Cardiovascular Risk Factors in Children with Obesity, Preventive Diagnostics and Possible Interventions
Abstract
1. Introduction
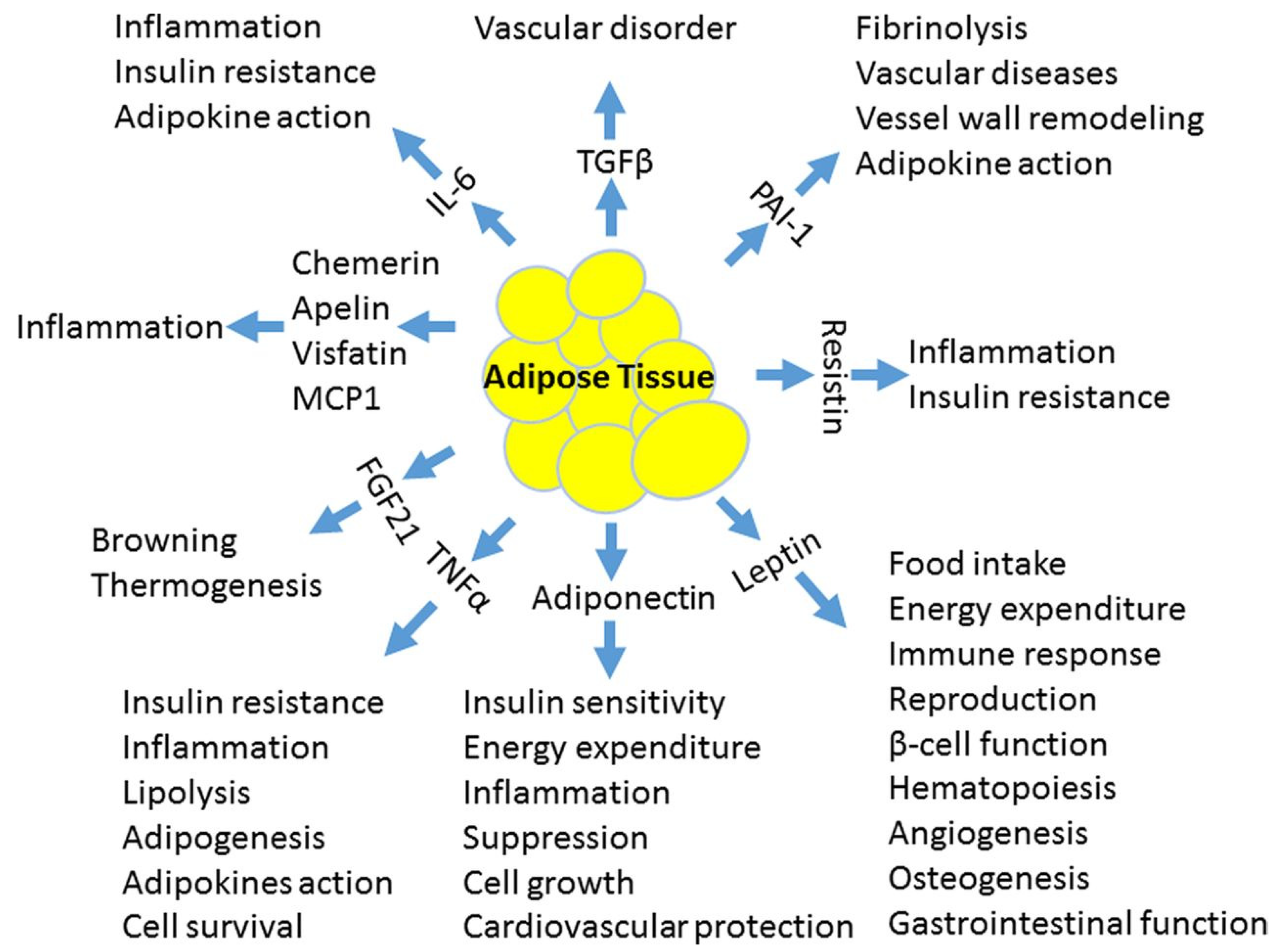
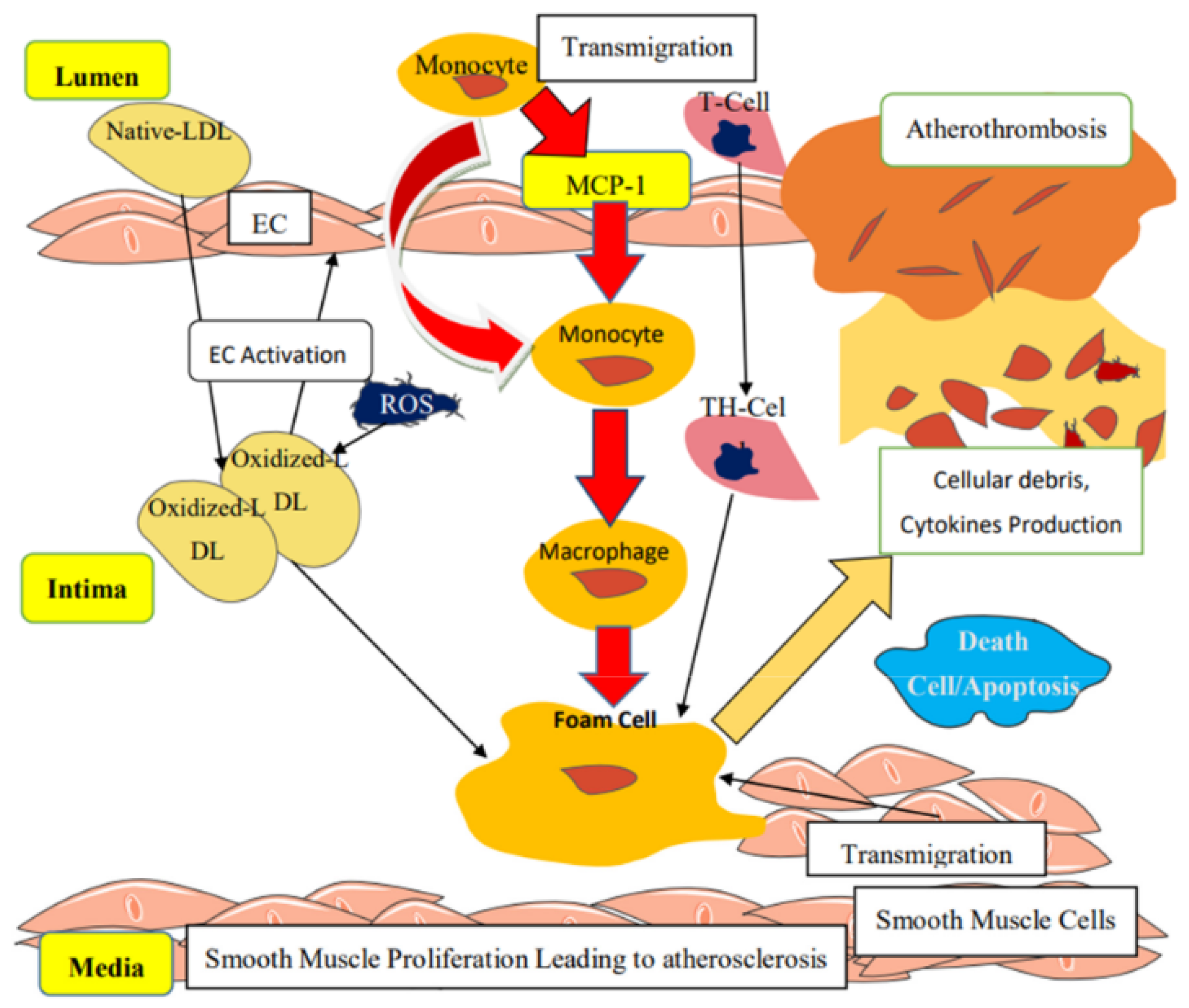
2. Pathophysiology of Vascular Dysfunction in Obesity
3. Obesity and Hypertension in Children
4. Obesity and Cardiac Dysfunction in Children
5. Obesity and Chronic Kidney Disease in Children
6. Obesity and Dyslipidemia in Children
7. Obesity and Hyperinsulinemia in Children
8. Evaluation and Possible Interventions in Obesity-Related Cardiovascular Risk Factors in Children
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 15 March 2021).
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Marčun-Varda, N.; Hanželj, N. Overweight of Slovenian school children. Acta Medico Biotech. 2015, 8, 46–54. [Google Scholar]
- Ferreira, S. Obesity and hypertension in children: A worldwide problem. Rev. Port. Cardiol. 2018, 37, 433–434. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 15 March 2021).
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307–1315. [Google Scholar]
- Milei, J.; Ottaviani, G.; Lavezzi, A.M.; Grana, D.R.; Stella, I.; Matturri, L. Perinatal and infant early atherosclerotic coronary lesions. Can. J. Cardiol. 2008, 24, 137–141. [Google Scholar] [CrossRef]
- Raghuveer, G. Lifetime cardiovascular risk of childhood obesity. Am. J. Clin. Nutr. 2010, 91, 1514S–1519S. [Google Scholar] [CrossRef]
- Bridger, T. Childhood obesity and cardiovascular disease. Paediatr. Child Health 2009, 14, 177–182. [Google Scholar] [CrossRef]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Meyers, M.R.; Gokce, N. Endothelial dysfunction in obesity: Etiological role in atherosclerosis. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 365–369. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Kim, J.; Alotaibi, W.H.; Kheirandish-Gozal, L.; Capdevila, O.S.; Gozal, D. Endothelial dysfunction in children without hypertension: Potential contributions of obesity and obstructive sleep apnea. Chest 2012, 141, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Skilton, M.; Celermajer, D.S. Endothelial dysfunction and arterial abnormalities in childhood obesity. Int. J. Obes. 2006, 30, 1041–1049. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Iantorno, M.; Campia, U.; Di Daniele, N.; Nistico, S.; Forleo, G.B.; Cardillo, C.; Tesauro, M. Obesity, inflammation and endothelial dysfunction. J. Biol. Regul. Homeost Agents 2014, 28, 169–176. [Google Scholar]
- Ciccone, M.M.; Faienza, M.F.; Altomare, M.; Nacci, C.; Montagnani, M.; Valente, F.; Cortese, F.; Gesualdo, M.; Zito, A.; Mancarella, R.; et al. Endothelial and Metabolic Function Interactions in Overweight/Obese Children. J. Atheroscler. Thromb. 2016, 23, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Varda, N.M.; Medved, M.; Ojsteršek, L. The associations between some biological markers, obesity, and cardiovascular risk in Slovenian children and adolescents. BMC Pediatr. 2020, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Korda, M.; Kubant, R.; Patton, S.; Malinski, T. Leptin-induced endothelial dysfunction in obesity. Am. J. Physiol. Circ. Physiol. 2008, 295, H1514–H1521. [Google Scholar] [CrossRef]
- Rojas, E.; Rodríguez-Molina, D.; Bolli, P.; Israili, Z.H.; Faria, J.; Fidilio, E.; Bermúdez, V.; Velasco, M. The Role of Adiponectin in Endothelial Dysfunction and Hypertension. Curr. Hypertens. Rep. 2014, 16, 1–8. [Google Scholar] [CrossRef]
- Li, R.; Lau, W.B.; Ma, X.L. Adiponectin resistance and vascular dysfunction in the hyperlipidemic state. Acta Pharmacol. Sin. 2010, 31, 1258–1266. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, K.K.Y.; Vanhoutte, P.M.; Lam, K.S.L.; Xu, A. Vascular effects of adiponectin: Molecular mechanisms and potential therapeutic intervention. Clin. Sci. 2008, 114, 361–374. [Google Scholar] [CrossRef]
- Zanetti, M.; Cappellari, G.G.; Graziani, A.; Barazzoni, R. Unacylated Ghrelin Improves Vascular Dysfunction and Attenuates Atherosclerosis during High-Fat Diet Consumption in Rodents. Int. J. Mol. Sci. 2019, 20, 499. [Google Scholar] [CrossRef] [PubMed]
- Tesauro, M.; Schinzari, F.; Iantorno, M.; Rizza, S.; Melina, D.; Lauro, D.; Cardillo, C. Ghrelin Improves Endothelial Function in Patients With Metabolic Syndrome. Circulation 2005, 112, 2986–2992. [Google Scholar] [CrossRef]
- Virdis, A.; Lerman, L.O.; Regoli, F.; Ghiadoni, L.; Lerman, A.; Taddei, S. Human Ghrelin: A Gastric Hormone with Cardiovascular Properties. Curr. Pharm. Des. 2015, 22, 52–58. [Google Scholar] [CrossRef]
- Jamaluddin, S.; Weakley, S.M.; Yao, Q.; Chen, C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012, 165, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Li, S.H.; Wang, C.H.; Fedak, P.W.; Li, R.K.; Weisel, R.D.; Mickle, D.A. Resistin promotes endothelial cell activation: Further evidence of adipokine-endothelial interaction. Circulation 2003, 108, 736–740. [Google Scholar] [CrossRef]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Int. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Taube, A.; Schlich, R.; Sell, H.; Eckardt, K.; Eckel, J. Inflammation and metabolic dysfunction: Links to cardiovascular diseases. Am. J. Physiol. Circ. Physiol. 2012, 302, H2148–H2165. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, D.; Yin, C.; Wang, S.; Wang, M.; Xiao, Y. IL-10/STAT3 is reduced in childhood obesity with hypertriglyceridemia and is related to triglyceride level in diet-induced obese rats. BMC Endocr. Disord. 2018, 18, 39. [Google Scholar] [CrossRef]
- Singh, A.; Foster, G.D.; Gunawardana, J.; McCoy, T.A.; Nguyen, T.; Veur, S.V.; Komaroff, E.; Rao, A.K. Elevated circulating tissue factor procoagulant activity, factor VII, and plasminogen activator inhibitor-1 in childhood obesity: Evidence of a procoagulant state. Br. J. Haematol. 2012, 158, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Eržen, B.; Šabovič, M. In young post-myocardial infarction male patients elevated plasminogen activator inhibitor-1 correlates with insulin resistance and endothelial dysfunction. Heart Vessels. 2013, 28, 570–577. [Google Scholar] [CrossRef]
- Aroor, A.R.; Demarco, V.G.; Jia, G.; Sun, Z.; Nistala, R.; Meininger, G.A.; Sowers, J.R. The Role of Tissue Renin-Angiotensin-Aldosterone System in the Development of Endothelial Dysfunction and Arterial Stiffness. Front. Endocrinol. 2013, 4, 161. [Google Scholar] [CrossRef]
- Feraco, A.; Armani, A.; Mammi, C.; Fabbri, A.; Rosano, G.M.; Caprio, M. Role of mineralocorticoid receptor and renin–angiotensin–aldosterone system in adipocyte dysfunction and obesity. J. Steroid Biochem. Mol. Biol. 2013, 137, 99–106. [Google Scholar] [CrossRef]
- Flynn, J. The changing face of pediatric hypertension in the era of the childhood obesity epidemic. Pediatr. Nephrol. 2012, 28, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Hertiš, T.; Petek, T.; Varda, N.M. The prevalence of elevated blood pressure in a sample of slovene children and adolescents: A pilot study. Slov. J. Public Health 2018, 57, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Estrada, E.; Eneli, I.; Hampl, S.; Mietus-Snyder, M.; Mirza, N.; Rhodes, E.; Sweeney, B.; Tinajero-Deck, L.; Woolford, S.J.; Pont, S.J.; et al. Children’s Hospital Association consensus statements for comorbidities of childhood obesity. Child Obes. 2014, 10, 304–317. [Google Scholar] [CrossRef]
- Brady, T.M. Obesity-Related Hypertension in Children. Front. Pediatr. 2017, 5, 197. [Google Scholar] [CrossRef] [PubMed]
- Wirix, A.J.G.; Kaspers, P.J.; Nauta, J.; Chinapaw, M.J.M.; Holthe, J.E.K.-V. Pathophysiology of hypertension in obese children: A systematic review. Obes. Rev. 2015, 16, 831–842. [Google Scholar] [CrossRef]
- Kotchen, T.A. Obesity-Related Hypertension: Epidemiology, Pathophysiology, and Clinical Management. Am. J. Hypertens. 2010, 23, 1170–1178. [Google Scholar] [CrossRef]
- Alpert, M.A. Obesity Cardiomyopathy: Pathophysiology and Evolution of the Clinical Syndrome. Am. J. Med. Sci. 2001, 321, 225–236. [Google Scholar] [CrossRef]
- Scaglione, R.; DiChiara, M.A.; Indovina, A.; Lipari, R.; Ganguzza, A.; Parrinello, G.; Capuana, G.; Merlino, G.; Licata, G. Left ventricular diastolic and systolic function in normotensive obese subjects: Influence of degree and duration of obesity. Eur. Heart J. 1992, 13, 738–742. [Google Scholar] [CrossRef][Green Version]
- Van Putte-Katier, N.; Rooman, R.P.; Haas, L.; Verhulst, S.L.; Desager, K.N.; Ramet, J.; Suys, B.E. Early cardiac abnormalities in obese children: Importance of obesity per se versus associated cardiovascular risk factors. Pediatr. Res. 2008, 64, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, G.; Pacileo, G.; Del Giudice, E.M.; Natale, F.; Limongelli, G.; Verrengia, M.; Rea, A.; Fratta, F.; Castaldi, B.; D’Andrea, A.; et al. Abnormal myocardial deformation properties in obese, non-hypertensive children: An ambulatory blood pressure monitoring, standard echocardiographic, and strain rate imaging study. Eur. Heart J. 2006, 27, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Pieruzzi, F.U.E.G.; Antolini, L.; Salerno, F.; Giussani, M.; Brambilla, P.; Galbiati, S.; Mastriani, S.; Rebora, P.; Stella, A.; Valsecchi, M.G.; et al. The role of blood pressure, body weight and fat distribution on left ventricular mass, diastolic function and cardiac geometry in children. J. Hypertens. 2015, 33, 1182–1192. [Google Scholar] [CrossRef]
- Di Sessa, A.; Umano, G.R.; Miraglia Del Giudice, E.; Santoro, N. From the liver to the heart: Cardiac dysfunction in obese children with non-alcoholic fatty liver disease. World J. Hepatol. 2017, 9, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.Y.; O’Moore-Sullivan, T.; Leano, R.; Byrne, N.; Beller, E.; Marwick, T.H. Alterations of Left Ventricular Myocardial Characteristics Associated with Obesity. Circulation 2004, 110, 3081–3087. [Google Scholar] [CrossRef]
- Kovesdy, C.; Furth, S.; Zoccali, C. World Kidney Day Steering Committee Obesity and kidney disease: Hidden consequences of the epidemic. Physiol. Int. 2017, 104, 1–14. [Google Scholar] [CrossRef]
- Gunta, S.S.; Mak, R.H. Is obesity a risk factor for chronic kidney disease in children? Pediatr. Nephrol. 2013, 28, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.F.; McAinch, A.; Poronnik, P.; Hryciw, D.H. Adipokines as a link between obesity and chronic kidney disease. Am. J. Physiol. Physiol. 2013, 305, F1629–F1636. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 297–310. [Google Scholar] [CrossRef]
- Weaver, D.J.; Mitsnefes, M. Cardiovascular Disease in Children and Adolescents with Chronic Kidney Disease. Semin. Nephrol. 2018, 38, 559–569. [Google Scholar] [CrossRef]
- Querfeld, U.; Anarat, A.; Bayazit, A.K.; Bakkaloglu, A.S.; Bilginer, Y.; Çalışkan, S.; Civilibal, M.; Doyon, A.; Düzova, A.; Kracht, D.; et al. The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) Study: Objectives, Design, and Methodology. Clin. J. Am. Soc. Nephrol. 2010, 5, 1642–1648. [Google Scholar] [CrossRef]
- Garofalo, C.; Borrelli, S.; Minutolo, R.; Chiodini, P.; De Nicola, L.; Conte, G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017, 91, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.S.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. The Relation of Overweight to Cardiovascular Risk Factors Among Children and Adolescents: The Bogalusa Heart Study. Pediatrics 1999, 103, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Elmaoğulları, S.; Tepe, D.; Uçaktürk, S.A.; Kara, F.K.; Demirel, F. Prevalence of Dyslipidemia and Associated Factors in Obese Children and Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2015, 7, 228–234. [Google Scholar] [CrossRef]
- D’Adamo, E.; Guardamagna, O.; Chiarelli, F.; Bartuli, A.; Liccardo, D.; Ferrari, F.; Nobili, V. Atherogenic Dyslipidemia and Cardiovascular Risk Factors in Obese Children. Int. J. Endocrinol. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Johnson, J.; Somers, V.K.; Kuniyoshi, F.S.; Garza, C.A.; Isley, W.L.; Gami, A.S.; Lopez-Jimenez, F. Comparison of Apolipoprotein-B/Apolipoprotein-AI in Subjects With Versus Without the Metabolic Syndrome. Am. J. Cardiol. 2006, 98, 1369–1373. [Google Scholar] [CrossRef]
- Nikolic, D.; Katsiki, N.; Montalto, G.; Isenovic, E.R.; Mikhailidis, D.P.; Rizzo, M. Lipoprotein Subfractions in Metabolic Syndrome and Obesity: Clinical Significance and Therapeutic Approaches. Nutrients 2013, 5, 928–948. [Google Scholar] [CrossRef]
- Castro, A.V.B.; Kolka, C.; Kim, S.P.; Bergman, R.N. Obesity, insulin resistance and comorbidities? Mechanisms of association. Arq. Bras. Endocrinol. Metabol. 2014, 58, 600–609. [Google Scholar] [CrossRef]
- Romualdo, M.C.; Nóbrega, F.J.; Escrivão, M.A. Insulin resistance in obese children and adolescents. J. Pediatr. 2014, 90, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Erion, K.A.; Corkey, B.E. Hyperinsulinemia: A cause of obesity? Curr. Obes. Rep. 2017, 6, 178–186. [Google Scholar] [CrossRef]
- Crofts, C.A.P.; Zinn, C.; Wheldon, M.C.; Schofield, G.M. Hyperinsulinemia: A unifying theory of chronic disease? Diabesity 2015, 1, 34–43. [Google Scholar] [CrossRef]
- Prieto, D.; Contreras, C.; Sánchez, A. Endothelial dysfunction, obesity and insulin resistance. Curr. Vasc. Pharmacol. 2014, 12, 412–426. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H.; Li, Y.; Li, S.; Fernandez, C.; Bazzano, L.; He, J.; Xue, F.; Chen, W. Long-term Impact of Temporal Sequence from Childhood Obesity to Hyperinsulinemia on Adult Metabolic Syndrome and Diabetes: The Bogalusa Heart Study. Sci. Rep. 2017, 7, srep43422. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1449–1455. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Katre, P.A.; Joshi, S.M.; Kumaran, K.; Bhat, D.S.; Lubree, H.G.; Memane, N.; Kinare, A.S.; Pandit, A.N.; Bhave, S.A.; et al. Higher glucose, insulin and insulin resistance (HOMA-IR) in childhood predict adverse cardiovascular risk in early adulthood: The Pune Children’s Study. Diabetology 2015, 58, 1626–1636. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, S213–S256. [Google Scholar] [CrossRef] [PubMed]
- Marčun-Varda, N. Hypertension and cardiovascular risk prevention in children. Slov. Pediatr. 2014, 21, 86–94. [Google Scholar]
- Varda, N.M.; Gregorič, A. A diagnostic approach for the child with hypertension. Pediatr. Nephrol. 2005, 20, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Rus, R.; Marčun, V.N. Novelties in the management of arterial hypertension in children and adolescents in accordance with US (2017) and European (2016) guidelines. Zdrav. Vestn. 2020, 89, 498–514. [Google Scholar] [CrossRef]
- Yanik, M.; Feig, D.I. Serum urate: A biomarker or treatment target in pediatric hypertension? Curr. Opin. Cardiol. 2013, 28, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Noone, D.G.; Marks, S.D. Hyperuricemia is Associated with Hypertension, Obesity, and Albuminuria in Children with Chronic Kidney Disease. J. Pediatr. 2013, 162, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Glowinska, B.; Urban, M.; Koput, A.; Galar, M. New atherosclerosis risk factors in obese, hypertensive and diabetic children and adolescents. Atherosclerosis 2003, 167, 275–286. [Google Scholar] [CrossRef]
- Urbina, E.M.; Khoury, P.R.; McCoy, C.E.; Dolan, L.M.; Daniels, S.R.; Kimball, T.R. Triglyceride to HDL-C Ratio and Increased Arterial Stiffness in Children, Adolescents, and Young Adults. Pediatrics 2013, 131, e1082–e1090. [Google Scholar] [CrossRef]
- de Giorgis, T.; Marcovecchio, M.L.; Di Giovanni, I.; Giannini, C.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. Triglycerides-to-HDL ratio as a new marker of endothelial dysfunction in obese prepubertal children. Eur. J. Endocrinol. 2013, 170, 173–180. [Google Scholar] [CrossRef]
- Glowinska, B.; Urban, M.; Koput, A. Cardiovascular risk factors in children with obesity, hypertension and diabetes: Lipoprotein(a) levels and body mass index correlate with family history of cardiovascular disease. Eur. J. Nucl. Med. Mol. Imaging 2002, 161, 511–518. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, X.; Li, M.; Neubauer, H. Elevated plasma homocysteine in obese schoolchildren with early atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2005, 165, 326–331. [Google Scholar] [CrossRef]
- Pereira da Silva, N.; Suano de Souza, F.I.; Pendezza, A.I.; Fonseca, F.L.A.; Hix, S.; Oliveira, A.C.; Sarni, R.O.S.; D’Almeida, V. Homocysteine and cysteine levels in prepubertal children: Association with waist circumference and lipid profile. Nutrition 2013, 29, 166–171. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Gul, A.; Ozer, S.; Yilmaz, R.; Sonmezgoz, E.; Kasap, T.; Takcı, S.; Demir, O. Association between vitamin D levels and cardiovascular risk factors in obese children and adolescents. Nutr. Hosp. 2017, 34, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, M.; Qu, P.; Hao, G.; Huang, Y.; Chen, J.; Li, T. The Association of Vitamin A and Vitamin D with Hypertension in Children: A Case-Control Study. Int. J. Hypertens. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, I.; Erol, O.B.; Toprak, S.; Cantez, M.S.; Omer, B.; Kilic, A.; Oguz, F.; Uysalol, M.; Yekeler, E.; Unuvar, E. Role of Vitamin D in Children with Hepatosteatosis. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Savastio, S.; Pozzi, E.; Tagliaferri, F.; Degrandi, R.; Cinquatti, R.; Rabbone, I.; Bona, G. Vitamin D and cardiovascular risk: Which implications in children? Int. J. Mol. Sci. 2020, 21, 3536. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transplant. 2009, 24, 3265–3268. [Google Scholar] [CrossRef] [PubMed]
- Goknar, N.; Öktem, F.; Özgen, I.T.; Torun, E.; Kucukkoc, M.; Demir, A.D.; Cesur, Y. Determination of early urinary renal injury markers in obese children. Pediatr. Nephrol. 2014, 30, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, S.; Dogan, B.; Ozkaramanli Gur, D.; Akyüz, A.; Fidan, Ç.; Guzel, S.; Ozkoyuncu, B. Assessment of salusin alpha and salusin beta levels in patients with newly diagnosed dipper and non-dipper hypertension. Clin. Exp. Hypertens. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Dervişoğlu, P.; Elmas, B.; Kösecik, M.; Işgüven, P.; Büyükavcı, M.; Köroğlu, M. Salusin-α levels are negatively correlated with diastolic blood pressure in children with obesity. Cardiol. Young 2019, 29, 1225–1229. [Google Scholar] [CrossRef]
- Altincik, A.; Sayin, O. Evaluation of the relationship between serum adropin levels and blood pressure in obese children. J. Pediatr. Endocrinol. Metab. 2015, 28, 1095–1100. [Google Scholar] [CrossRef]
- Leiherer, A.; Muendlein, A.; Saely, C.H.; Ebner, J.; Brandtner, E.M.; Fraunberger, P.; Drexel, H. Serum uromodulin is a predictive biomarker for cardiovascular events and overall mortality in coronary patients. Int. J. Cardiol. 2017, 231, 6–12. [Google Scholar] [CrossRef]
- Wiromrat, P.; Bjornstad, P.; Roncal, C.; Pyle, L.; Johnson, R.J.; Cherney, D.Z.; Lipina, T.; Bishop, F.; Maahs, D.M.; Wadwa, R.P. Serum uromodulin is associated with urinary albumin excretion in adolescents with type 1 diabetes. J. Diabetes Its Complicat. 2019, 33, 648–650. [Google Scholar] [CrossRef]
- Szczurek, W.; Szyguła-Jurkiewicz, B. Oxidative stress and inflammatory markers—The future of heart failure diagnostics? Kardiochir. Torakochirurgia Pol. 2015, 12, 145–149. [Google Scholar]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Kilic, E.; Özer, Ö.F.; Erek Toprak, A.; Erman, H.; Torun, E.; Kesgin Ayhan, S.; Caglar, H.; Selek, S.; Kocyigit, A. Oxidative stress status in childhood obesity: A potential risk predictor. Med. Sci. Monit. 2016, 22, 3673–3679. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Valls-Bellés, V.; Arilla-Codoñer, A.; Alonso-Iglesias, E. Oxidant mechanisms in childhood obesity: The link between inflammation and oxidative stress. Transl. Res. 2011, 158, 369–384. [Google Scholar] [CrossRef]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. J. Am. Coll. Cardiol. 2016, 68, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Magenta, A.; Pannarale, G.; Martino, E.; Zanoni, C.; Perla, F.M.; Puddu, P.E.; Barilla’, F. Epigenetics and cardiovascular risk in childhood. J. Cardiovasc. Med. 2016, 17, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Aypak, C.; Türedi Yüce, A.; Görpelioğlu, S. Thyroid-stimulating hormone (TSH) level in nutritionally obese children and metabolic co-morbidity. J. Pediatr. Endocrinol. Metab. 2013, 26, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Hirschler, V.; Molinari, C.; Maccallini, G.; Aranda, C. Is albuminuria associated with obesity in school children? Pediatr. Diabetes 2010, 11, 322–330. [Google Scholar] [CrossRef]
- Larkins, N.; Teixeira-Pinto, A.; Craig, J. The population-based prevalence of albuminuria in children. Pediatr. Nephrol. 2017, 32, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, B.; Czarniak, P.; Król, E.; Szcześniak, P.; Zdrojewski, T. Overweight, obesity, hypertension and albuminuria in Polish adolescents─Results of the Sopkard 15 study. Nephrol. Dial. Transplant. 2013, 28, iv204–iv211. [Google Scholar] [CrossRef]
- Genovesi, S.; Antolini, L.; Orlando, A.; Tassistro, E.; Giussani, M.; Nava, E.; Turolo, L.; Manolopoulou, J.; Parati, G.; Morganti, A. Aldosterone-to-renin ratio depends on age and sex in children attending a clinic for cardiovascular risk assessment. J. Hypertens. 2018, 36, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Baracco, R.; Kapur, G. Pheochromocytoma and paraganglioma─An update on diagnosis, evaluation, and management. Pediatr. Nephrol. 2020, 35, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Granata, A.; Fiorini, F.; Andrulli, S.; Logias, F.; Gallieni, M.; Romano, G.; Sicurezza, E.; Fiore, C. Doppler ultrasound and renal artery stenosis: An overview. J. Ultrasound 2009, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Rountas, C.; Vlychou, M.; Vassiou, K.; Liakopoulos, V.; Kapsalaki, E.; Koukoulis, G.; Fezoulidis, I.V.; Stefanidis, I. Imaging Modalities for Renal Artery Stenosis in Suspected Renovascular Hypertension: Prospective Intraindividual Comparison of Color Doppler US, CT Angiography, GD-Enhanced MR Angiography, and Digital Substraction Angiography. Ren. Fail. 2007, 29, 295–302. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D.; et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar] [CrossRef]
- Sigrist, R.M.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Yoneda, M.; Suzuki, K.; Kato, S.; Fujita, K.; Nozaki, Y.; Hosono, K.; Saito, S.; Nakajima, A. Nonalcoholic Fatty Liver Disease: US-based Acoustic Radiation Force Impulse Elastography. Radiology 2010, 256, 640–647. [Google Scholar] [CrossRef]
- Grenier, N.; Gennisson, J.-L.; Cornelis, F.; Le Bras, Y.; Couzi, L. Renal ultrasound elastography. Diagn. Interv. Imaging 2013, 94, 545–550. [Google Scholar] [CrossRef]
- Tutar, O.; Beşer, F.; Adaletli, I.; Tunc, N.; Gulcu, D.; Kantarci, F.; Mihmanli, I.; Cokugras, F.C.; Kutlu, T.; Ozbay, G.; et al. Shear Wave Elastography in the Evaluation of Liver Fibrosis in Children. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 750–755. [Google Scholar] [CrossRef]
- Verçoza, A.M.; Baldisserotto, M.; Santos, C.A.D.L.; Poli-De-Figueiredo, C.E.; D’Avila, D.O. Cardiovascular Risk Factors and Carotid Intima-Media Thickness in Asymptomatic Children. Pediatr. Cardiol. 2009, 30, 1055–1060. [Google Scholar] [CrossRef]
- Kusters, D.M.; Wiegman, A.; Kastelein, J.J.; Hutten, B.A. Carotid Intima-Media Thickness in Children with Familial Hypercholesterolemia. Circ. Res. 2014, 114, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Niemirska, A. Intima–media thickness measurements in children with cardiovascular risk factors. Pediatr. Nephrol. 2009, 24, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Miniello, V.; Marchioli, R.; Scicchitano, P.; Cortese, F.; Palumbo, V.; Primitivo, S.G.; Sassara, M.; Ricci, G.; Carbonara, S.; et al. Morphological and functional vascular changes induced by childhood obesity. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 831–835. [Google Scholar] [CrossRef]
- Močnik, M.; Varda, N.M. Hemodynamic Data Analysis and Site of Measurement in Children and Adolescents. Artery Res. 2020, 27, 20–24. [Google Scholar] [CrossRef]
- Močnik, M.; Nikolić, S.; Varda, N.M. Arterial Compliance Measurement in Overweight and Hypertensive Children. Indian J. Pediatr. 2015, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Brady, T.M. The Role of Obesity in the Development of Left Ventricular Hypertrophy Among Children and Adolescents. Curr. Hypertens. Rep. 2015, 18, 1–7. [Google Scholar] [CrossRef]
- Haley, E.J.; Zhiqian, G.; Philip, K.R.; Nicolas, M.L.; Thomas, K.R.; Lawrence, D.M.; Elaine, U.M.; Gao, Z.; Khoury, P.R.; Madsen, N.L.; et al. Reduction in myocardial strain is evident in adolescents and young adults with obesity and type 2 diabetes. Pediatr. Diabetes 2019, 21, 243–250. [Google Scholar] [CrossRef]
- Xu, E.; Kachenoura, N.; della Valle, V.; Dubern, B.; Karsenty, A.; Tounian, P.; Dacher, J.; Layese, R.; Lamy, J.; le Pointe, H.D.; et al. Multichamber Dysfunction in Children and Adolescents With Severe Obesity: A Cardiac Magnetic Resonance Imaging Myocardial Strain Study. J. Magn. Reson. Imaging 2021. online ahead of print. [Google Scholar] [CrossRef]
- Bizzarri, C.; Pedicelli, S.; Romanzo, A.; Bocchini, S.; Bottaro, G.; Cianfarani, S.; Cappa, M. The impact of IGF-I, puberty and obesity on early retinopathy in children: A cross-sectional study. Ital. J. Pediatr. 2019, 45, 52. [Google Scholar] [CrossRef] [PubMed]
- Dirani, M.; Xie, J.; Fenwick, E.; Benarous, R.; Rees, G.; Wong, T.Y.; Lamoureux, E.L. Are Obesity and Anthropometry Risk Factors for Diabetic Retinopathy? The Diabetes Management Project. Investig. Opthalmol. Vis. Sci. 2011, 52, 4416–4421. [Google Scholar] [CrossRef]
- Price, S.A.; Gorelik, A.; Fourlanos, S.; Colman, P.G.; Wentworth, J.M. Obesity is associated with retinopathy and macrovascular disease in type 1 diabetes. Obes. Res. Clin. Pract. 2014, 8, e178–e182. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, H.K.; Hwang, J.S. HbA1c and glucose intolerance in obese children and adolescents. Diabet. Med. 2012, 29, e102–e105. [Google Scholar] [CrossRef] [PubMed]
- Mauras, N.; DelGiorno, C.; Hossain, J.; Bird, K.; Killen, K.; Merinbaum, D.; Weltman, A.; Damaso, L.; Balagopal, P. Metformin use in children with obesity and normal glucose tolerance–effects on cardiovascular markers and intrahepatic fat. J. Pediatr. Endocrinol. Metab. 2012, 25, 33–40. [Google Scholar] [CrossRef]
- Kendall, D.; Vail, A.; Amin, R.; Barrett, T.; Dimitri, P.; Ivison, F.; Kibirige, M.; Mathew, V.; Matyka, K.; McGovern, A.; et al. Metformin in Obese Children and Adolescents: The MOCA Trial. J. Clin. Endocrinol. Metab. 2013, 98, 322–329. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lin, R.H.; Lin, Y.C.; Chen, J.Y.; Li, W.C.; Lee, L.A.; Liu, K.-H.; Chuang, H.-H. Are body composition parameters better than conventional anthropometric measures in predicting pediatric hypertension? Int. J. Environ. Res. Public Health 2020, 17, 5771. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Methven, E.; McDowell, Z.C.; Hacking, B.; Alexander, D.; Stewart, L.; Kelnar, C.J.H. Health consequences of obesity. Arch. Dis. Child. 2003, 88, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.Y.; Allen, K.L.; Davis, E.; Blair, E.; Zubrick, S.; Byrne, S.M. The psychosocial burden of childhood overweight and obesity: Evidence for persisting difficulties in boys and girls. Eur. J. Nucl. Med. Mol. Imaging 2017, 176, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Boirie, Y.; Cederholm, T.; Chourdakis, M.; Cuerda, C.; Delzenne, N.M.; Deutz, N.E.; Fouque, D.; Genton, L.; Gil, C.; et al. Towards a multidisciplinary approach to understand and manage obesity and related diseases. Clin. Nutr. 2016, 36, 917–938. [Google Scholar] [CrossRef] [PubMed]
- Mather, A.A.; Cox, B.J.; Enns, M.W.; Sareen, J. Associations of obesity with psychiatric disorders and suicidal behaviors in a nationally representative sample. J. Psychosom. Res. 2009, 66, 277–285. [Google Scholar] [CrossRef]
- Gidding, S.S.; Dennison, B.A.; Birch, L.L.; Daniels, S.R.; Gillman, M.W.; Lichtenstein, A.H.; Rattay, K.T.; Steinberger, J.; Stettler, N.; Van Horn, L.; et al. Dietary recommendations for children and adolescents: A guide for practitioners: Consensus statement from the American Heart Association. Circulation 2005, 112, 2061–2075. [Google Scholar] [CrossRef] [PubMed]
- Iughetti, L.; China, M.; Berri, R.; Predieri, B. Pharmacological Treatment of Obesity in Children and Adolescents: Present and Future. J. Obes. 2010, 2011, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Axon, E.; Atkinson, G.; Richter, B.; Metzendorf, M.-I.; Baur, L.; Finer, N.; Corpeleijn, E.; O’Malley, C.; Ells, L.J. Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst. Rev. 2016, 11, CD012436. [Google Scholar] [CrossRef] [PubMed]
| Investigations | Routine | Optional + Research Level |
|---|---|---|
| Laboratory work-up |
|
|
| Imaging |
|
|
| Functional diagnostics |
|
| Age (Years)/Risk Factor | Less Than 1 Year | 1–4 | 5–8 | 9–11 | 12–17 | 18–21 |
|---|---|---|---|---|---|---|
| Family history of early cardiovascular event | Family history given by parents: early cardiovascular events in close family (men ≤ 55 years, women ≤ 65 years) | Continuing history | Continuing history provided by the patient | |||
| Tobacco exposure | Advice on smoking indoors, help with cessationof smoking in parents | Continuing anti-smoking advice | Active anti-smoking advice to the child | Determination of smoking status in child, advice, helping with cessation of smoking | ||
| Diet | Promoting breastfeeding, introduction of healthy food | Introduction of healthy food, counselling against sweet beverages and sweets | Determination of diet, encouraging healthy diet, diet counselling | |||
| Growth, overweight, obesity | Counselling about appropriate growth, height/weight growth charts | Weight/growth charts, BMI determination after 2 years of age | Weight/growth charts, body mass BMI > 85th percentile: healthy lifestyle interventions; if no change in six months, counselling by a dietician is necessary, BMI > 95th percentile: obesity treatment | |||
| Lipid profile | No lipid profile determined | Lipid profile determined if parents have dyslipidemia or other high-risk cardiovascular risk factors | Routine lipid profile at age of 5 years | Regular assessment of lipid profile if previously elevated; lipid profile determination if other cardiovascular risk factors; diet and possibly pharmacological management | ||
| Blood pressure | Measurement in babies with kidney, urological, heart disease or if history of intensive care treatment | Annual measurement from 3 years of age, evaluation with percentile charts | Annual measurement, evaluation with percentile charts, exclusion of secondary hypertension, pharmacological treatment if very high values, unsuccessful non-pharmacological management, or presence of left heart hypertrophy | |||
| Physical activity | Promotion of regular physical activity; no sedentary lifestyle (e.g., watching television) under the age of 2 | Promotion of child’s active play, limitation of sedentary activities to less than two hours daily, no television or computer in the bedroom | History of physical activity, physical activity > one hour per day, sedentary activities < two hours per day | Discussion about the importance of healthy lifestyle with promotion of physical activity and limitation of sedentary lifestyle | ||
| Diabetes mellitus | When indicated, blood-sugar measurement with oral glucose tolerance test and determination of insulin level; referral to endocrinologist | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Močnik, M.; Marčun Varda, N. Cardiovascular Risk Factors in Children with Obesity, Preventive Diagnostics and Possible Interventions. Metabolites 2021, 11, 551. https://doi.org/10.3390/metabo11080551
Močnik M, Marčun Varda N. Cardiovascular Risk Factors in Children with Obesity, Preventive Diagnostics and Possible Interventions. Metabolites. 2021; 11(8):551. https://doi.org/10.3390/metabo11080551
Chicago/Turabian StyleMočnik, Mirjam, and Nataša Marčun Varda. 2021. "Cardiovascular Risk Factors in Children with Obesity, Preventive Diagnostics and Possible Interventions" Metabolites 11, no. 8: 551. https://doi.org/10.3390/metabo11080551
APA StyleMočnik, M., & Marčun Varda, N. (2021). Cardiovascular Risk Factors in Children with Obesity, Preventive Diagnostics and Possible Interventions. Metabolites, 11(8), 551. https://doi.org/10.3390/metabo11080551






