Metabolite Profiling and Anti-Aging Activity of Rice Koji Fermented with Aspergillus oryzae and Aspergillus cristatus: A Comparative Study
Abstract
:1. Introduction
2. Results
2.1. Metabolic Profiling for Rice Koji Fermented with Different Aspergillus spp.
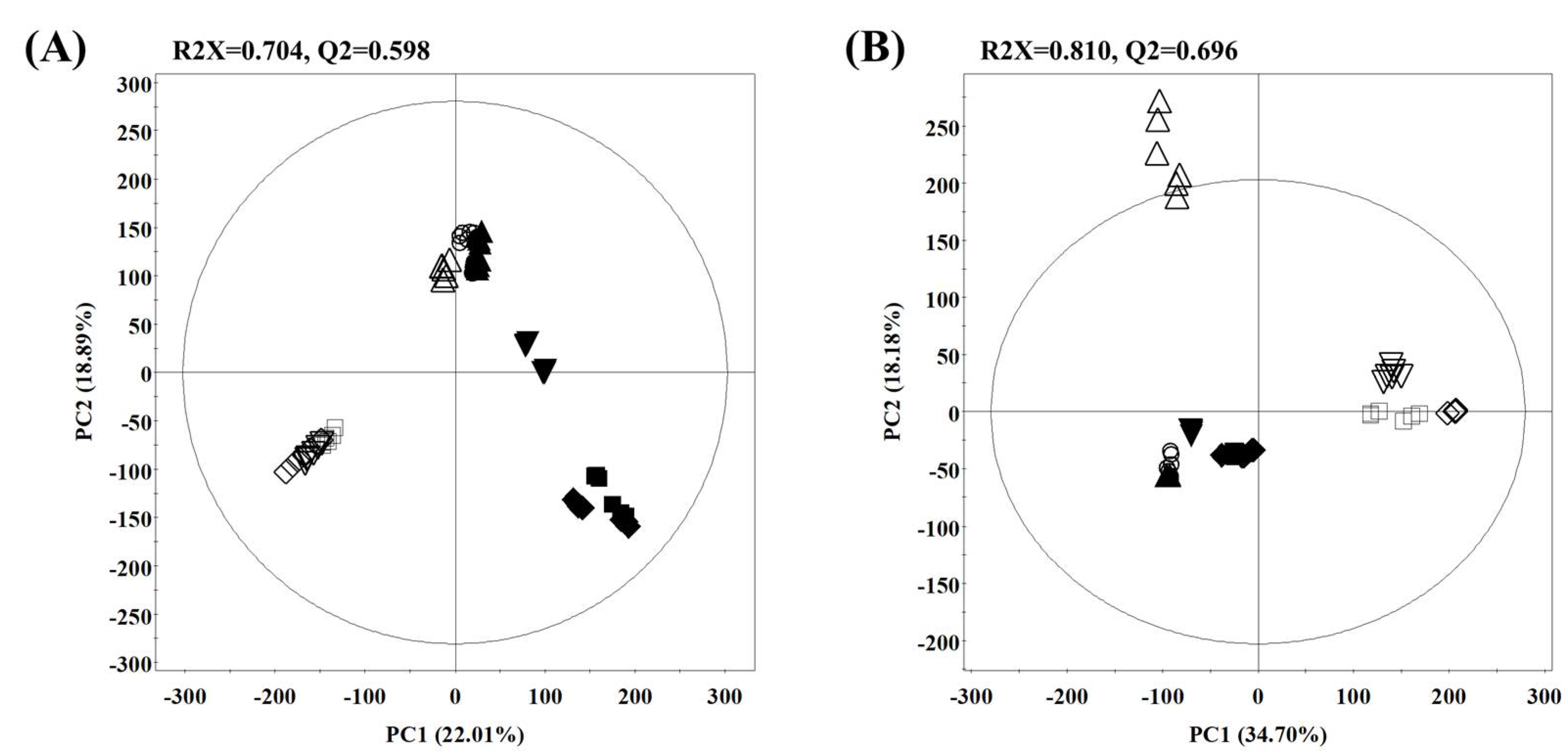
2.1.1. Temporal Metabolomes for Rice Koji with Different Aspergillus spp. Inoculation According to Fermentation Time
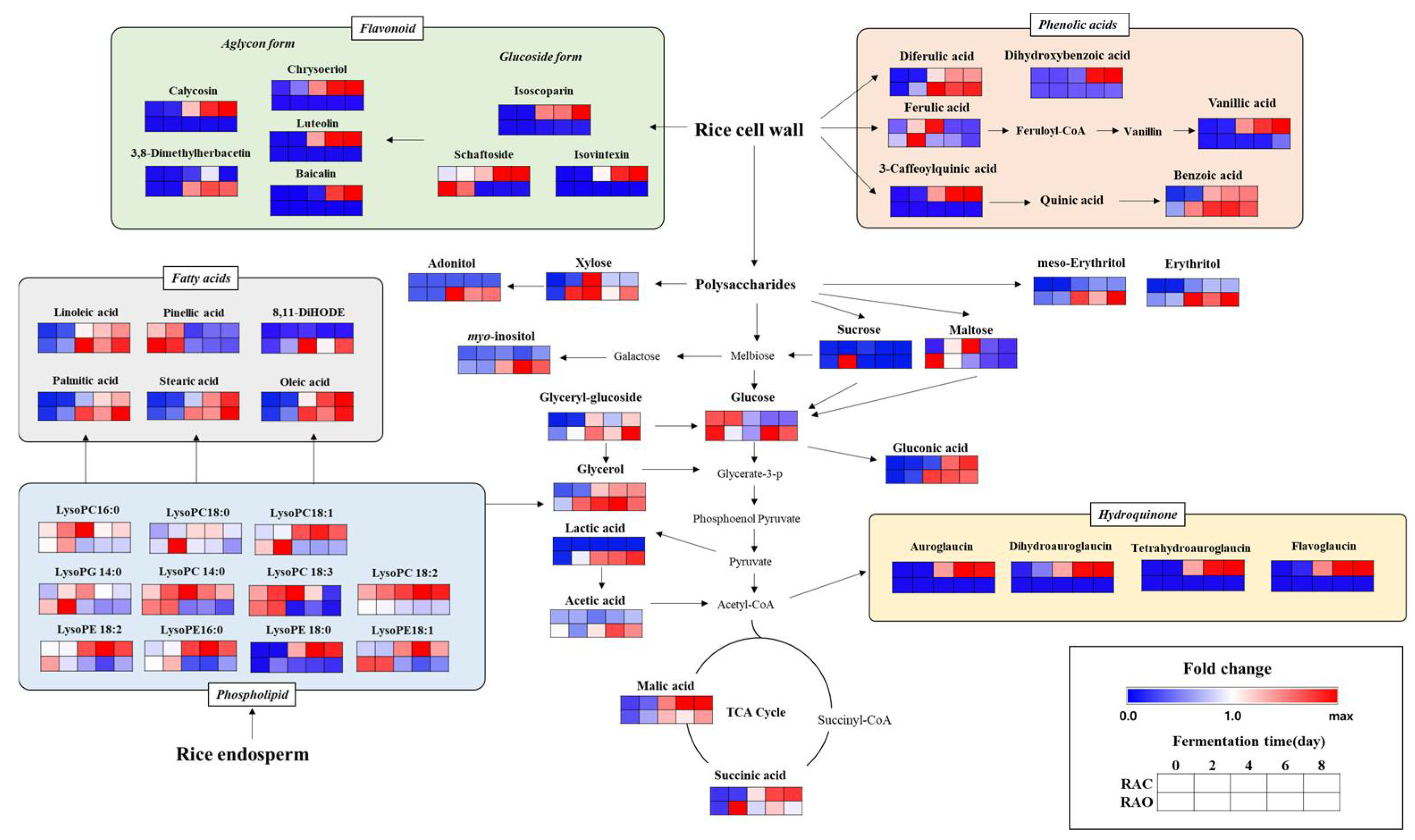
2.1.2. Relative Disparity in the Level of Discriminant Metabolites in Rice Koji Fermented by A. cristatus or A. oryzae
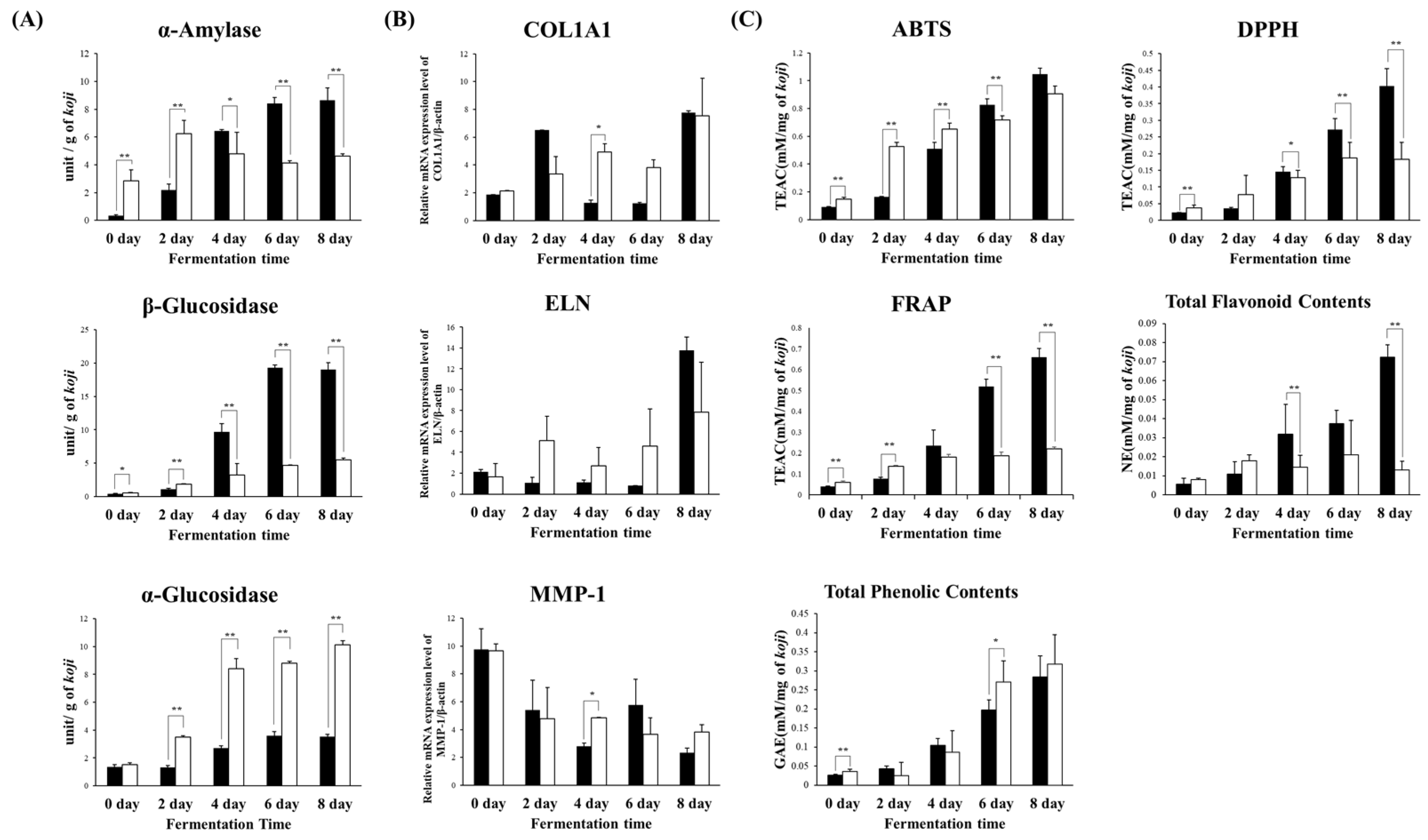
2.2. Comparison of Enzymatic Production and Bioactivity in Rice Koji Fermented with Different Microorganisms
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation and Extraction
4.3. GC–TOF–MS Analysis
4.4. UHPLC–LTQ–Orbitrap–MS Analysis
4.5. Data Processing and Statistical Analysis
4.6. Determination of Enzymatic Activities
4.7. Determination of Antioxidant Activities and Total Phenolic and Flavonoid Contents
4.8. Cell Cultures
4.9. Real-Time Polymerase Chain Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanlier, N.; Gokcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Yu, K.-W.; Lee, S.-E.; Choi, H.-S.; Suh, H.J.; Ra, K.S.; Choi, J.W.; Hwang, J.-H. Optimization for rice koji preparation using Aspergillus oryzae CJCM-4 isolated from a Korean traditional meju. Food Sci. Biotechnol. 2012, 21, 129–135. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Lin, X.; Wang, G.; Zhang, H.; Xiong, Z.; Yu, H.; Yu, J.; Ai, L. Improvement of flavor profiles in Chinese rice wine by creating fermenting yeast with superior ethanol tolerance and fermentation activity. Food Res. Int. 2018, 108, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, E.; Hirata, S.; Hata, Y.; Yazawa, H.; Tamura, H.; Kaneoke, M.; Iwashita, K.; Hirata, D. Effect of koji starter on metabolites in Japanese alcoholic beverage sake made from the sake rice Koshitanrei. Biosci. Biotechnol. Biochem. 2020, 84, 1714–1723. [Google Scholar] [CrossRef]
- Phetpornpaisan, P.; Tippayawat, P.; Jay, M.; Sutthanut, K. A local Thai cultivar glutinous black rice bran: A source of functional compounds in immunomodulation, cell viability and collagen synthesis, and matrix metalloproteinase-2 and-9 inhibition. J. Funct. Foods 2014, 7, 650–661. [Google Scholar] [CrossRef]
- Kim, A.J.; Choi, J.N.; Kim, J.; Kim, H.Y.; Park, S.B.; Yeo, S.H.; Choi, J.H.; Liu, K.H.; Lee, C.H. Metabolite profiling and bioactivity of rice koji fermented by Aspergillus strains. J. Microbiol. Biotechnol. 2012, 22, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.-C.; Chang, Y.-C.; Su, S.-W. Antioxidant activity and active compounds of rice koji fermented with Aspergillus candidus. Food Chem. 2003, 83, 49–54. [Google Scholar] [CrossRef]
- Lee, D.E.; Lee, S.; Singh, D.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Time-resolved comparative metabolomes for Koji fermentation with brown-, white-, and giant embryo-rice. Food Chem. 2017, 231, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, M.; Behl, S.; Shaheen, N.; Fatima, A.; Nasab, R. Anti-Aging Effects of Retinol and Alpha Hydroxy Acid on Elastin Fibers of Artificially Photo-Aged Human Dermal Fibroblast Cell Lines. Int. J. Med Health Biomed. Pharm. Eng. 2015, 7, 328. [Google Scholar]
- Bolla, S.R.; Al-Subaie, A.M.; Al-Jindan, R.Y.; Balakrishna, J.P.; Ravi, P.K.; Veeraraghavan, V.P.; Pillai, A.A.; Gollapalli, S.S.R.; Joseph, J.P.; Surapaneni, K.M. In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon 2019, 5, e01648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of Orally Taken Carotenoid-Rich Curly Kale Extract on Collagen I/Elastin Index of the Skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majeed, M.; Bhat, B.; Anand, S.; Sivakumar, A.; Paliwal, P.; Geetha, K.G. Inhibition of UV-induced ROS and collagen damage by Phyllanthus emblica extract in normal human dermal fibroblasts. J. Cosmet. Sci. 2011, 62, 49–56. [Google Scholar] [PubMed]
- Masuda, M.; Murata, K.; Naruto, S.; Uwaya, A.; Isami, F.; Matsuda, H. Matrix metalloproteinase-1 inhibitory activities of Morinda citrifolia seed extract and its constituents in UVA-irradiated human dermal fibroblasts. Biol. Pharm. Bull. 2012, 35, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.-K.; Jung, S.-H.; Song, K.-Y.; Park, J.-K.; Park, C.-S. Anti-photoaging effect of fermented rice bran extract on UV-induced normal skin fibroblasts. Eur. Food Res. Technol. 2010, 231, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Bechman, A.; Phillips, R.D.; Chen, J. Changes in selected physical property and enzyme activity of rice and barley koji during fermentation and storage. J. Food. Sci. 2012, 77, M318–M322. [Google Scholar] [CrossRef]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef]
- Zhao, P.; Alam, M.B.; Lee, S.H. Protection of UVB-Induced Photoaging by Fuzhuan-Brick Tea Aqueous Extract via MAPKs/Nrf2-Mediated Down-Regulation of MMP-1. Nutrients 2018, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-D.; Xu, X.; Lin, Y.-F.; Xia, H.-Y.; Huang, L.; Dong, M.-S. On-line screening and identification of free radical scavenging compounds in Angelica dahurica fermented with Eurotium cristatum using an HPLC-PDA-Triple-TOF-MS/MS-ABTS system. Food Chem. 2019, 272, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.E.; Singh, D.; Lee, C.H. Metabolomics Reveal Optimal Grain Preprocessing (Milling) toward Rice Koji Fermentation. J. Agric. Food Chem. 2018, 66, 2694–2703. [Google Scholar] [CrossRef]
- Lee da, E.; Lee, S.; Jang, E.S.; Shin, H.W.; Moon, B.S.; Lee, C.H. Metabolomic Profiles of Aspergillus oryzae and Bacillus amyloliquefaciens During Rice Koji Fermentation. Molecules 2016, 21, 773. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M. Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivities in humans, animals, and cells. J. Agric. Food Chem. 2013, 61, 10626–10641. [Google Scholar] [CrossRef]
- Rao, R.S.P.; Muralikrishna, G. Water soluble feruloyl arabinoxylans from rice and ragi: Changes upon malting and their consequence on antioxidant activity. Phytochemistry 2006, 67, 91–99. [Google Scholar] [CrossRef]
- Kim, A.J.; Choi, J.N.; Kim, J.; Yeo, S.H.; Choi, J.H.; Lee, C.H. Metabolomics-based optimal koji fermentation for tyrosinase inhibition supplemented with Astragalus radix. Biosci. Biotechnol. Biochem. 2012, 76, 863–869. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.; Marchesan, E. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Biol. Technol. 2011, 54, 371–377. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chen, J.; Tang, H.; Wang, C.; Li, Z.; Xiao, Y. Bioprocessing of soybeans (Glycine max L.) by solid-state fermentation with Eurotium cristatum YL-1 improves total phenolic content, isoflavone aglycones, and antioxidant activity. RSC Adv. 2020, 10, 16928–16941. [Google Scholar] [CrossRef]
- Miyake, Y.; Mochizuki, M.; Ito, C.; Itoigawa, M.; Osawa, T. Peroxynitrite scavengers produced by filamentous fungus used in the katsuobushi manufacturing process. Food Sci. Technol. Res. 2010, 16, 493–498. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.J.; Hubka, V.; Frisvad, J.C.; Visagie, C.M.; Houbraken, J.; Meijer, M.; Varga, J.; Demirel, R.; Jurjevic, Z.; Kubatova, A.; et al. Polyphasic taxonomy of Aspergillus section Aspergillus (formerly Eurotium), and its occurrence in indoor environments and food. Stud. Mycol. 2017, 88, 37–135. [Google Scholar] [CrossRef] [Green Version]
- Yahagi, S.; Koike, M.; Okano, Y.; Masaki, H. Lysophospholipids improve skin moisturization by modulating of calcium-dependent cell differentiation pathway. Int. J. Cosmet. Sci. 2011, 33, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.C.; Petros, J.; Clark, R.E.; Viehman, G.E. Dry skin and moisturizers. Clin. Dermatol. 2001, 19, 387–392. [Google Scholar] [CrossRef]
- Yokota, M.; Shimizu, K.; Kyotani, D.; Yahagi, S.; Hashimoto, S.; Masaki, H. The possible involvement of skin dryness on alterations of the dermal matrix. Exp. Dermatol. 2014, 23, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.C.; Kim, Y.X.; Lee, S.; Jung, E.S.; Singh, D.; Sung, J.; Lee, C.H. Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism. Metabolites 2019, 9, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudick, M.J.; Fitzgerald, Z.E.; Rudick, V.L. Intra-and extracellular forms of α-glucosidase from Aspergillus niger. Arch. Biochem. Biophys. 1979, 193, 509–520. [Google Scholar] [CrossRef]
- Del Moral, S.; Barradas-Dermitz, D.; Aguilar-Uscanga, M. Production and biochemical characterization of α-glucosidase from Aspergillus niger ITV-01 isolated from sugar cane bagasse. 3 Biotech. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Son, S.Y.; Lee, S.; Singh, D.; Lee, S.; Lee, J.S.; Lee, C.H. Strategy for Screening of Antioxidant Compounds from Two Ulmaceae Species Based on Liquid Chromatography-Mass Spectrometry. Molecules 2018, 23, 1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Lee, S.; Kyung, S.; Ryu, J.; Kang, S.; Park, M.; Lee, C. Metabolite Profiling and Anti-Aging Activity of Rice Koji Fermented with Aspergillus oryzae and Aspergillus cristatus: A Comparative Study. Metabolites 2021, 11, 524. https://doi.org/10.3390/metabo11080524
Lee H, Lee S, Kyung S, Ryu J, Kang S, Park M, Lee C. Metabolite Profiling and Anti-Aging Activity of Rice Koji Fermented with Aspergillus oryzae and Aspergillus cristatus: A Comparative Study. Metabolites. 2021; 11(8):524. https://doi.org/10.3390/metabo11080524
Chicago/Turabian StyleLee, Hyunji, Sunmin Lee, Seoyeon Kyung, Jeoungjin Ryu, Seunghyun Kang, Myeongsam Park, and Choonghwan Lee. 2021. "Metabolite Profiling and Anti-Aging Activity of Rice Koji Fermented with Aspergillus oryzae and Aspergillus cristatus: A Comparative Study" Metabolites 11, no. 8: 524. https://doi.org/10.3390/metabo11080524
APA StyleLee, H., Lee, S., Kyung, S., Ryu, J., Kang, S., Park, M., & Lee, C. (2021). Metabolite Profiling and Anti-Aging Activity of Rice Koji Fermented with Aspergillus oryzae and Aspergillus cristatus: A Comparative Study. Metabolites, 11(8), 524. https://doi.org/10.3390/metabo11080524






