Serum Retinal and Retinoic Acid Predict the Development of Type 2 Diabetes Mellitus in Korean Subjects with Impaired Fasting Glucose from the KCPS-II Cohort
Abstract
:1. Introduction
2. Results
2.1. Clinical and Biochemical Characteristics at Baseline
2.2. Method Validation of UHPLC-QE Orbitrap Plus MS Analysis
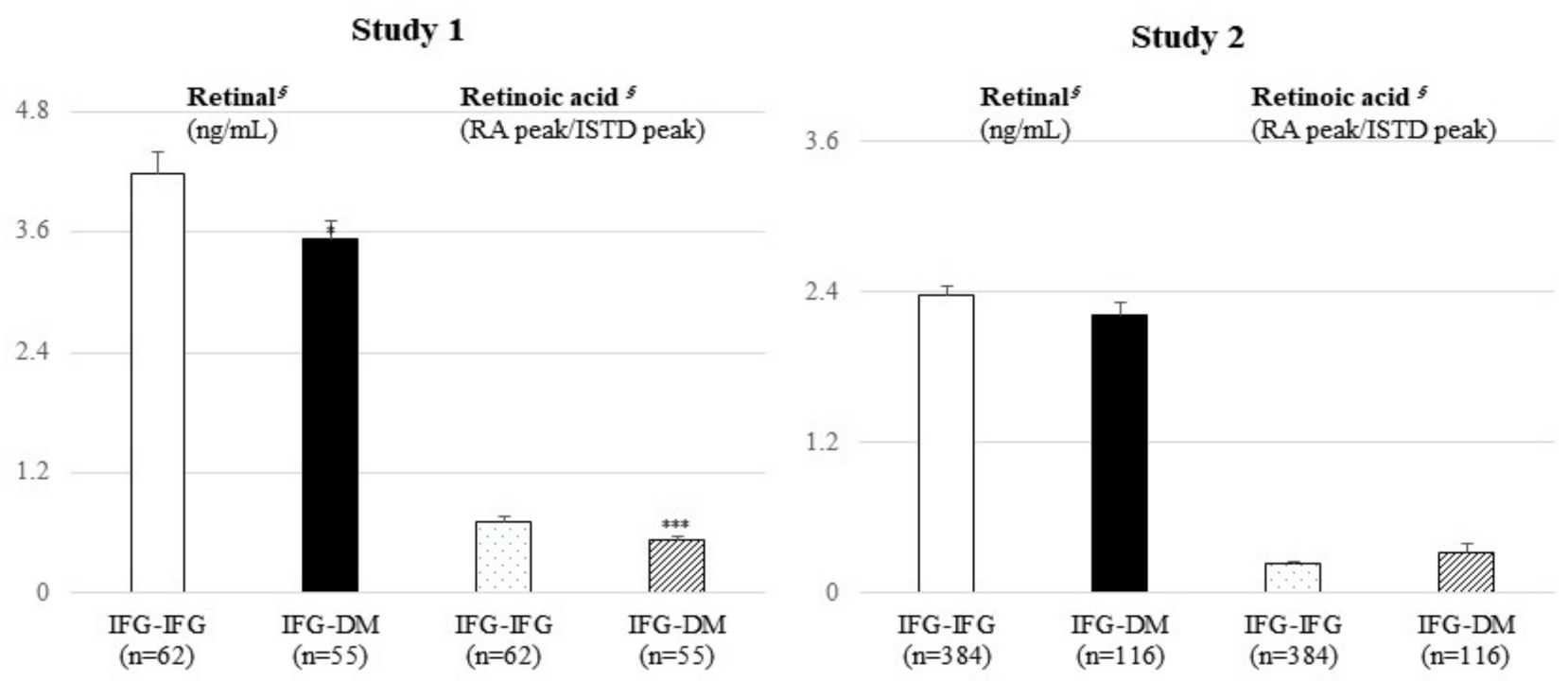
2.3. Serum Retinal and Retinoic Acid Analysis Using UHPLC-QE Orbitrap Plus MS
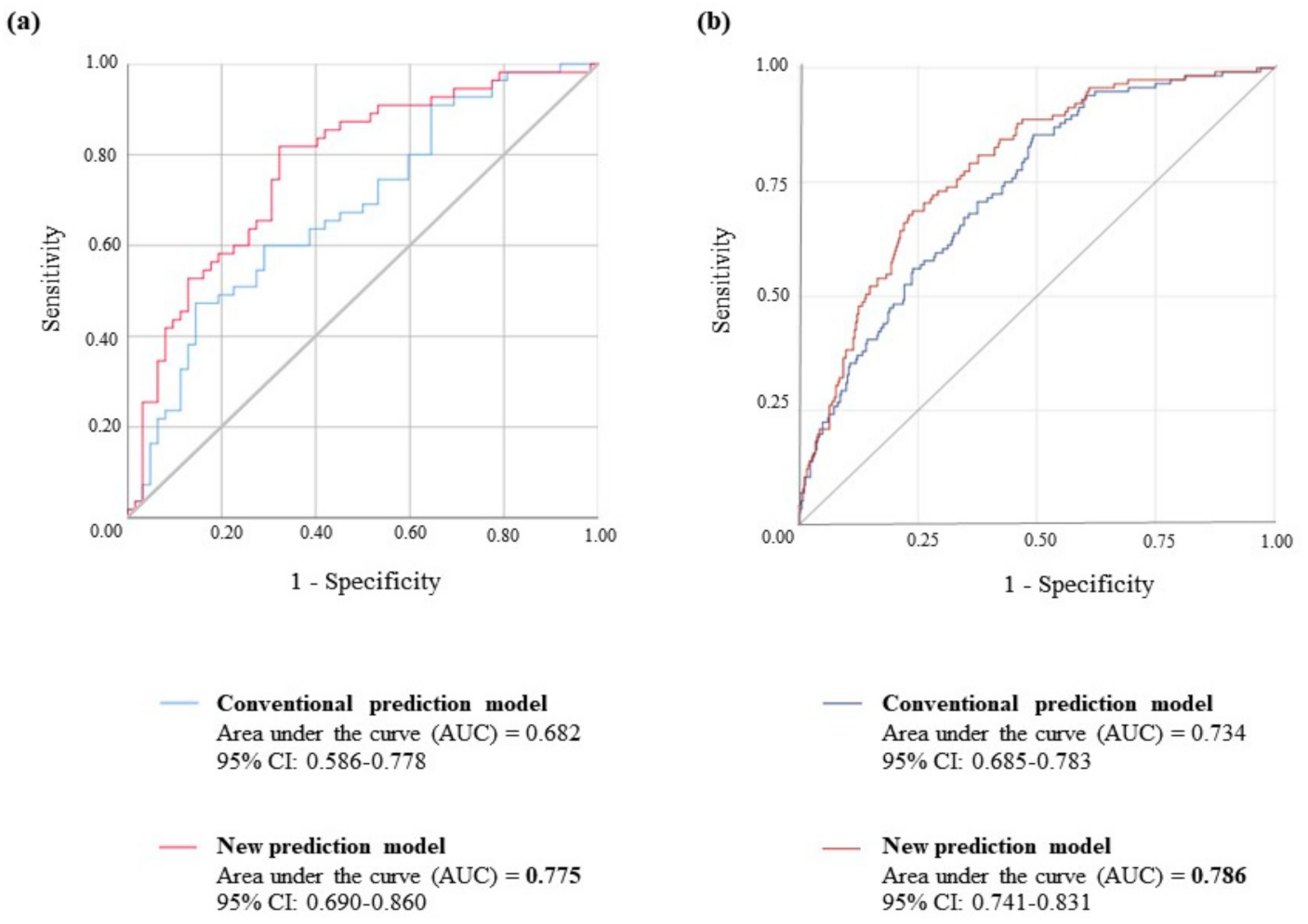
2.4. Logistic Regression Analysis
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Sample Collection and Clinical and Biochemical Assessments
4.3. Targeted Metabolic Profiling with UHPLC-QE Orbitrap Plus MS Using Serum Samples
4.3.1. Preparation of Blood Samples
4.3.2. Preparation of Stock Solutions and Standard Samples
4.3.3. UHPLC-QE Orbitrap Plus MS Analysis
4.3.4. UHPLC-QE Orbitrap Plus MS Method Validation
5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walford, G.A.; Porneala, B.C.; Dauriz, M.; Vassy, J.L.; Cheng, S.; Rhee, E.P.; Wang, T.; Meigs, J.B.; Gerszten, R.E.; Florez, J.C. Metabolite Traits and Genetic Risk Provide Complementary Information for the Prediction of Future Type 2 Diabetes. Diabetes Care 2014, 37, 2508–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Brunius, C.; Lehtonen, M.; Auriola, S.; Bergdahl, I.A.; Rolandsson, O.; Hanhineva, K.; Landberg, R. Plasma metabolites associated with type 2 diabetes in a Swedish population: A case–control study nested in a prospective cohort. Diabetologia 2018, 61, 849–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Jee, S.; Yoo, H.; Kang, M.; Kim, J.; Lee, J. Serum vitamin A-related metabolite levels are associated with incidence of type 2 diabetes. Diabetes Metab. 2017, 43, 287–291. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [Green Version]
- Libien, J.; Kupersmith, M.; Blaner, W.; McDermott, M.; Gao, S.; Liu, Y.; Corbett, J.; Wall, M. Role of vitamin A metabolism in IIH: Results from the idiopathic intracranial hypertension treatment trial. J. Neurol. Sci. 2017, 372, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, H.; Wang, J.; Zhou, W.; Sun, R.; Xia, M. Association of serum retinoic acid with hepatic steatosis and liver injury in nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2015, 102, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, H.; Mu, D.; Fan, J.; Song, J.; Zhong, Y.; Li, D.; Xia, M. Circulating Retinoic Acid Levels and the Development of Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.-J.; Plutzky, J. Retinoid Metabolism and Diabetes Mellitus. Diabetes Metab. J. 2012, 36, 167–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, H.; Mu, D.; Li, D.; Zhong, Y.; Jiang, N.; Xia, M. Association of Serum Retinoic Acid with Risk of Mortality in Patients with Coronary Artery Disease. Circ. Res. 2016, 119, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; He, J.C.; Tharaux, P.-L. Nuclear receptors in podocyte biology and glomerular disease. Nat. Rev. Nephrol. 2021, 17, 185–204. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ogawa, K.; Ikoma, Y.; Yano, M. High serum carotenoids associated with lower risk for the metabolic syndrome and its components among Japanese subjects: Mikkabi cohort study. Br. J. Nutr. 2015, 114, 1674–1682. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shi, W.-Q.; Cao, Y.; He, L.-P.; Guan, K.; Ling, W.H.; Chen, Y.-M. Higher serum carotenoid concentrations associated with a lower prevalence of the metabolic syndrome in middle-aged and elderly Chinese adults. Br. J. Nutr. 2014, 112, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- Erikstrup, C.; Mortensen, O.H.; Nielsen, A.R.; Fischer, C.; Plomgaard, P.; Petersen, A.M.; Krogh-Madsen, R.; Lindegaard, B.; Erhardt, J.G.; Ullum, H.; et al. RBP-to-retinol ratio, but not total RBP, is elevated in patients with type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Coppack, S.W. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grapov, D.; Adams, S.H.; Pedersen, T.L.; Garvey, W.T.; Newman, J.W. Type 2 Diabetes Associated Changes in the Plasma Non-Esterified Fatty Acids, Oxylipins and Endocannabinoids. PLoS ONE 2012, 7, e48852. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, G.R. Inflammation in Obesity is a Common Link Between Defects in Fatty Acid Metabolism and Insulin Resistance. Cell Cycle 2007, 6, 888–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, R.N.; Ader, M. Free Fatty Acids and Pathogenesis of Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2000, 11, 351–356. [Google Scholar] [CrossRef]
- Zhang, L.; Keung, W.; Samokhvalov, V.; Wang, W.; Lopaschuk, G.D. Role of fatty acid uptake and fatty acid β-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2010, 1801, 1–22. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Li, R.; Chen, G. Transcriptional Factors Mediating Retinoic Acid Signals in the Control of Energy Metabolism. Int. J. Mol. Sci. 2015, 16, 14210–14244. [Google Scholar] [CrossRef] [Green Version]
- Starkey, J.M.; Zhao, Y.; Sadygov, R.G.; Haidacher, S.J.; Lejeune, W.S.; Dey, N.; Luxon, B.A.; Kane, M.A.; Napoli, J.L.; Denner, L.; et al. Altered Retinoic Acid Metabolism in Diabetic Mouse Kidney Identified by 18O Isotopic Labeling and 2D Mass Spectrometry. PLoS ONE 2010, 5, e11095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Zhang, R.; Li, Y.; Zhu, B.; Chen, W.; Zhang, Y.; Chen, G. A RARE of hepatic Gck promoter interacts with RARα, HNF4α and COUP-TFII that affect retinoic acid- and insulin-induced Gck expression. J. Nutr. Biochem. 2014, 25, 964–976. [Google Scholar] [CrossRef]
- Roder, K.; Zhang, L.; Schweizer, M. SREBP-1c mediates the retinoid-dependent increase in fatty acid synthase promoter activity in HepG2. FEBS Lett. 2007, 581, 2715–2720. [Google Scholar] [CrossRef] [Green Version]
- Agius, L. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem. J. 2008, 414, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.; Espenshade, P.J. Expanding Roles for SREBP in Metabolism. Cell Metab. 2012, 16, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, W.; Li, Y.; Zhang, Y.; Chen, G. Retinoids synergized with insulin to induce Srebp-1c expression and activated its promoter via the two liver X receptor binding sites that mediate insulin action. Biochem. Biophys. Res. Commun. 2011, 406, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.; Lu, D.; Li, N.-Q.; Ross, A.C. Retinoids synergize with insulin to induce hepatic Gck expression. Biochem. J. 2009, 419, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Cinti, F.; Bouchi, R.; Kim-Muller, J.Y.; Ohmura, Y.; Sandoval, P.R.; Masini, M.; Marselli, L.; Suleiman, M.; Ratner, L.E.; Marchetti, P.; et al. Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 1044–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorberbaum, D.S.; Kishore, S.; Rosselot, C.; Sarbaugh, D.; Brooks, E.P.; Aragon, E.; Xuan, S.; Simon, O.; Ghosh, D.; Mendelsohn, C.; et al. Retinoic acid signaling within pancreatic endocrine progenitors regulates mouse and human β cell specification. Development 2020, 147, 189977. [Google Scholar] [CrossRef]
- Chien, C.-Y.; Yuan, T.-A.; Cho, C.H.-H.; Chang, F.-P.; Mao, W.-Y.; Wu, R.-R.; Lee, H.-S.; Shen, C.-N. All-trans retinoic acid ameliorates glycemic control in diabetic mice via modulating pancreatic islet production of vascular endothelial growth factor-A. Biochem. Biophys. Res. Commun. 2016, 477, 874–880. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhou, J.; Zhang, Y.; Tang, J.; Sun, B.; Xu, W.; Wang, X.; Chen, Y.; Sun, Z. Changes in Intestinal Microbiota Are Associated with Islet Function in a Mouse Model of Dietary Vitamin A Deficiency. J. Diabetes Res. 2020, 2020, 2354108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chertow, B.S.; Blaner, W.S.; Baranetsky, N.G.; Sivitz, W.; Cordle, M.B.; Thompson, D.; Meda, P. Effects of vitamin A deficiency and repletion on rat insulin secretion in vivo and in vitro from isolated islets. J. Clin. Investig. 1987, 79, 163–169. [Google Scholar] [CrossRef]
- Olsen, T.; Blomhoff, R. Retinol, Retinoic Acid, and Retinol-Binding Protein 4 are Differentially Associated with Cardiovascular Disease, Type 2 Diabetes, and Obesity: An Overview of Human Studies. Adv. Nutr. 2019, 11, 644–666. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H. PPAR agonists for the treatment of cardiovascular disease in patients with diabetes. Diabetes Obes. Metab. 2012, 14, 973–982. [Google Scholar] [CrossRef]
- Trasino, S.E.; Tang, X.-H.; Jessurun, J.; Gudas, L.J. Retinoic acid receptor β2 agonists restore glycaemic control in diabetes and reduce steatosis. Diabetes Obes. Metab. 2015, 18, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Jee, S.H.; Kim, M.; Kim, M.; Kang, M.; Seo, Y.W.; Jung, K.J.; Lee, S.J.; Hong, S.; Lee, J.H. Clinical relevance of glycerophospholipid, sphingomyelin and glutathione metabolism in the pathogenesis of pharyngolaryngeal cancer in smokers: The Korean Cancer Prevention Study-II. Metabolomics 2016, 12, 164. [Google Scholar] [CrossRef]
- Jee, S.H.; Batty, G.; Jang, Y.; Oh, D.J.; Oh, B.-H.; Lee, S.H.; Park, S.-W.; Seung, K.-B.; Kimm, H.; Kim, S.Y.; et al. The Korean Heart Study: Rationale, objectives, protocol, and preliminary results for a new prospective cohort study of 430,920 men and women. Eur. J. Prev. Cardiol. 2014, 21, 1484–1492. [Google Scholar] [CrossRef] [PubMed]


| Study 1 | Study 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 117) | p | Total (n = 500) | p | |||||||
| IFG-IFG (n = 62) | IFG-DM (n = 55) | IFG-IFG (n = 384) | IFG-DM (n = 116) | |||||||
| Age (year) | 48.2 | ±1.41 | 49.3 | ±1.23 | 0.564 | 48.1 | ±0.53 | 52.9 | ±0.91 | <0.001 |
| Male/female, n (%) | 29 (46.8)/33 (53.2) | 26 (47.3)/29 (52.7) | 0.957 | 196 (51.0)/188 (49.0) | 54 (46.6)/62 (53.5) | 0.458 | ||||
| BMI (kg/m2) | 25.3 | ±0.43 | 26.1 | ±0.43 | 0.197 | 24.8 | ±0.16 | 26.1 | ±0.30 | <0.001 |
| Waist circumference (cm) | 84.4 | ±1.17 | 86.4 | ±1.01 | 0.188 | 83.3 | ±0.45 | 87.1 | ±0.85 | <0.001 |
| Systolic blood pressure (mmHg) | 124.8 | ±1.38 | 124.4 | ±1.82 | 0.852 | 127.6 | ±0.79 | 129.1 | ±1.42 | 0.347 |
| Diastolic blood pressure (mmHg) | 78.2 | ±1.20 | 77.8 | ±0.87 | 0.757 | 79.3 | ±0.63 | 77.1 | ±1.13 | 0.105 |
| Glucose (mg/dL) | 109.0 | ±0.89 | 113.0 | ±1.13 | 0.006 ∮ | 107.0 | ±0.39 | 112.8 | ±0.62 | <0.001 |
| Triglyceride (mg/dL) | 140.6 | ±9.95 | 149.9 | ±10.5 | 0.465 ∮ | 149.3 | ±5.39 | 181.2 | ±11.1 | 0.006 |
| Total cholesterol (mg/dL) | 191.9 | ±3.85 | 191.7 | ±4.34 | 0.897 ∮ | 194.2 | ±1.76 | 201.9 | ±3.37 | 0.037 |
| HDL-cholesterol (mg/dL) | 50.2 | ±1.08 | 50.6 | ±1.32 | 0.932 ∮ | 52.4 | ±0.62 | 50.2 | ±1.16 | 0.094 |
| LDL-cholesterol (mg/dL) | 117.7 | ±3.33 | 112.1 | ±4.05 | 0.202 ∮ | 118.2 | ±1.64 | 122.3 | ±3.14 | 0.235 |
| AST (IU/L) | 22.9 | ±1.29 | 23.9 | ±1.02 | 0.204 ∮ | 23.1 | ±0.49 | 28.1 | ±1.40 | <0.001 ∮ |
| ALT (IU/L) | 25.2 | ±2.02 | 28.8 | ±1.63 | 0.020 ∮ | 25.8 | ±0.97 | 37.7 | ±3.57 | <0.001 † |
| GGT (IU/L) | 30.0 | ±2.34 | 36.6 | ±2.54 | 0.016 ∮ | 39.1 | ±1.99 | 47.4 | ±4.94 | 0.008 † |
| Study 1 | Nonobese (n = 58) | Obese (n = 59) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IFG-IFG (n = 35) | IFG-DM (n = 23) | p | IFG-IFG (n = 27) | IFG-DM (n = 32) | p | |||||
| Age (year) | 47.1 | ±2.10 | 50.5 | ±1.95 | 0.268 | 49.6 | ±1.75 | 48.4 | ±1.59 | 0.921 † |
| Male/female, n (%) | 20 (57.1)/15 (42.9) | 13 (56.5)/10 (43.5) | 0.963 | 9 (33.3)/18 (66.7) | 13 (40.6)/19 (59.4) | 0.564 | ||||
| BMI (kg/m2) | 23.1 | ±0.21 | 23.4 | ±0.26 | 0.328 † | 28.1 | ±0.61 | 28.0 | ±0.47 | 0.563 † |
| Waist circumference (cm) | 80.7 | ±1.08 | 80.7 | ±1.22 | 0.974 | 89.2 | ±1.92 | 90.3 | ±0.92 | 0.607 |
| Systolic blood pressure (mmHg) | 125.3 | ±2.06 | 125.5 | ±2.77 | 0.951 | 124.2 | ±1.75 | 123.6 | ±2.44 | 0.850 |
| Diastolic blood pressure (mmHg) | 79.3 | ±1.83 | 77.1 | ±1.52 | 0.404 | 76.9 | ±1.41 | 48.3 | ±1.04 | 0.429 † |
| Glucose (mg/dL) | 107.3 | ±1.08 | 112.1 | ±1.80 | 0.020 ∮ | 111.0 | ±1.41 | 113.6 | ±1.47 | 0.219 |
| Triglyceride (mg/dL) | 129.7 | ±10.8 | 137.8 | ±16.5 | 0.859 ∮ | 154.9 | ±18.0 | 158.6 | ±13.7 | 0.586 ∮ |
| Total cholesterol (mg/dL) | 189.9 | ±4.59 | 187.3 | ±8.13 | 0.762 | 194.5 | ±6.61 | 194.8 | ±4.68 | 0.837 † |
| HDL-cholesterol (mg/dL) | 51.5 | ±1.26 | 51.2 | ±2.20 | 0.903 | 48.6 | ±1.84 | 50.3 | ±1.66 | 0.501 |
| LDL-cholesterol (mg/dL) | 116.2 | ±3.94 | 108.6 | ±6.64 | 0.302 | 119.7 | ±5.76 | 114.6 | ±5.12 | 0.461 † |
| AST (IU/L) | 21.3 | ±1.31 | 21.7 | ±1.39 | 0.799 † | 24.9 | ±2.39 | 25.5 | ±1.40 | 0.156 † |
| ALT (IU/L) | 23.5 | ±2.58 | 24.9 | ±2.14 | 0.106 † | 27.4 | ±3.22 | 31.6 | ±2.23 | 0.081 ∮ |
| GGT (U/L) | 29.1 | ±3.29 | 28.2 | ±2.30 | 0.553 ∮ | 31.1 | ±3.31 | 42.7 | ±3.71 | 0.017 ∮ |
| Study 2 | Nonobese (n= 263) | Obese (n= 237) | ||||||||
| IFG-IFG (n = 218) | IFG-DM (n= 45) | p | IFG-IFG (n= 166) | IFG-DM (n= 71) | p | |||||
| Age (year) | 47.7 | ±0.72 | 54.0 | ±1.50 | <0.001 | 48.8 | ±0.80 | 52.2 | ±1.15 | 0.017 |
| Male/female, n (%) | 100 (45.9)/118 (54.1) | 17 (37.8)/28 (62.2) | 0.407 | 96 (57.8)/70 (42.2) | 37 (52.1)/34 (47.9) | 0.416 | ||||
| BMI (kg/m2) | 22.7 | ±0.12 | 22.9 | ±0.22 | 0.689 | 27.5 | ±0.17 | 28.2 | ±0.24 | 0.028 |
| Waist circumference (cm) | 78.5 | ±0.50 | 79.2 | ±1.05 | 0.555 | 89.6 | ±0.54 | 92.0 | ±0.78 | 0.015 |
| Systolic blood pressure (mmHg) | 124.7 | ±1.06 | 124.3 | ±2.27 | 0.877 | 131.4 | ±1.11 | 132.1 | ±1.75 | 0.728 |
| Diastolic blood pressure (mmHg) | 78.5 | ±0.84 | 77.3 | ±1.89 | 0.540 | 80.2 | ±0.94 | 77.1 | ±1.42 | 0.065 |
| Glucose (mg/dL) | 106.3 | ±0.53 | 112.4 | ±0.99 | <0.001 | 107.9 | ±0.57 | 113.0 | ±0.79 | <0.001 |
| Triglyceride (mg/dL) | 130.2 | ±5.08 | 157.0 | ±13.2 | 0.035 | 174.5 | ±10.2 | 196.5 | ±15.9 | 0.244 |
| Total cholesterol (mg/dL) | 192.5 | ±2.37 | 198.6 | ±4.80 | 0.281 | 196.5 | ±2.62 | 204.1 | ±4.59 | 0.133 |
| HDL-cholesterol (mg/dL) | 54.4 | ±0.88 | 52.3 | ±1.93 | 0.331 | 49.8 | ±0.81 | 48.9 | ±1.44 | 0.563 |
| LDL-cholesterol (mg/dL) | 118.2 | ±2.07 | 120.3 | ±4.81 | 0.666 | 118.3 | ±2.65 | 123.6 | ±4.14 | 0.280 |
| AST (IU/L) | 21.8 | ±0.62 | 23.5 | ±1.34 | 0.269 | 24.7 | ±0.76 | 31.1 | ±2.05 | <0.001 † |
| ALT (IU/L) | 22.9 | ±1.17 | 24.7 | ±2.40 | 0.539 | 29.5 | ±1.60 | 45.9 | ±5.43 | <0.001 ∮ |
| GGT (U/L) | 32.6 | ±2.36 | 43.9 | ±11.01 | 0.126 ∮ | 47.6 | ±3.28 | 49.6 | ±4.13 | 0.731 |
| Retinal | Retinoic Acid | |||
|---|---|---|---|---|
| R2 of calibration curves (linearity) | 0.9956 | 0.9959 | ||
| LOQ (ng/mL) | 1.63 | 24.5 | ||
| Precision assessments | ||||
| Intra-assay variation (%RSD) | 0.0268 | 0.0497 | ||
| Inter-assay variation (%RSD) | 0.0304 | 0.0645 | ||
| Accuracy assessments | ||||
| Recovery (%) | SD (%) | Recovery (%) | SD (%) | |
| Recovery at low concentration | 93.5 | 3.83 | 48.2 | 6.50 |
| Recovery at high concentration | 90.8 | 7.63 | 93.1 | 3.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Yang, Y.; Kim, M.; Jee, S.H.; Yoo, H.J.; Lee, J.H. Serum Retinal and Retinoic Acid Predict the Development of Type 2 Diabetes Mellitus in Korean Subjects with Impaired Fasting Glucose from the KCPS-II Cohort. Metabolites 2021, 11, 510. https://doi.org/10.3390/metabo11080510
Han Y, Yang Y, Kim M, Jee SH, Yoo HJ, Lee JH. Serum Retinal and Retinoic Acid Predict the Development of Type 2 Diabetes Mellitus in Korean Subjects with Impaired Fasting Glucose from the KCPS-II Cohort. Metabolites. 2021; 11(8):510. https://doi.org/10.3390/metabo11080510
Chicago/Turabian StyleHan, Youngmin, Yeunsoo Yang, Minjoo Kim, Sun Ha Jee, Hye Jin Yoo, and Jong Ho Lee. 2021. "Serum Retinal and Retinoic Acid Predict the Development of Type 2 Diabetes Mellitus in Korean Subjects with Impaired Fasting Glucose from the KCPS-II Cohort" Metabolites 11, no. 8: 510. https://doi.org/10.3390/metabo11080510
APA StyleHan, Y., Yang, Y., Kim, M., Jee, S. H., Yoo, H. J., & Lee, J. H. (2021). Serum Retinal and Retinoic Acid Predict the Development of Type 2 Diabetes Mellitus in Korean Subjects with Impaired Fasting Glucose from the KCPS-II Cohort. Metabolites, 11(8), 510. https://doi.org/10.3390/metabo11080510








