Effect of Korean Red Ginseng on Plasma Ceramide Levels in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Randomized Trial Design
4.2. Study Outcomes
4.3. Covariates and Definition of Metabolic Syndrome
4.4. Reagents
4.5. Lipid Extraction
4.6. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, M.; Mulvagh, S.L.; Merz, C.N.; Buring, J.E.; Manson, J.E. Cardiovascular disease in women: Clinical perspectives. Circ. Res. 2016, 118, 1273–1293. [Google Scholar] [CrossRef] [PubMed]
- Boo, S.; Froelicher, E.S. Cardiovascular risk factors and 10-year risk for coronary heart disease in Korean women. Asian Nurs. Res. 2012, 6, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A. Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the American heart association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef]
- Mielke, M.M.; Bandaru, V.V.; Han, D.; An, Y.; Resnick, S.M.; Ferrucci, L.; Haughey, N.J. Demographic and clinical variables affecting mid- to late-life trajectories of plasma ceramide and dihydroceramide species. Aging Cell 2015, 14, 1014–1023. [Google Scholar] [CrossRef]
- Vozella, V.; Basit, A.; Piras, F.; Realini, N.; Armirotti, A.; Bossù, P.; Assogna, F.; Sensi, S.L.; Spalletta, G.; Piomelli, D. Elevated plasma ceramide levels in post-menopausal women: A cross-sectional study. Aging 2019, 11, 73–88. [Google Scholar] [CrossRef]
- Bismuth, J.; Lin, P.; Yao, Q.; Chen, C. Ceramide: A common pathway for atherosclerosis? Atherosclerosis 2008, 196, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond ldl-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, E.; Blachnio-Zabielska, A. The role of ceramides in insulin resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Haus, J.M.; Kashyap, S.R.; Kasumov, T.; Zhang, R.; Kelly, K.R.; Defronzo, R.A.; Kirwan, J.P. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 2009, 58, 337–343. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef]
- Fernandez, C.; Sandin, M.; Sampaio, J.L.; Almgren, P.; Narkiewicz, K.; Hoffmann, M.; Hedner, T.; Wahlstrand, B.; Simons, K.; Shevchenko, A.; et al. Plasma lipid composition and risk of developing cardiovascular disease. PLoS ONE 2013, 8, e71846. [Google Scholar] [CrossRef]
- Walker, M.; Xanthakis, V.; Ma, J.; Quatromoni, P.A.; Moore, L.; Ramachandran, V.; Jacques, P. A mediterranean style diet is favorably associated with concentrations of circulating ceramides and ceramide ratios in the framingham offspring cohort (P18-048-19). Curr. Dev. Nutr. 2019, 3, nzz039. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma ceramides, mediterranean diet, and incident cardiovascular disease in the predimed trial (prevención con dieta mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Meikle, P.J.; Pedersen, E.R.; Tell, G.S.; Dhar, I.; Brenner, H.; Schöttker, B.; Lääperi, M.; Kauhanen, D.; Koistinen, K.M.; et al. Development and validation of a ceramide- and phospholipid-based cardiovascular risk estimation score for coronary artery disease patients. Eur. Heart J. 2020, 41, 371–380. [Google Scholar] [CrossRef]
- Mantovani, A.; Dugo, C. Ceramides and risk of major adverse cardiovascular events: A meta-analysis of longitudinal studies. J. Clin. Lipidol. 2020, 14, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, X.; Xing, S.; Bian, F.; Yao, W.; Bai, X.; Zheng, T.; Wu, G.; Jin, S. Endogenous ceramide contributes to the transcytosis of oxldl across endothelial cells and promotes its subendothelial retention in vascular wall. Oxid. Med. Cell. Longev. 2014, 2014, 823071. [Google Scholar] [CrossRef]
- Schissel, S.L.; Tweedie-Hardman, J.; Rapp, J.H.; Graham, G.; Williams, K.J.; Tabas, I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Investig. 1996, 98, 1455–1464. [Google Scholar] [PubMed]
- Funai, K.; Summers, S.A.; Rutter, J. Reign in the membrane: How common lipids govern mitochondrial function. Curr. Opin. Cell Biol. 2020, 63, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Pahan, K.; Khan, M.; Singh, A.K. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J. Biol. Chem. 1998, 273, 20354–20362. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, C.; Colell, A.; Marí, M.; Morales, A.; Fernández-Checa, J.C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J. Biol. Chem. 1997, 272, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Warshauer, J.T.; Lopez, X.; Gordillo, R.; Hicks, J.; Holland, W.L.; Anuwe, E.; Blankfard, M.B.; Scherer, P.E.; Lingvay, I. Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab. Res. Rev. 2015, 31, 734–744. [Google Scholar] [CrossRef]
- Chavez, J.A.; Siddique, M.M.; Wang, S.T.; Ching, J.; Shayman, J.A.; Summers, S.A. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J. Biol. Chem. 2014, 289, 723–734. [Google Scholar] [CrossRef]
- Powell, D.J.; Hajduch, E.; Kular, G.; Hundal, H.S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase b (pkb)/akt by a pkczeta-dependent mechanism. Mol. Cell. Biol. 2003, 23, 7794–7808. [Google Scholar] [CrossRef]
- Croyal, M.; Kaabia, Z.; León, L.; Ramin-Mangata, S.; Baty, T.; Fall, F.; Billon-Crossouard, S.; Aguesse, A.; Hollstein, T.; Sullivan, D.R.; et al. Fenofibrate decreases plasma ceramide in type 2 diabetes patients: A novel marker of cvd? Diabetes Metab. 2018, 44, 143–149. [Google Scholar] [CrossRef]
- Tarasov, K.; Ekroos, K.; Suoniemi, M.; Kauhanen, D.; Sylvänne, T.; Hurme, R.; Gouni-Berthold, I.; Berthold, H.K.; Kleber, M.E.; Laaksonen, R.; et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and pcsk9 deficiency. J. Clin. Endocrinol. Metab. 2014, 99, E45–E52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, H.; Tian, Z.; Wang, X.; Xu, L.; Li, K.; Gao, X.; Fan, D.; Ma, X.; Ling, W.; et al. Dose-dependent reductions in plasma ceramides after anthocyanin supplementation are associated with improvements in plasma lipids and cholesterol efflux capacity in dyslipidemia: A randomized controlled trial. Clin. Nutr. 2021, 40, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.W.; Ooi, E.M.; Watts, G.F.; Chan, D.C.; Meikle, P.J.; Barrett, P.H. Association of plasma ceramides and sphingomyelin with vldl apob-100 fractional catabolic rate before and after rosuvastatin treatment. J. Clin. Endocrinol. Metab. 2015, 100, 2497–2501. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.A.; Mehak, F.; Khan, Z.M.; Ahmad, W.; Khan, M.R.; Zia, S.; Rahaman, A.; Aadil, R.M. Interplay between ceramides and phytonutrients: New insights in metabolic syndrome. Trends Food Sci. Technol. 2021. [CrossRef]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.K.; Han, C.K. Physiological and pharmacological features of the non-saponin components in Korean red ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Park, S.K.; Hyun, S.H.; In, G.; Park, C.K.; Kwak, Y.S.; Jang, Y.J.; Kim, B.; Kim, J.H.; Han, C.K. The antioxidant activities of Korean red ginseng (panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J. Ginseng Res. 2021, 45, 41–47. [Google Scholar] [CrossRef]
- Li, P.L.; Zhang, Y. Cross talk between ceramide and redox signaling: Implications for endothelial dysfunction and renal disease. Handb. Exp. Pharmacol. 2013, 171–197. [Google Scholar] [CrossRef]
- Zhou, W.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Ginsenoside rb1 blocks homocysteine-induced endothelial dysfunction in porcine coronary arteries. J. Vasc. Surg. 2005, 41, 861–868. [Google Scholar] [CrossRef]
- Xie, J.T.; Shao, Z.H.; Vanden Hoek, T.L.; Chang, W.T.; Li, J.; Mehendale, S.; Wang, C.Z.; Hsu, C.W.; Becker, L.B.; Yin, J.J.; et al. Antioxidant effects of ginsenoside re in cardiomyocytes. Eur. J. Pharmacol. 2006, 532, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. Review of ginseng anti-diabetic studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Lv, W.; Yang, Y.; Gao, H.; Yang, J.; Shen, Y.; Ning, G. Ginsenoside re reduces insulin resistance through inhibition of c-jun nh2-terminal kinase and nuclear factor-kappab. Mol. Endocrinol. 2008, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gao, J.; Wei, F.; Zhao, J.; Wang, D.; Wei, J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front. Pharm. 2018, 9, 423. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Jang, S.N.; Liu, K.H.; Jung, D.H. Effect of Korean red ginseng on cholesterol metabolites in postmenopausal women with hypercholesterolemia: A pilot randomized controlled trial. Nutrients 2020, 12, 3423. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American heart association/national heart, lung, and blood institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Chen, S.; Hoene, M.; Li, J.; Li, Y.; Zhao, X.; Häring, H.U.; Schleicher, E.D.; Weigert, C.; Xu, G.; Lehmann, R. Simultaneous extraction of metabolome and lipidome with methyl tert-butyl ether from a single small tissue sample for ultra-high performance liquid chromatography/mass spectrometry. J. Chromatogr. A 2013, 1298, 9–16. [Google Scholar] [CrossRef]
- Im, S.S.; Park, H.Y.; Shon, J.C.; Chung, I.S.; Cho, H.C.; Liu, K.H.; Song, D.K. Plasma sphingomyelins increase in pre-diabetic Korean men with abdominal obesity. PLoS ONE 2019, 14, e0213285. [Google Scholar] [CrossRef] [PubMed]
- Buré, C.; Ayciriex, S.; Testet, E.; Schmitter, J.M. A single run lc-ms/ms method for phospholipidomics. Anal. Bioanal. Chem. 2013, 405, 203–213. [Google Scholar] [CrossRef] [PubMed]
| Ginseng (n = 36) | Placebo (n = 32) | p-Value 1 | |
|---|---|---|---|
| Age, years | 55.9 ± 5.9 | 58.1 ± 4.7 | 0.093 |
| Body mass index (kg/m2) | 24.3 ± 3.2 | 24.5 ± 3.7 | 0.741 |
| Waist circumference (cm) | 82.5 ± 8.7 | 82.6 ± 10.2 | 0.950 |
| SBP (mmHg) | 119.8 ± 13.5 | 116.8 ± 16.5 | 0.409 |
| DBP (mmHg) | 76.7 ± 9.7 | 72.2 ± 9.3 | 0.065 |
| Fasting glucose (mg/dL) | 108.4 ± 18.5 | 103.0 ± 10.2 | 0.145 |
| Triglycerides (mg/dL) | 124.9 ± 60.4 | 147.7 ± 82.5 | 0.197 |
| HDL cholesterol (mg/dL) | 64.5 ± 14.2 | 58.6 ± 14.7 | 0.098 |
| WBC (103 L) | 5.7 ± 1.4 | 5.8 ± 1.6 | 0.786 |
| Hypertension | 5 (13.9) | 5 (15.6) | 0.572 |
| Diabetes | 2 (5.6) | 1 (3.1) | 0.535 |
| Physical activity, n (%) | 15 (41.7) | 10 (31.3) | 0.374 |
| Smoking, n (%) | 2 (5.6) | 1 (3.1) | 0.534 |
| Alcohol consumption, n (%) | 9 (25.0) | 10 (13.2) | 0.567 |
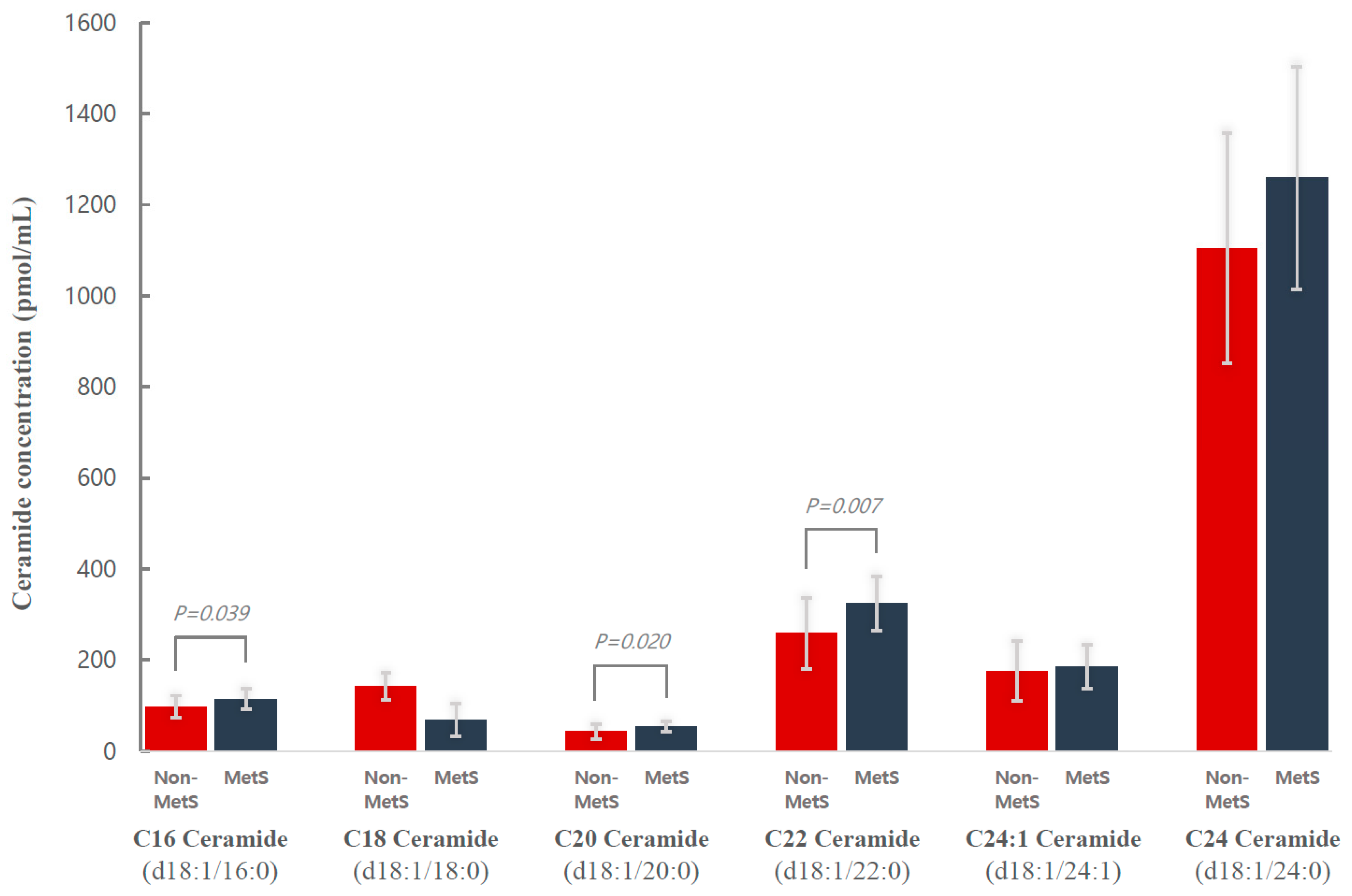
| Ceramides (pmol/mL) | |||
| C16 ceramide (d18:1/16:0) | 105.9 ± 24.5 | 154.5 ± 50.9 | <0.001 |
| C18 ceramide (d18:1/18:0) | 105.6 ± 223.5 | 64.3 ± 25.1 | 0.303 |
| C20 ceramide (d18:1/20:0) | 48.2 ± 15.2 | 63.2 ± 22.3 | 0.002 |
| C22 ceramide (d18:1/22:0) | 291.8 ± 76.0 | 510.7 ± 204.9 | <0.001 |
| C24 ceramide (d18:1/24:0) | 1181.6 ± 257.3 | 1594.5 ± 580.9 | <0.001 |
| C24:1 ceramide (d18:1/24:1) | 180.8 ± 57.0 | 658.8 ± 225.6 | <0.001 |
| Lipids | Before | After | p-Value 1 |
|---|---|---|---|
| Neutral lipids | |||
| Monoacylglycerol (nmol/mL) | 50.7 ± 17.6 | 50.4 ± 12.0 | 0.931 |
| Diacylglycerol (nmol/mL) | 4.1 ± 1.5 | 4.1 ± 1.6 | 0.816 |
| Triacylglycerol (nmol/mL) | 42,250.9 ± 19,080.588 | 43,081.1 ± 19,820.933 | 0.766 |
| Acylcarnitine (nmol/mL) | 17.4 ± 9.1 | 18.1 ± 5.6 | 0.648 |
| Phospholipids | |||
| Phosphatidylcholine (μmol/mL) | 2033.8 ± 435.8 | 2124.3 ± 634.6 | 0.309 |
| Plasmenyl phosphatidylcholine (μmol/mL) | 93.2 ± 39.8 | 95.4 ± 25.6 | 0.730 |
| Lysophosphatidylcholine (nmol/mL) | 109.0 ± 25.4 | 116.8 ± 23.0 | 0.088 |
| Phosphatidylethanolamine (nmol/mL) | 29.8 ± 11.1 | 29.6 ± 11.7 | 0.917 |
| Plasmenyl phosphatidylethanolamine (nmol/mL) | 40.9 ± 16.7 | 40.4 ± 13.2 | 0.832 |
| Lysophosphatidylethanolamine (nmol/mL) | 5.6 ± 1.6 | 6.2 ± 1.9 | 0.066 |
| Sphingolipids | |||
| Sphingomyelin (μmol/mL) | 690.2 ± 143.0 | 748.5 ± 221.1 | 0.080 |
| Ceramide (μmol/mL) | 1913.9 ± 442.1 | 1770.3 ± 410.1 | 0.025 |
| Ginseng (n = 36) | Placebo (n = 32) | ||||||
|---|---|---|---|---|---|---|---|
| Pre- Intervention | Post- Intervention | ΔChanges | Pre- Intervention | Post- Intervention | ΔChanges | p1 | |
| Ceramides | |||||||
| Ceramide (d18:1/16:0) | 111.9 ± 34.1 | 105.9 ± 24.5 | −6.4 ± 6.3 | 154.5 ± 50.9 | 155.2 ± 43.9 | 14.6 ± 6.8 | 0.040 |
| Ceramide (d18:1/18:0) | 105.6 ± 2223.5 | 48.4 ± 18.8 | −66.0 ± 29.7 | 64.3 ± 25.1 | 58.3 ± 24.7 | 3.9 ± 31.8 | 0.142 |
| Ceramide (d18:1/20:0) | 48.2 ± 15.2 | 46.1 ± 15.5 | −5.8 ± 3.3 | 63.2 ± 22.3 | 59.8 ± 21.8 | 0.7 ± 3.5 | 0.214 |
| Ceramide (d18:1/22:0) | 291.8 ± 76.0 | 289.9 ± 80.0 | −20.8 ± 24.4 | 510.7 ± 204.9 | 560.6 ± 214.3 | 71.1 ± 26.2 | 0.020 |
| Ceramide (d18:1/24:0) | 1181.6 ± 257.3 | 1109.4 ± 295.9 | −94.3 ± 77.1 | 1594.5 ± 580.9 | 1711.0 ± 682.9 | 141.3 ± 82.6 | 0.058 |
| Ceramide (d18:1/24:1) | 180.8 ± 57.0 | 164.7 ± 55.8 | −40.3 ± 27.7 | 658.8 ± 225.6 | 651.6 ± 232.6 | 20.1 ± 29.7 | 0.173 |
| Metabolic Syndrome Group (n = 18) | Non-Metabolic Syndrome Group (n = 18) | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Pre- Intervention | Post- Intervention | ΔChanges | Pre- Intervention | Post- Intervention | ΔChanges | p2 |
| Ceramide (d18:1/16:0) | 115.1 (90.5, 131.4) | 103.6 (88.4, 121.3) | −10.4 (−31.5, 11.5) | 95.6 (78.7, 114.8) | 119.8 (78.2, 150.0) | 19.2 (−10.4, 47.2) | 0.019 |
| Ceramide (d18:1/18:0) | 65.9 (51.9, 80.1) | 46.9 (40.0, 65.8) | −11.7 (−25.7, 1.2) | 51.1 (38.6, 58.7) | 37.7 (29.4, 50.3) | −9.1 (−30.3, 6.6) | 0.999 |
| Ceramide (d18:1/20:0) | 53.1 (48.0, 59.4) | 47.5 (42.2, 60.0) | 0.9 (−16.0, 5.8) | 42.7 (29.2, 54.6) | 39.7 (29.9, 52.1) | −4.7 (−11.0, 18.3) | 0.728 |
| Ceramide (d18:1/22:0) | 320.0 (286.0, 357.3) | 282.9 (255.6, 365.8) | −41.1 (−77.4, 15.5) | 243.0 (205.4, 310.3) | 253.0 (228.8, 349.5) | 31.5 (−10.2, 84.4) | 0.040 |
| Ceramide (d18:1/24:0) | 1246.9 (1138.0, 1381.4) | 1053.8 (879.2, 1304.3) | −198.6 (−399.0, 38.9) | 1102.6 (973.1, 1250.3) | 1086.6 (858.1, 1401.5) | −24.5 (−213.9, 236.1) | 0.066 |
| Ceramide (d18:1/24:1) | 180.2 (157.6, 213.4) | 160.2 (117.0, 212.5) | −25.4 (−64.2, 13.7) | 171.6 (129.5, 208.0) | 161.5 (131.5, 186.7) | −16.6 (−56.7, 36.0) | 0.359 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-J.; Lee, G.-M.; Liu, K.-H.; Jung, D.-H. Effect of Korean Red Ginseng on Plasma Ceramide Levels in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial. Metabolites 2021, 11, 417. https://doi.org/10.3390/metabo11070417
Kwon Y-J, Lee G-M, Liu K-H, Jung D-H. Effect of Korean Red Ginseng on Plasma Ceramide Levels in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial. Metabolites. 2021; 11(7):417. https://doi.org/10.3390/metabo11070417
Chicago/Turabian StyleKwon, Yu-Jin, Gyung-Min Lee, Kwang-Hyeon Liu, and Dong-Hyuk Jung. 2021. "Effect of Korean Red Ginseng on Plasma Ceramide Levels in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial" Metabolites 11, no. 7: 417. https://doi.org/10.3390/metabo11070417
APA StyleKwon, Y.-J., Lee, G.-M., Liu, K.-H., & Jung, D.-H. (2021). Effect of Korean Red Ginseng on Plasma Ceramide Levels in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial. Metabolites, 11(7), 417. https://doi.org/10.3390/metabo11070417