Metabolomic Profiling of Adults with Congenital Heart Disease
Abstract
:1. Introduction
2. Results
2.1. Study Cohort
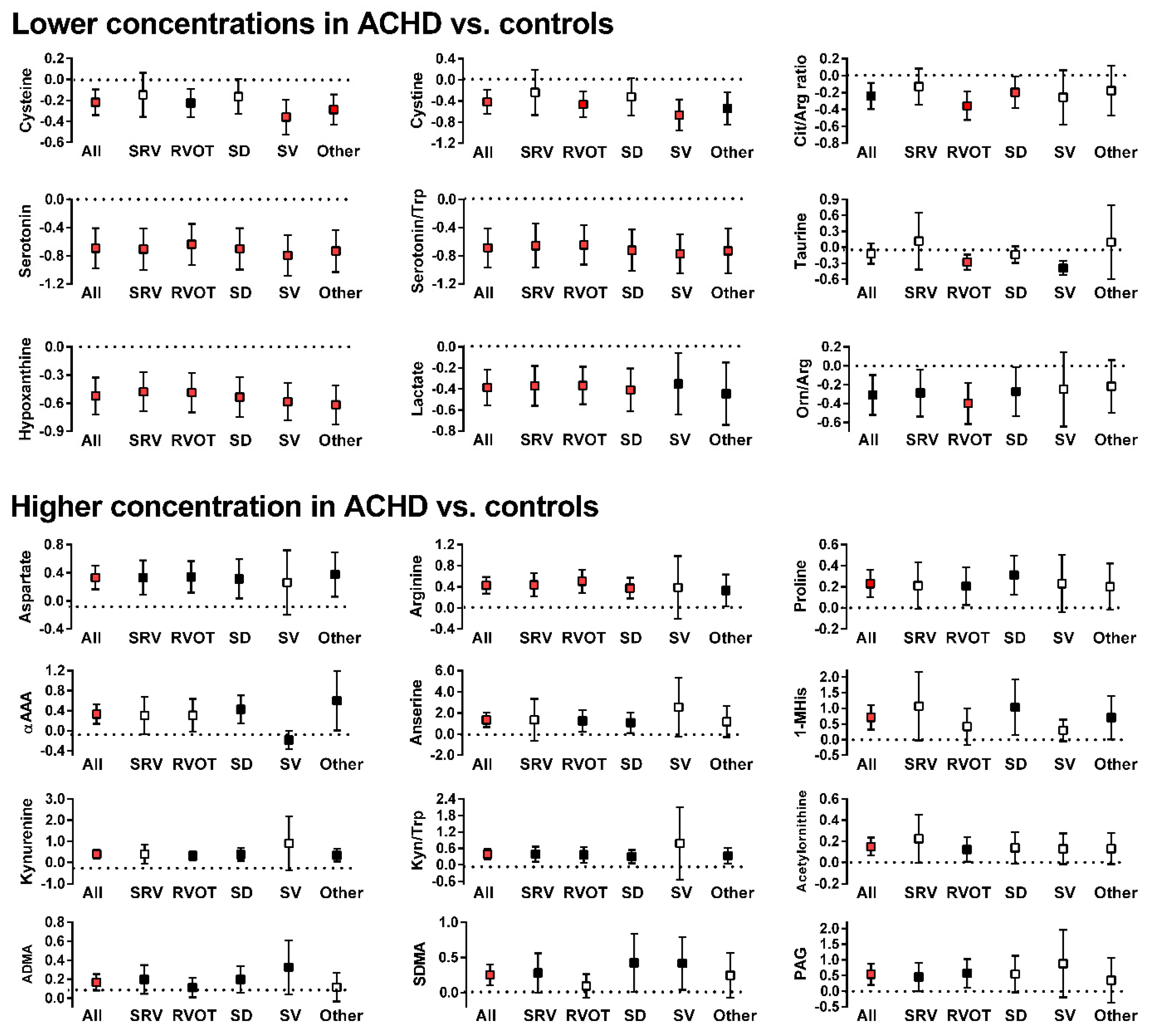
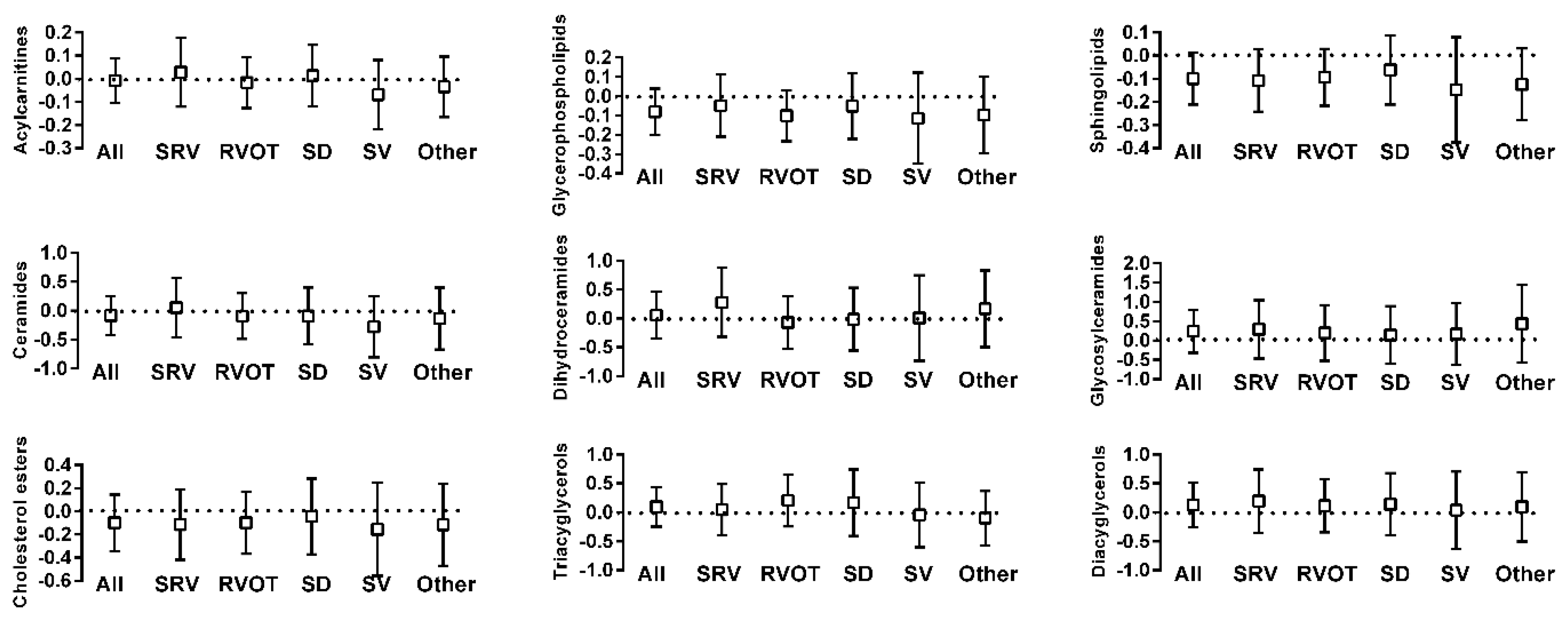
2.2. Metabolite Differences between ACHD and Control
2.3. ACHD Principal Component Cluster Determination
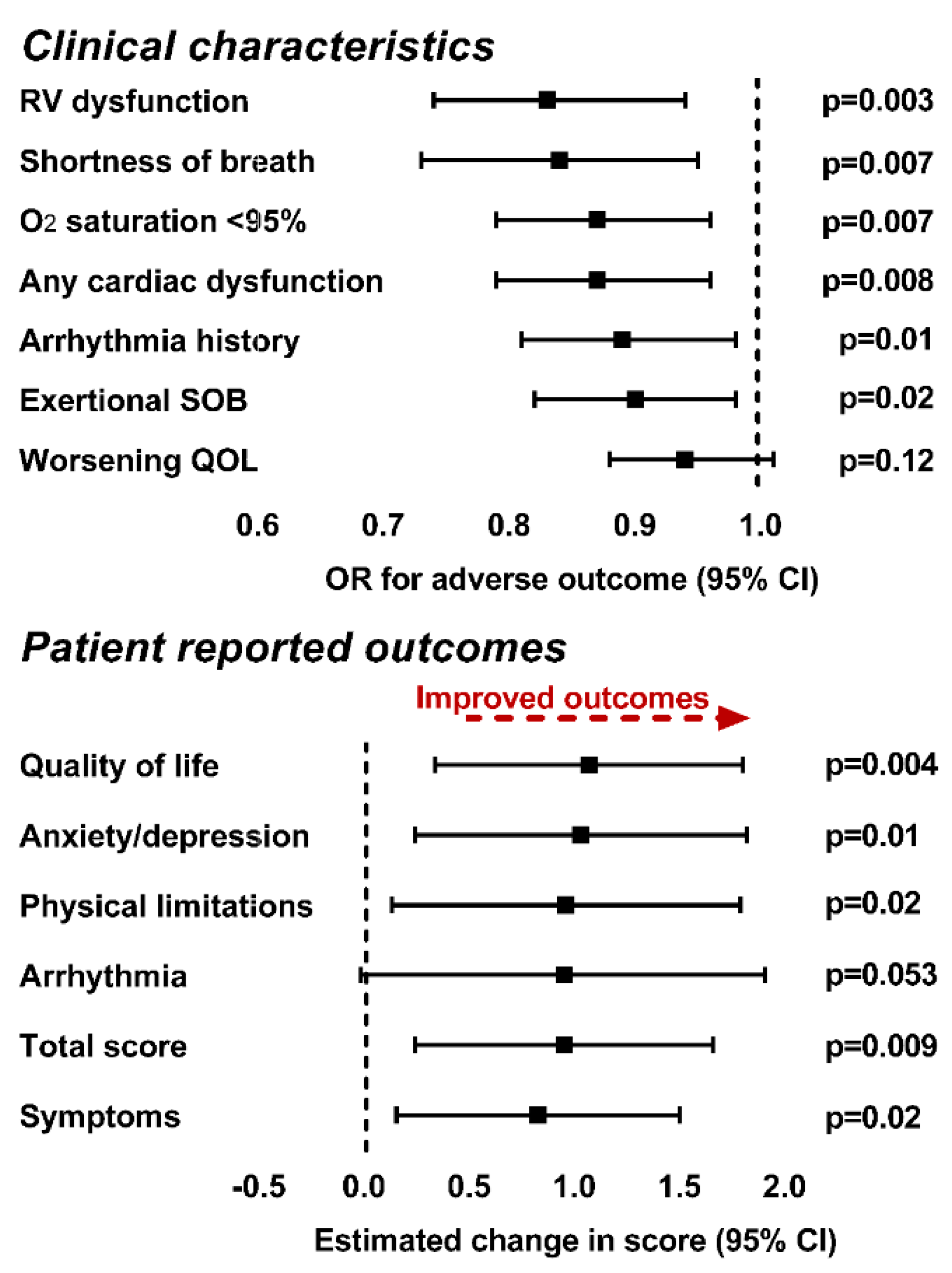
2.4. Metabolite Association with Clinical Variables
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Cohort
4.2. Clinical Data Acquisition
4.3. Biological Sample Acquisition and Metabolite Quantification
4.4. Metabolic Data Preprocessing
4.5. Statistical Analysis—Sample Selection
4.6. Comparisons of Metabolic Profiles and Identification of Metabolic Pathways
4.7. Principal Component Analysis
4.8. Association between Clinical Variables and Metabolic Profiles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement from the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.; Domínguez-Perles, R.; Gil, J.; Ferreres, F.; Gil-Izquierdo, A. Metabolomics and the diagnosis of human diseases—A guide to the markers and pathophysiological pathways affected. Curr. Med. Chem. 2014, 21, 823–848. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Sun, J.-L.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Pieper, K.S.; Haynes, C.; Hauser, E.R.; Kraus, E.W.; Granger, C.B.; et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012, 163, 844–850.e1. [Google Scholar] [CrossRef]
- A Koeth, R.; Wang, Z.; Levison, B.; A Buffa, J.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.W.; Wang, Z.; Cho, L.; Brennan, D.M.; Hazen, S.L. Diminished Global Arginine Bioavailability and Increased Arginine Catabolism as Metabolic Profile of Increased Cardiovascular Risk. J. Am. Coll. Cardiol. 2009, 53, 2061–2067. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; A Koeth, R.; Levison, B.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutarel, O.; Denecke, A.; Bode-Böger, S.M.; Martens-Lobenhoffer, J.; Lovric, S.; Bauersachs, J.; Schieffer, B.; Westhoff-Bleck, M.; Kielstein, J.T. Asymmetrical Dimethylarginine—More Sensitive than NT-proBNP to Diagnose Heart Failure in Adults with Congenital Heart Disease. PLoS ONE 2012, 7, e33795. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Dubowy, K.-O.; Zlamy, M.; Karall, D.; Adam, M.G.; Entenmann, A.; Keller, M.A.; Koch, J.; Komazec, I.O.; Geiger, R.; et al. Targeted metabolomic analysis of serum phospholipid and acylcarnitine in the adult Fontan patient with a dominant left ventricle. Ther. Adv. Chronic Dis. 2020, 11. [Google Scholar] [CrossRef]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, S.M.; Devine, O.J.; Kucik, J.E.; Oster, M.E.; Riehle-Colarusso, T.; Nembhard, W.N.; Xu, P.; Correa, A.; Jenkins, K.; Marelli, A.J. Congenital Heart Defects in the United States. Circulation 2016, 134, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Opotowsky, A.R.; Siddiqi, O.K.; Webb, G.D. Trends in Hospitalizations for Adults with Congenital Heart Disease in the U.S. J. Am. Coll. Cardiol. 2009, 54, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Verheugt, C.L.; Uiterwaal, C.S.P.M.; Van Der Velde, E.T.; Meijboom, F.J.; Pieper, P.G.; van Dijk, A.; Vliegen, H.W.; Grobbee, D.E.; Mulder, B.J. Mortality in adult congenital heart disease. Eur. Heart J. 2010, 31, 1220–1229. [Google Scholar] [CrossRef] [Green Version]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: Executive Summary. J. Am. Coll. Cardiol. 2019, 73, 1494–1563. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz-Siemion, M.; Kaminski, M.; Ciborowski, M.; Ptaszynska-Kopczynska, K.; Szpakowicz, A.; Lisowska, A.; Jasiewicz, M.; Tarasiuk, E.; Kretowski, A.; Sobkowicz, B.; et al. Machine-learning facilitates selection of a novel diagnostic panel of metabolites for the detection of heart failure. Sci. Rep. 2020, 10, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Ganna, A.; Salihovic, S.; Sundstrom, J.; Broeckling, C.; Hedman, A.K.; Magnusson, P.K.E.; Pedersen, N.L.; Larsson, A.; Siegbahn, A.; Zilmer, M.; et al. Large-scale Metabolomic Profiling Identifies Novel Biomarkers for Incident Coronary Heart Disease. PLoS Genet. 2014, 10, e1004801. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; Van Der Westhuizen, J.; Mathew, J.P.; Schwinn, D.; Glower, D.D.; Newgard, C.B.; Podgoreanu, M. Metabolomic Profiling Reveals Distinct Patterns of Myocardial Substrate Use in Humans with Coronary Artery Disease or Left Ventricular Dysfunction During Surgical Ischemia/Reperfusion. Circulation 2009, 119, 1736–1746. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a Peripheral Blood Metabolic Profile with Coronary Artery Disease and Risk of Subsequent Cardiovascular Events. Circ. Cardiovasc. Genet. 2010, 3, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Zomer, A.; Vaartjes, I.; van der Velde, E.; de Jong, H.; Konings, T.; Wagenaar, L.; Heesen, W.; Eerens, F.; Baur, L.; Grobbee, D.; et al. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int. J. Cardiol. 2013, 168, 2487–2493. [Google Scholar] [CrossRef]
- Engelings, C.C.; Helm, P.C.; Abdul-Khaliq, H.; Asfour, B.; Bauer, U.M.; Baumgartner, H.; Kececioglu, D.; Körten, M.-A.; Diller, G.-P.; Tutarel, O. Cause of death in adults with congenital heart disease—An analysis of the German National Register for Congenital Heart Defects. Int. J. Cardiol. 2016, 211, 31–36. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, Z.; Shrestha, K.; Thakur, A.; Borowski, A.G.; Sweet, W.; Thomas, J.D.; Moravec, C.S.; Hazen, S.L.; Tang, W.W. Pulmonary Hypertension Associated with Advanced Systolic Heart Failure: Dysregulated Arginine Metabolism and Importance of Compensatory Dimethylarginine Dimethylaminohydrolase-1. J. Am. Coll. Cardiol. 2012, 59, 1150–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.W.; Tong, W.; Shrestha, K.; Wang, Z.; Levison, B.S.; DelFraino, B.; Hu, B.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. Eur. Hear. J. 2008, 29, 2506–2513. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.; Dubowy, K.-O.; Entenmann, A.; Karall, D.; Adam, M.G.; Zlamy, M.; Komazec, I.O.; Geiger, R.; Niederwanger, C.; Salvador, C.; et al. Targeted metabolomic analysis of serum amino acids in the adult Fontan patient with a dominant left ventricle. Sci. Rep. 2020, 10, 8930. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Shrestha, K.; Wang, Z.; Troughton, R.W.; Klein, A.L.; Hazen, S.L. Diminished Global Arginine Bioavailability as a Metabolic Defect in Chronic Systolic Heart Failure. J. Card. Fail. 2013, 19, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Paulin, R.; Meloche, J.; Jacob, M.H.; Bisserier, M.; Courboulin, A.; Bonnet, S. Dehydroepiandrosterone inhibits the Src/STAT3 constitutive activation in pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1798–H1809. [Google Scholar] [CrossRef]
- Bonnet, S.; Dumas-De-La-Roque, E.; Begueret, H.; Marthan, R.; Fayon, M.; Dos Santos, P.; Savineau, J.-P.; Baulieu, E.-E. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc. Natl. Acad. Sci. USA 2003, 100, 9488–9493. [Google Scholar] [CrossRef] [Green Version]
- Alzoubi, A.; Toba, M.; Abe, K.; O’Neill, K.D.; Rocic, P.; Fagan, K.A.; McMurtry, I.F.; Oka, M. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am. J. Physiol. Circ. Physiol. 2013, 304, H1708–H1718. [Google Scholar] [CrossRef] [Green Version]
- Ventetuolo, C.; Ouyang, P.; Bluemke, D.; Tandri, H.; Barr, R.G.; Bagiella, E.; Cappola, A.R.; Bristow, M.R.; Johnson, C.; Kronmal, R.A.; et al. Sex Hormones Are Associated with Right Ventricular Structure and Function: The MESA-right ventricle study. Am. J. Respir. Crit. Care Med. 2011, 183, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Aubrecht, J.; Li, D.; Warner, R.L.; Johnson, K.J.; Kenny, J.; Colangelo, J.L. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS ONE 2018, 13, e0193824. [Google Scholar] [CrossRef] [PubMed]
- Hakuno, D.; Hamba, Y.; Toya, T.; Adachi, T. Plasma Amino Acid Profiling Identifies Specific Amino Acid Associations with Cardiovascular Function in Patients with Systolic Heart Failure. PLoS ONE 2015, 10, e0117325. [Google Scholar] [CrossRef]
- Razavi, A.C.; Bazzano, L.A.; He, J.; Fernandez, C.; Whelton, S.P.; Krousel-Wood, M.; Li, S.; Nierenberg, J.; Shi, M.; Li, C.; et al. Novel Findings from a Metabolomics Study of Left Ventricular Diastolic Function: The Bogalusa Heart Study. J. Am. Heart Assoc. 2020, 9, e015118. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef]
- Hardikar, S.; Albrechtsen, R.D.; Achaintre, D.; Lin, T.; Pauleck, S.; Playdon, M.; Holowatyj, A.N.; Gigic, B.; Schrotz-King, P.; Boehm, J.; et al. Impact of Pre-Blood Collection Factors on Plasma Metabolomic Profiles. Metabolites. 2020, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Cedars, A.M.; Ko, J.M.; John, A.S.; Vittengl, J.; Stefanescu-Schmidt, A.C.; Jarrett, R.B.; Kutty, S.; Spertus, J.A. Development of a Novel Adult Congenital Heart Disease–Specific Patient-Reported Outcome Metric. J. Am. Heart Assoc. 2020, 9, e015730. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 21 April 2020).
| Characteristics. | N | Value |
|---|---|---|
| Sex (male) | 99 | 50 (51%) |
| Diagnosis | 99 | |
| Transposition of the great arteries (SRV) | 21 (21%) | |
| TOF/DORV, RVOT stenosis (RVOT) | 34 (35%) | |
| Septal defects (SD) | 20 (20%) | |
| Single ventricle/Ebstein’s (SV) | 9 (9%) | |
| Other diagnoses | 15 (15%) | |
| Non-cardiac diagnosis | 96 | 71 (74%) |
| Caucasian race | 75 (76%) | |
| Education | 89 | |
| High school or less | 36 (40%) | |
| College | 22 (25%) | |
| Post-college | 31 (35%) | |
| Smoking | 98 | |
| Never | 64 (65%) | |
| Past smoker | 11 (11%) | |
| Current smoker | 23 (24%) | |
| Hypertension | 99 | 35 (35%) |
| Diabetes | 99 | 14 (14%) |
| Dyslipidemia | 98 | 18 (18%) |
| History of arrhythmia | 99 | 45 (45%) |
| Previous vascular surgery | 92 | 10 (11%) |
| Previous non-cardiac surgery | 98 | 59 (60%) |
| Significantly Impacted Pathways | |
|---|---|
| All congenital heart disease | Septal |
| Aminoacyl-tRNA biosynthesis *** | Aminoacyl-tRNA biosynthesis * |
| Histidine metabolism *** | Histidine metabolism * |
| Arginine biosynthesis ** | Arginine and proline metabolism |
| Arginine and proline metabolism * | Arginine biosynthesis |
| Phenylalanine, tyrosine and tryptophan biosynthesis * | beta-Alanine metabolism |
| Galactose metabolism | |
| Alanine, aspartate and glutamate metabolism | Single ventricle |
| D-Glutamine and D-glutamate metabolism | Taurine and hypotaurine metabolism |
| Taurine and hypotaurine metabolism | Sphingolipid metabolism |
| beta-Alanine metabolism | Linoleic acid metabolism |
| Phenylalanine metabolism | Cysteine and methionine metabolism |
| Systemic right ventricle | Other |
| Alanine, aspartate and glutamate metabolism *** | Histidine metabolism ** |
| Histidine metabolism *** | Aminoacyl-tRNA biosynthesis * |
| Aminoacyl-tRNA biosynthesis ** | Pantothenate and CoA biosynthesis |
| Arginine biosynthesis ** | beta-Alanine metabolism |
| Arginine and proline metabolism ** | Alanine, aspartate and glutamate metabolism |
| D-Glutamine and D-glutamate metabolism ** | Arginine biosynthesis |
| beta-Alanine metabolism ** | Glycerophospholipid metabolism |
| Butanoate metabolism | |
| Sphingolipid metabolism | |
| TOF/DORV, RVOT stenosis | |
| Arginine biosynthesis *** | |
| Aminoacyl-tRNA biosynthesis *** | |
| Histidine metabolism * | |
| D-Glutamine and D-glutamate metabolism * | |
| Arginine and proline metabolism * | |
| Taurine and hypotaurine metabolism | |
| Phenylalanine metabolism | |
| Alanine, aspartate and glutamate metabolism | |
| Pantothenate and CoA biosynthesis |
| Significantly Impacted Pathways | |
|---|---|
| PC1 | PC2 |
| Glycerolipid metabolism | Sphingolipid metabolism * |
| Linoleic acid metabolism | |
| PC3 | alpha-Linolenic acid metabolism |
| Glycine, serine and threonine metabolism | |
| Glycerophospholipid metabolism | PC4 |
| Arginine biosynthesis * | |
| Phenylalanine metabolism | |
| Phenylalanine, tyrosine and tryptophan biosynthesis | |
| Galactose metabolism | |
| Butanoate metabolism | |
| D-Glutamine and D-glutamate metabolism | |
| Taurine and hypotaurine metabolism | |
| Arginine and proline metabolism |
| PC Only | PC + Highly Reliable Metabolites | |||||
|---|---|---|---|---|---|---|
| Outcomes | PC Loads | Clinical Covariates | QIC (PC) | PC Loads | Significant Metabolites | QIC (mets) |
| O2 saturation < 95% | 4 | SV, SD | 95.6 | ADMA/Arg, serotonin/tryptophan, GCDCA, Betaine | 84.6 | |
| Cardiac dysfunction | 4 | None | 119.6 | 2,5 | Citrulline, Cystine, 1-Methylhistidine | 103.4 |
| RV dysfunction | 4 | SRV, dyslipidemia | 79.9 | 4 | DHEAS | 76.4 |
| Arrhythmia history | 2,4 | SRV, SV, age | 179.3 | 2 | TCA, Orn/Arg | 175.4 |
| Shortness of breath | 1,4 | None | 143.0 | Ornithine, SDMA/Arg, Homo-L-arginine | 120.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedars, A.; Manlhiot, C.; Ko, J.-M.; Bottiglieri, T.; Arning, E.; Weingarten, A.; Opotowsky, A.; Kutty, S. Metabolomic Profiling of Adults with Congenital Heart Disease. Metabolites 2021, 11, 525. https://doi.org/10.3390/metabo11080525
Cedars A, Manlhiot C, Ko J-M, Bottiglieri T, Arning E, Weingarten A, Opotowsky A, Kutty S. Metabolomic Profiling of Adults with Congenital Heart Disease. Metabolites. 2021; 11(8):525. https://doi.org/10.3390/metabo11080525
Chicago/Turabian StyleCedars, Ari, Cedric Manlhiot, Jong-Mi Ko, Teodoro Bottiglieri, Erland Arning, Angela Weingarten, Alexander Opotowsky, and Shelby Kutty. 2021. "Metabolomic Profiling of Adults with Congenital Heart Disease" Metabolites 11, no. 8: 525. https://doi.org/10.3390/metabo11080525
APA StyleCedars, A., Manlhiot, C., Ko, J.-M., Bottiglieri, T., Arning, E., Weingarten, A., Opotowsky, A., & Kutty, S. (2021). Metabolomic Profiling of Adults with Congenital Heart Disease. Metabolites, 11(8), 525. https://doi.org/10.3390/metabo11080525







