 ± 0.01 | 0.05 ± 0.01 | NTP (normalized) | 0.09 ± 0.01 | 0.08 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, A.; Pourfathi, M.; Kiefer, R.M.; Noji, M.C.; Siddiqui, S.; Profka, E.; Weber, C.N.; Pantel, A.; Kadlecek, S.J.; Rizi, R.; et al. Radial Flow Perfusion Enables Real-Time Profiling of Cellular Metabolism at Low Oxygen Levels with Hyperpolarized 13C NMR Spectroscopy. Metabolites 2021, 11, 576. https://doi.org/10.3390/metabo11090576
Mancuso A, Pourfathi M, Kiefer RM, Noji MC, Siddiqui S, Profka E, Weber CN, Pantel A, Kadlecek SJ, Rizi R, et al. Radial Flow Perfusion Enables Real-Time Profiling of Cellular Metabolism at Low Oxygen Levels with Hyperpolarized 13C NMR Spectroscopy. Metabolites. 2021; 11(9):576. https://doi.org/10.3390/metabo11090576
Chicago/Turabian StyleMancuso, Anthony, Mehrdad Pourfathi, Ryan M. Kiefer, Michael C. Noji, Sarmad Siddiqui, Enri Profka, Charles N. Weber, Austin Pantel, Stephen J. Kadlecek, Rahim Rizi, and et al. 2021. "Radial Flow Perfusion Enables Real-Time Profiling of Cellular Metabolism at Low Oxygen Levels with Hyperpolarized 13C NMR Spectroscopy" Metabolites 11, no. 9: 576. https://doi.org/10.3390/metabo11090576
APA StyleMancuso, A., Pourfathi, M., Kiefer, R. M., Noji, M. C., Siddiqui, S., Profka, E., Weber, C. N., Pantel, A., Kadlecek, S. J., Rizi, R., & Gade, T. P. F. (2021). Radial Flow Perfusion Enables Real-Time Profiling of Cellular Metabolism at Low Oxygen Levels with Hyperpolarized 13C NMR Spectroscopy. Metabolites, 11(9), 576. https://doi.org/10.3390/metabo11090576





