Monitoring Early Glycolytic Flux Alterations Following Radiotherapy in Cancer and Immune Cells: Hyperpolarized Carbon-13 Magnetic Resonance Imaging Study
Abstract
:1. Introduction
2. Results
2.1. Cancer and Immune Cells
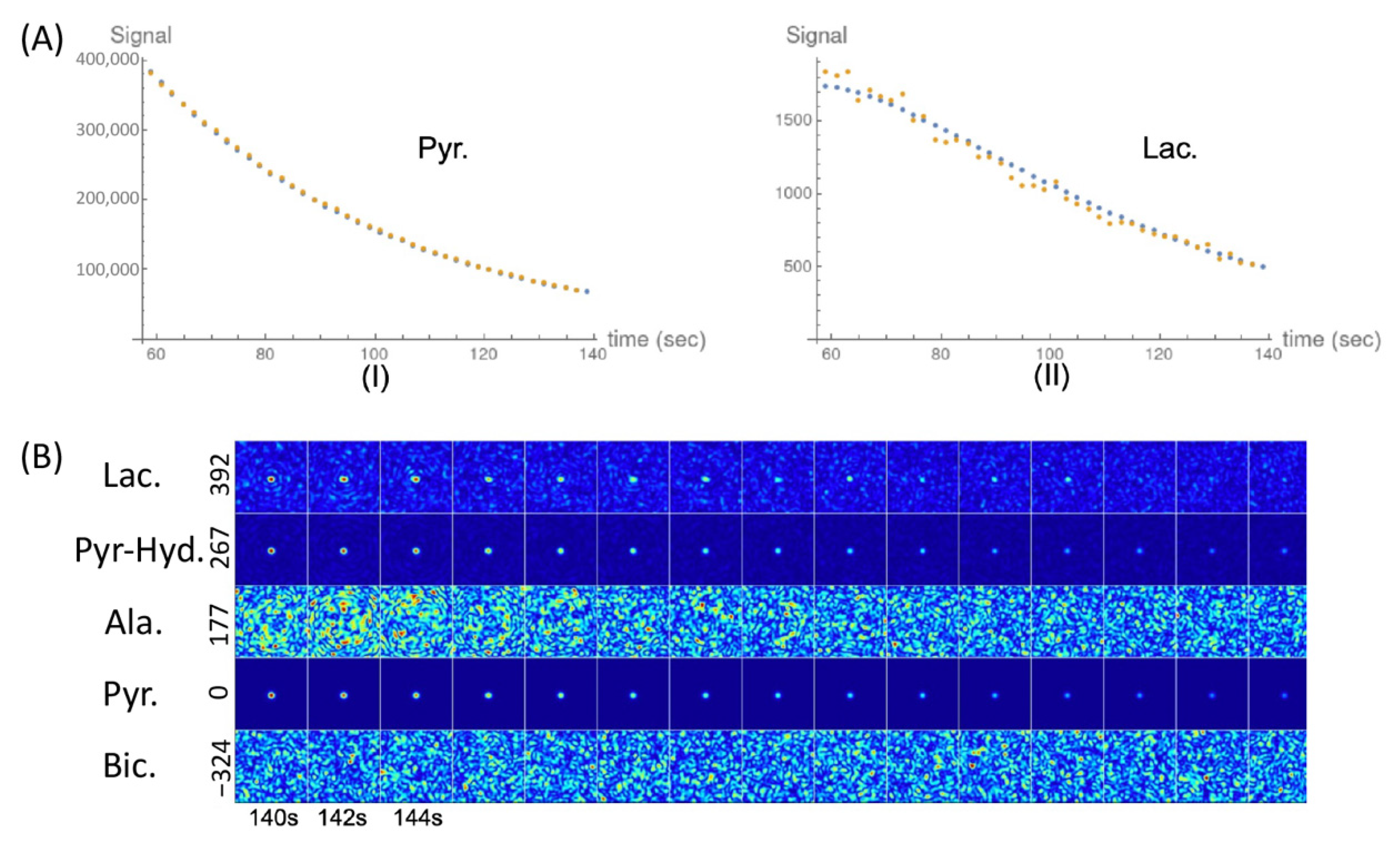
2.2. Glycolytic Flux Alterations Following Radiotherapy in Cancer and Immune Cells
2.3. The Changes of LDH Corresponding to the Changes of kPL [Pyr.] on DNP 13C-MRI
3. Discussion
4. Materials and Methods
4.1. Cell Preparation and Irradiation
4.2. [1-13. C]Pyruvate Hyperpolarization and In Vitro Experiments
4.3. Imaging Acquisition
4.4. Western Blot
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hutchinson, M.N.D.; Mierzwa, M.; D’Silva, N.J. Radiation resistance in head and neck squamous cell carcinoma: Dire need for an appropriate sensitizer. Oncogene 2020, 39, 3638–3649. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 1–15. [Google Scholar] [CrossRef]
- Lin, J.; Xia, L.; Liang, J.; Han, Y.; Wang, H.; Oyang, L.; Tan, S.; Tian, Y.; Rao, S.; Chen, X.; et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J. Exp. Clin. Cancer Res. 2019, 38, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Gregorio, A.; Martinez-Ramirez, I.; Pedraza-Chaverri, J.; Lizano, M. Reprogramming of energy metabolism in response to radiotherapy in head and neck squamous cell carcinoma. Cancers 2019, 11, 182. [Google Scholar] [CrossRef] [Green Version]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draoui, N.; Feron, O. Lactate shuttles at a glance: From physiological paradigms to anti-cancer treatments. Dis. Model. Mech. 2011, 4, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Ming, J.; Zhou, Y.; Fan, L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother. Pharmacol. 2016, 77, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.I.; Giatromanolaki, A.; Winter, S.; Leek, R.; Sivridis, E.; Harris, A.L. Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology 2009, 77, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Quennet, V.; Yaromina, A.; Zips, D.; Rosner, A.; Walenta, S.; Baumann, M.; Mueller-Klieser, E. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother. Oncol. 2006, 81, 130–135. [Google Scholar] [CrossRef]
- Biswas, S.K. Metabolic reprogramming of immune cells in cancer progression. Immunity 2015, 43, 435–449. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, K.; Akakabe, M.; Tsuda, M.; Tsuda, M.; Fukushi, E.; Kawabata, J.; Abe, T.; Ichikawa, K. Observation of glycolytic metabolites in tumor cell lysate by using hyperpolarization of deuterated glucose. Biol. Pharm. Bull. 2014, 37, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehlerm, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Suh, Y.; Jung, K. Context drives diversification of monocytes and neutrophils in orchestrating the tumor microenvironment. Front. Immunol. 2019, 10, 1817. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Najac, C.; Ronen, S.M. Studies of metabolism using 13C MRS of hyperpolarized probes. Methods Enzymol. 2015, 561, 1–71. [Google Scholar]
- Merritt, M.E.; Harrison, C.; Storey, C.; Jeffrey, F.M.; Sherry, A.D.; Malloy, C.R. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc. Natl. Acad. Sci. USA 2007, 104, 19773–19777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [Green Version]
- Sandulache, V.C.; Chen, Y.; Lee, J.; Rubinstein, A.; Ramirez, M.S.; Skinner, H.D.; Walker, C.M.; Williams, M.D.; Tailor, R.; Court, L.E.; et al. Evaluation of hyperpolarized [1-13C]-pyruvate by magnetic resonance to detect ionizing radiation effects in real time. PLoS ONE 2014, 9, e87031. [Google Scholar] [CrossRef]
- Chen, A.P.; Chu, W.; Gu, Y.P.; Cunningham, C.H. Probing early tumor response to radiation therapy using hyperpolarized [1-13C]pyruvate in MDA-MB-231 xenografts. PLoS ONE 2013, 8, e56551. [Google Scholar]
- Shulman, R.G.; Brown, T.R.; Ugurbil, K.; Ogawa, S.; Cohen, S.M.; Den Hollander, J.A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science 1979, 205, 160–166. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Larson, P.E.; Yoshihara, H.A.; Danforth, O.M.; Vigneron, D.B.; Nelson, S.J.; Pieper, R.O.; Phillips, J.J.; Ronen, S.M. Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand, K. Aerobic glycolysis by proliferating cells: Protection against oxidative stress at the expense of energy yield. J. Bioenerg. Biomembr. 1997, 29, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Qin, L.; Liu, H.C.; Candas, D.; Fan, M.; Li, J.J. Tumor cells switch to mitochondrial oxidative phosphorylation under radiation via mTOR-mediated hexokinase II inhibition—A Warburg-reversing effect. PLoS ONE 2015, 10, e0121046. [Google Scholar] [CrossRef]
- Kelly, P.M.; Davison, R.S.; Bliss, E.; McGee, J.O. Macrophages in human breast disease: A quantitative immunohistochemical study. Br. J. Cancer 1988, 57, 174–177. [Google Scholar] [CrossRef]
- Van Overmeire, E.; Laoui, D.; Keirsse, J.; Van Ginderachter, J.A.; Sarukhan, A. Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Front. Immunol. 2014, 5, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, A.M.; Sun, Y.; Burns, T.C.; He, L.; Kee, N.; Oliva-Vilarnau, N.; Alevyzaki, A.; Zhou, K.; Louhivouri, L.; Hedlund, E.; et al. Radiation triggers a dynamic sequence of transient microglial alterations in juvenile brain. Cell Rep. 2020, 31, 107699. [Google Scholar] [CrossRef]
- Suzuki, C.; Han, S.; Kesavamoorthy, G.; Kosugi, M.; Araki, K.; Harada, N.; Kanazawa, M.; Tsukada, H.; Magata, Y.; Ouchi, Y. Differences in in vitro microglial accumulation of the energy metabolism tracers [18F]FDG and [18F]BCPP-EF during LPS- and IL4 stimulation. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Bernier, L.P.; York, E.M.; Kamyabi, A.; Choi, H.B.; Weilinger, N.L.; MacVicar, B.A. Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nat. Commun. 2020, 11, 1559. [Google Scholar] [CrossRef]
- Kramer, P.A.; Ravi, S.; Chacko, B.; Johnson, M.S.; Darley-Usmar, V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014, 2, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Woo, J.K.; Jang, Y.S.; Oh, S.H. Radiation potentiates monocyte infiltration into tumors by Ninjurin1 expression in endothelial cells. Cells 2020, 9, 1086. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Al-Sharea, A.; Shihata, W.A.; Bertuzzo Veiga, C.; Cooney, O.D.; Fleetwood, A.J.; Flynn, M.C.; Claeson, E.; Palmer, C.S.; Lancaster, G.I.; et al. Glycolysis is required for LPS-induced activation and adhesion of human CD14 (+) CD16 (-) monocytes. Front. Immunol. 2019, 10, 2054. [Google Scholar] [CrossRef]
- Witney, T.H.; Kettunen, M.I.; Brindle, K.M. Kinetic modeling of hyperpolarized 13C label exchange between pyruvate and lactate in tumor cells. J. Biol. Chem. 2011, 286, 24572–24580. [Google Scholar] [CrossRef] [Green Version]
- Lev-Cohain, N.; Sapir, G.; Harris, T.; Azar, A.; Gamliel, A.; Nardi-Schreiber, A.; Uppala, S.; Sosna, J.; Gomori, J.M.; Katz-Brull, R.; et al. Real-time ALT and LDH activities determined in viable precision-cut mouse liver slices using hyperpolarized [1-13C] pyruvate—Implications for studies on biopsied liver tissues. NMR Biomed. 2019, 32, e4043. [Google Scholar] [CrossRef]
- Rao, Y.; Gammon, S.; Zacharias, N.M.; Liu, T.; Salzillo, T.; Xi, Y.; Wang, J.; Bhattacharya, P.; Piwnica-Worms, D. Hyperpolarized [1-13C] pyruvate-to-[1-13C] lactate conversion is rate-limited by monocarboxylate transporter-1 in the plasma membrane. Proc. Natl. Acad. Sci. USA 2020, 117, 22378–22389. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.; Yang, C.; Jindal, A.; DeBerardinis, R.J.; Hooshyar, M.A.; Merritt, M.; Sherry, A.D.; Malloy, C.R. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13C NMR. NMR Biomed. 2012, 25, 1286–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiesinger, F.; Weidl, E.; Menzel, M.I.; Janich, M.A.; Khegai, O.; Glaser, S.J.; Haase, A.; Schwaiger, M.; Schulte, R.F. IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C] pyruvate. Magn. Reson. Med. 2012, 68, 8–16. [Google Scholar] [CrossRef] [PubMed]


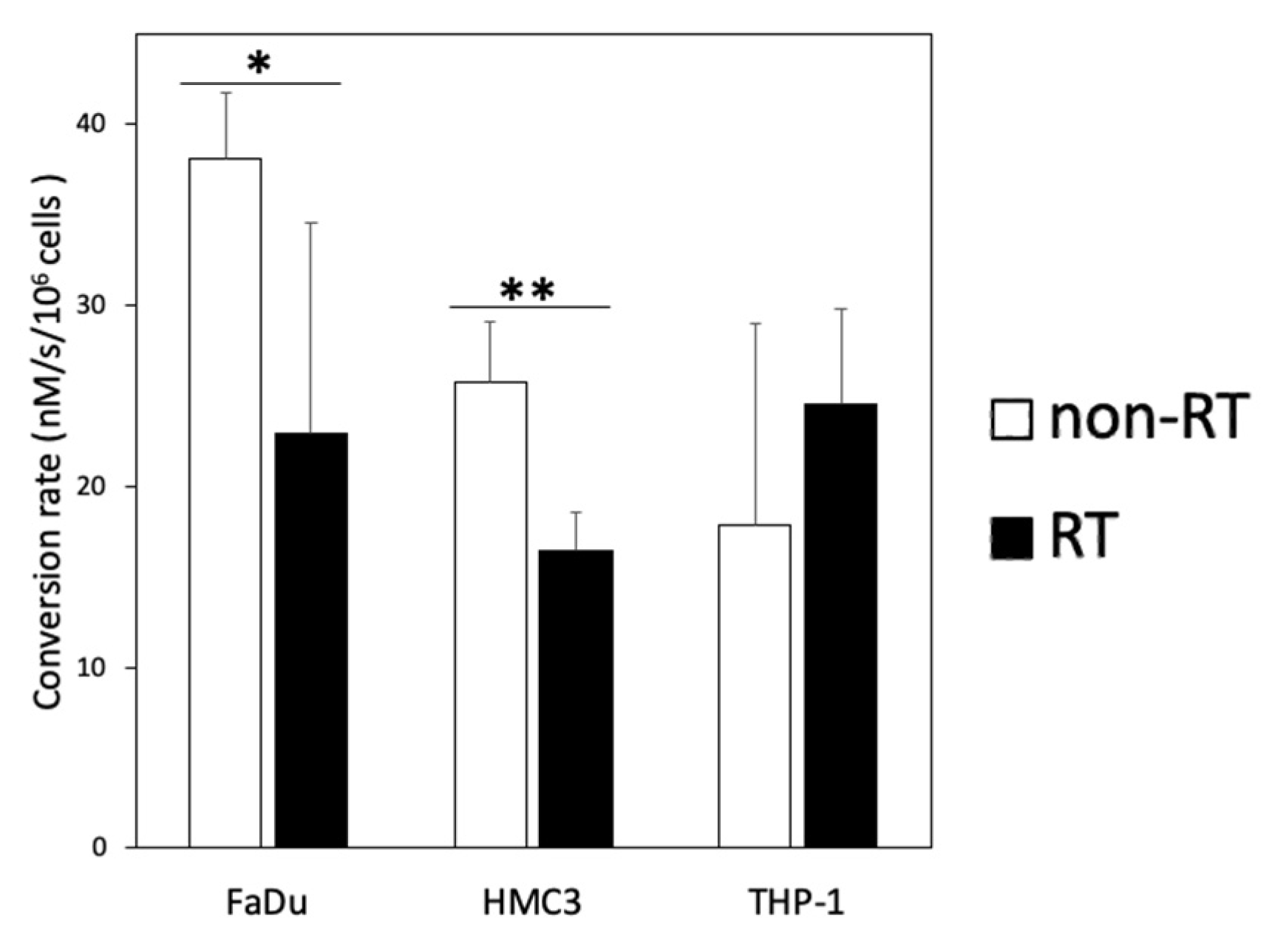

| Cells | Non-RT | RT | p-Value * | ||
|---|---|---|---|---|---|
| kPL [Pyr.] | R2 | kPL [Pyr.] | R2 | ||
| FaDu | 0.049 | ||||
| 34.1 | 0.821 | 25.6 | 0.963 | ||
| 41.2 | 0.780 | 10.2 | 0.901 | ||
| 39.0 | 0.948 | 33.0 | 0.923 | ||
| HMC3 | 0.008 | ||||
| 29.2 † | 0.827 | 17.6 | 0.298 | ||
| 22.5 | 0.980 | 13.9 † | 0.988 | ||
| 25.5 | 0.945 | 17.7 | 0.900 | ||
| THP-1 | 0.199 | ||||
| 17.2 | 0.996 | 22.6 | 0.993 | ||
| 29.3 | 0.990 | 30.5 | 0.984 | ||
| 7.2 | 0.928 | 20.6 | 0.746 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-C.; Hsieh, C.-Y.; Lu, K.-Y.; Sung, C.-H.; Ho, H.-Y.; Cheng, M.-L.; Chen, A.P.; Ng, S.-H.; Chen, F.-H.; Lin, G. Monitoring Early Glycolytic Flux Alterations Following Radiotherapy in Cancer and Immune Cells: Hyperpolarized Carbon-13 Magnetic Resonance Imaging Study. Metabolites 2021, 11, 518. https://doi.org/10.3390/metabo11080518
Lai Y-C, Hsieh C-Y, Lu K-Y, Sung C-H, Ho H-Y, Cheng M-L, Chen AP, Ng S-H, Chen F-H, Lin G. Monitoring Early Glycolytic Flux Alterations Following Radiotherapy in Cancer and Immune Cells: Hyperpolarized Carbon-13 Magnetic Resonance Imaging Study. Metabolites. 2021; 11(8):518. https://doi.org/10.3390/metabo11080518
Chicago/Turabian StyleLai, Ying-Chieh, Ching-Yi Hsieh, Kuan-Ying Lu, Cheng-Hsuan Sung, Hung-Yao Ho, Mei-Ling Cheng, Albert P. Chen, Shu-Hang Ng, Fang-Hsin Chen, and Gigin Lin. 2021. "Monitoring Early Glycolytic Flux Alterations Following Radiotherapy in Cancer and Immune Cells: Hyperpolarized Carbon-13 Magnetic Resonance Imaging Study" Metabolites 11, no. 8: 518. https://doi.org/10.3390/metabo11080518
APA StyleLai, Y.-C., Hsieh, C.-Y., Lu, K.-Y., Sung, C.-H., Ho, H.-Y., Cheng, M.-L., Chen, A. P., Ng, S.-H., Chen, F.-H., & Lin, G. (2021). Monitoring Early Glycolytic Flux Alterations Following Radiotherapy in Cancer and Immune Cells: Hyperpolarized Carbon-13 Magnetic Resonance Imaging Study. Metabolites, 11(8), 518. https://doi.org/10.3390/metabo11080518









