Metabolic Profiles of Brassica juncea Roots in Response to Cadmium Stress
Abstract
1. Introduction
2. Results and Discussion
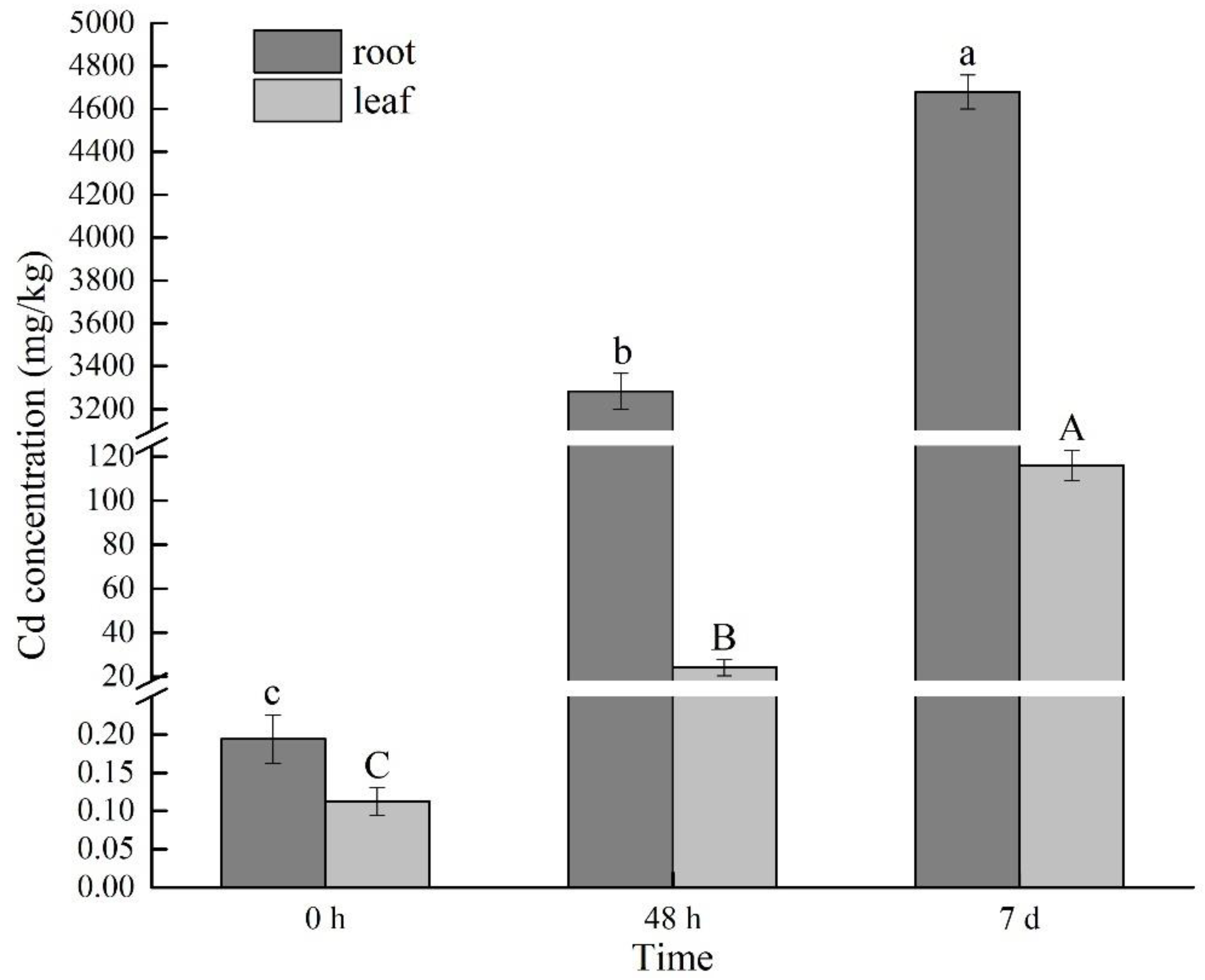
2.1. Changes of Cd Content under Cd Stress at Different Times
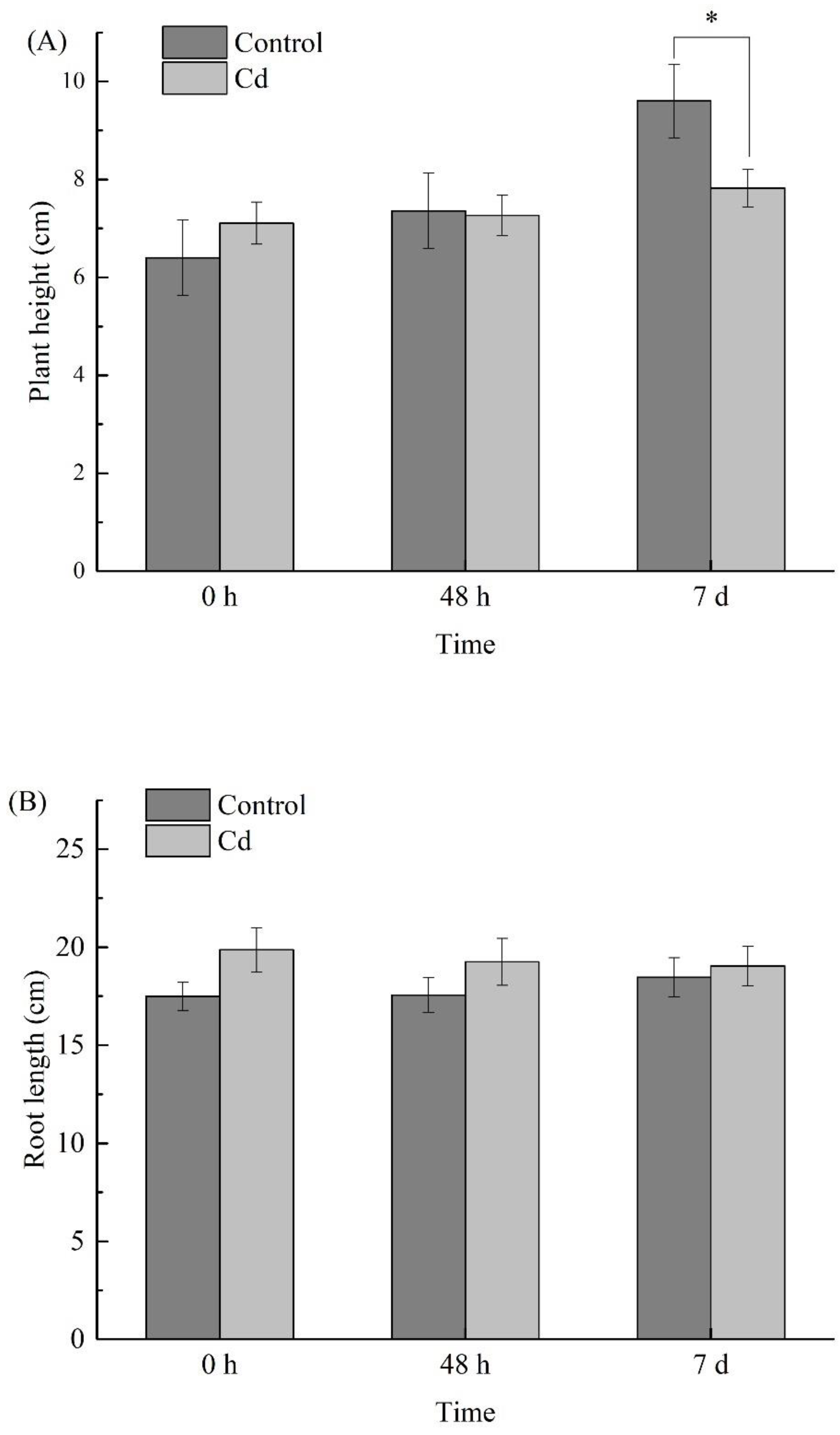
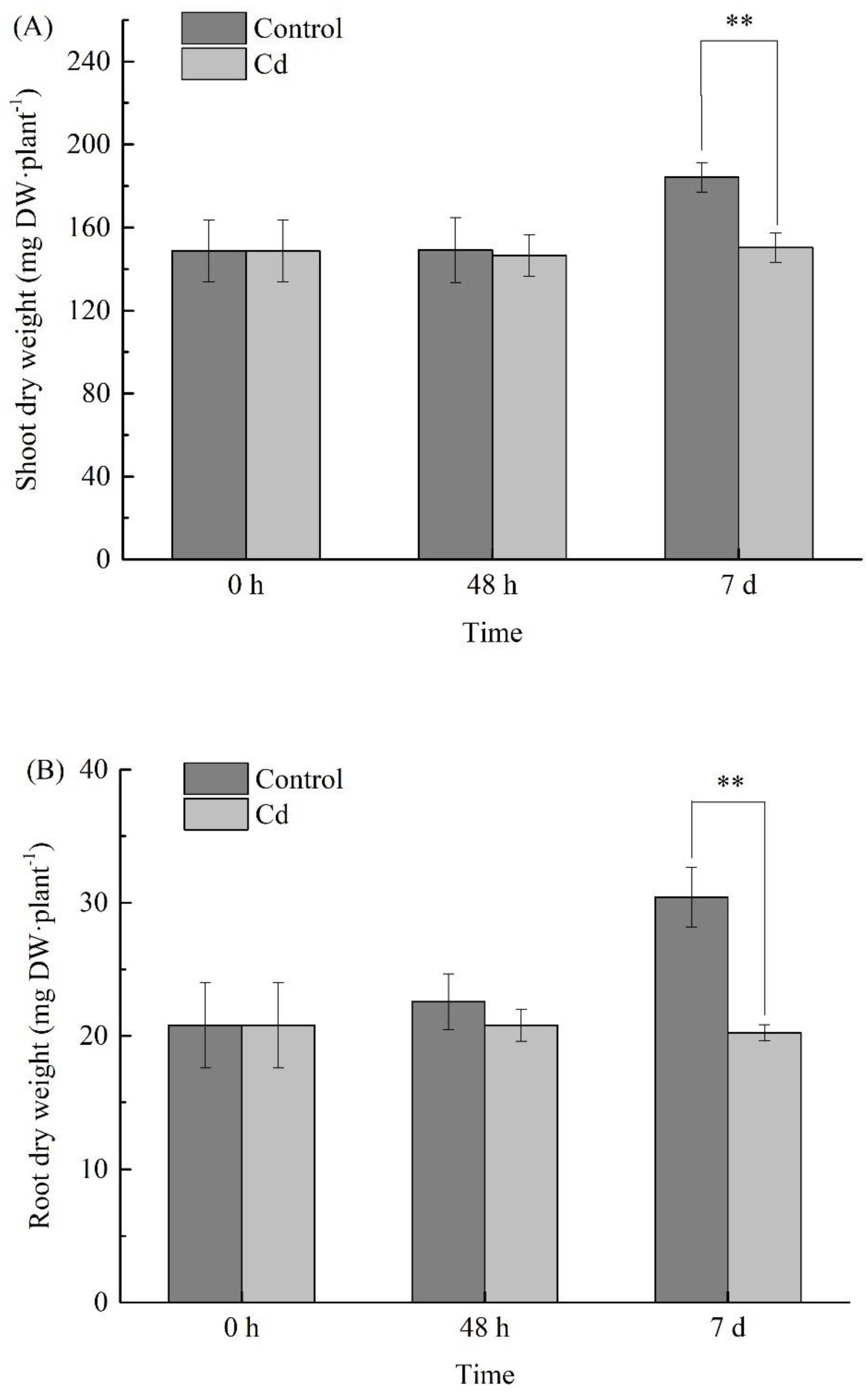
2.2. Changes of Biomass under Cd Stress at Different Times
2.3. Nontargeted Analysis of B. juncea by LC-MS
2.4. Multivariate Analysis in Different B. juncea Samples
2.5. Differential Metabolites Analysis
2.5.1. Amino Acids
2.5.2. Organic Acids
2.5.3. Carbohydrates
2.5.4. Lipids
2.5.5. Flavonoid Glycosides, Alkaloids, and Indoles
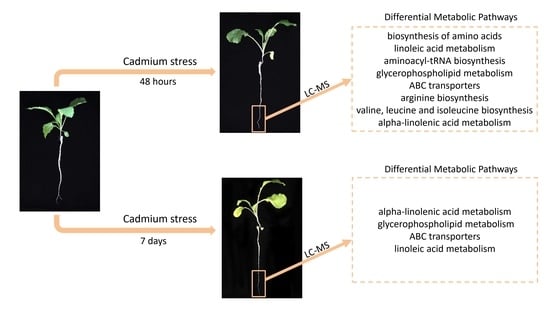
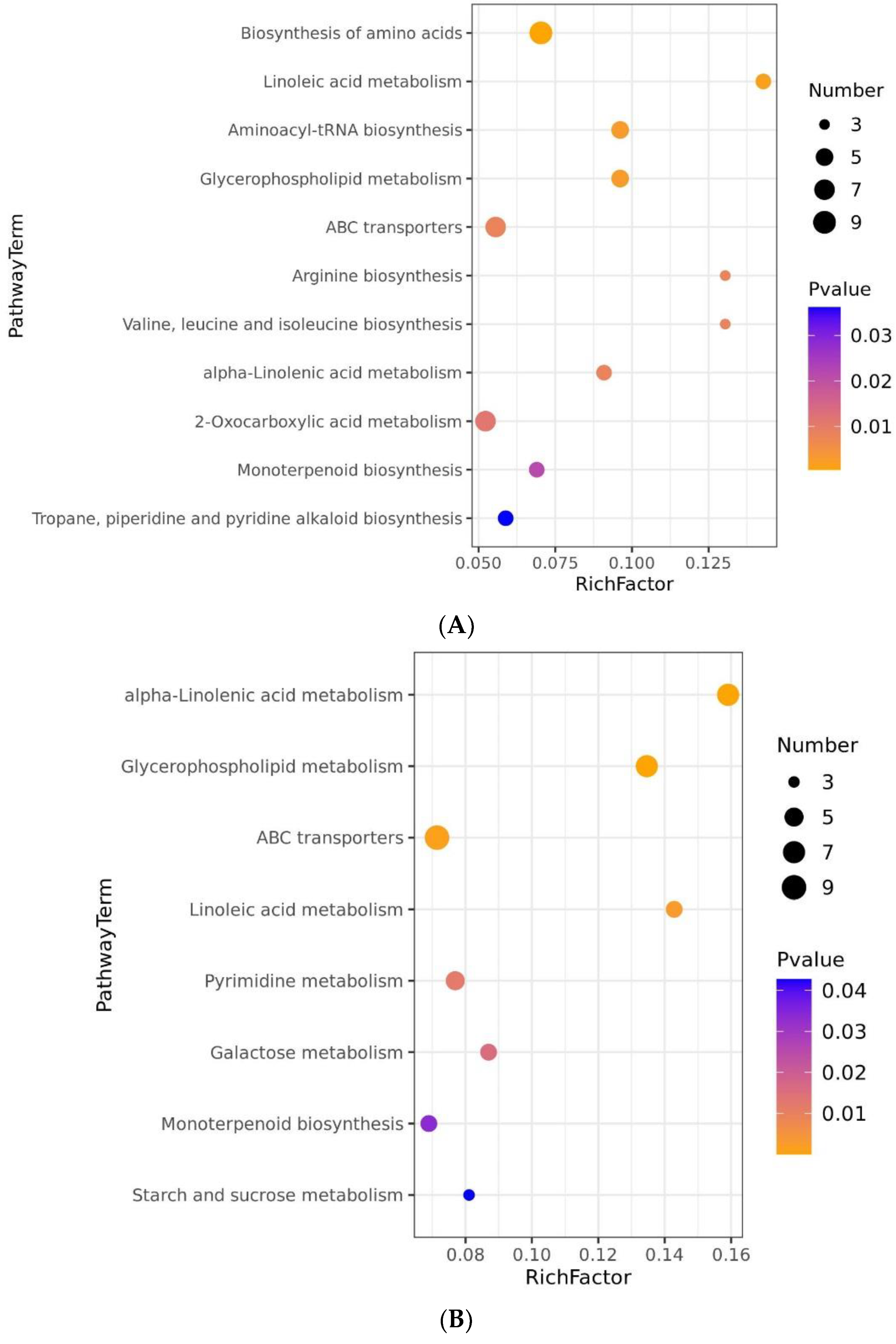
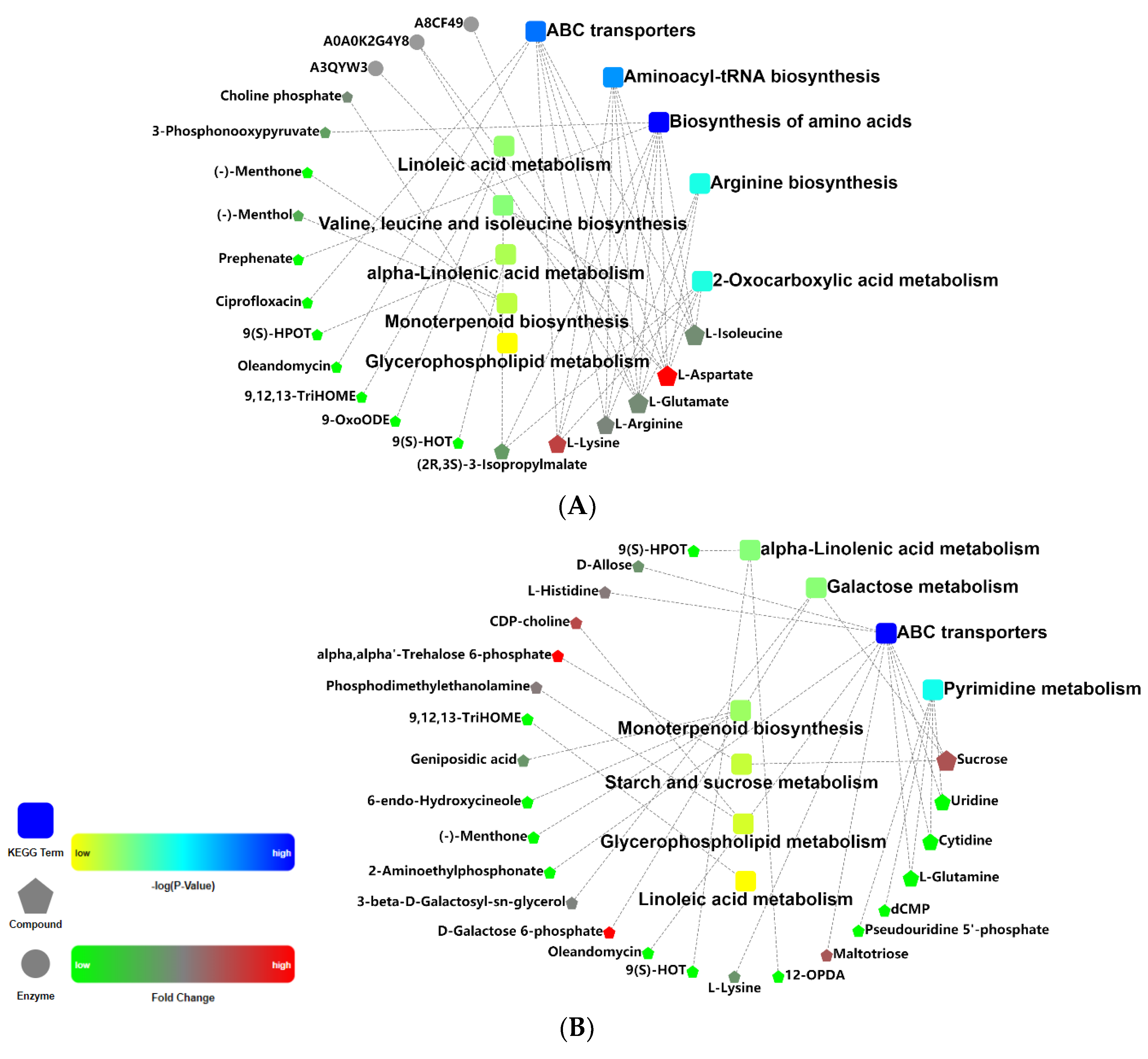
2.6. Differential Metabolic Pathways and Metabolic Network Analyses
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Chemicals
3.3. Measurements of Cd Content and Growth Parameters of Plant
3.4. Metabolites Extraction
3.5. LC-MS Analysis
3.6. LC-MS Data Analyses
3.7. Metabolic Pathways Enrichment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonkiewicz, J.; Pelka, R.; Bik-Malodzinska, M.; Zukowska, G.; Glen-Karolczyk, K. The effect of cellulose production waste and municipal sewage sludge on biomass and heavy metal uptake by a plant mixture. Environ. Sci. Pollut. R. 2018, 25, 31101–31112. [Google Scholar] [CrossRef]
- Murakami, M.; Ae, N.; Ishikawa, S. Phytoextraction of cadmium by rice (Oryza sativa L.), soybean (Glycine max (L.) Merr.), and maize (Zea mays L.). Environ. Pollut. 2007, 145, 96–103. [Google Scholar] [CrossRef]
- Hossain, M.F. Arsenic contamination in bangladesh—An overview. Agric. Ecosyst. Environ. 2006, 113, 1–16. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Huang, Y.; He, C.; Shen, C.; Guo, J.; Yang, Z. Toxicity of cadmium and its health risks from leafy vegetable consumption. Food Funct. 2017, 8, 1373–1401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, Y.; Shen, C.; He, C.; Yuan, J.; Yang, Z. Comparative analysis between low- and high-cadmium-accumulating cultivars of Brassica parachinensis to identify difference of cadmium-induced microRNA and their targets. Plant Soil. 2017, 420, 223–237. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-ur-Rehman, M.; Hannan, F.; Kellerc, C.; Al-Wabeld, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Hadi, F.; Ali, N. Effective phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int. J. Phytoremediation 2015, 17, 56–65. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Taamalli, M.; Gevi, F.; Timperio, A.M.; Zolla, L.; Ghnaya, T. Cadmium stress responses in Brassica juncea: Hints from proteomics and metabolomics. J. Proteome Res. 2013, 12, 4979–4997. [Google Scholar] [CrossRef]
- Baczek-Kwinta, R.; Juzo, K.; Borek, M.; Antonkiewicz, J. Photosynthetic response of cabbage in cadmium-spiked soil. Photosynthetica 2019, 57, 731–739. [Google Scholar] [CrossRef]
- Parvaiz, A.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Gucel, S. Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J. Plant Growth Regul. 2016, 35, 936–950. [Google Scholar]
- Kumar, P.B.A.N.; Dushenkov, V.; Motto, H.; Raskin, I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995, 29, 1232–1238. [Google Scholar] [CrossRef]
- Raj, D.; Kumar, A.; Maiti, S.K. Brassica juncea (L.) Czern. (Indian mustard): A putative plant species to facilitate the phytoremediation of mercury contaminated soils. Int. J. Phytoremed. 2020, 22, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.M.; Chandra, H.M.; Srinivasan, B.; Ramalingam, S. Cadmium induced physio-biochemical and molecular response in Brassica juncea. Int. J. Phytoremediat. 2013, 15, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Castagna, A.; Ranieri, A.; Toppi, L.S.d. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Bioch. 2012, 57, 15–22. [Google Scholar] [CrossRef]
- Cao, M.; Fraser, K.; Jones, C.; Stewart, A.; Lyons, T.; Faville, M.; Barrett, B. Untargeted metabotyping Lolium perenne reveals population-level variation in plant flavonoids and alkaloids. Front. Plant Sci. 2017, 8, 133. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Metabolomics analysis of Camellia sinensis with respect to harvesting time. Food Res. Int. 2020, 128, 108814. [Google Scholar] [CrossRef]
- Rakesh, K.; Bohra, A.; Pandey, A.K.; Pandey, M.K.; Kumar, A. Metabolomics for plant improvement: Status and prospects. Front. Plant Sci. 2017, 8, 1–27. [Google Scholar]
- Luo, Q.; Sun, L.; Hu, X. Metabonomics study on root exudates of cadmium hyperaccumulator Sedum alfredii. Chin. J. Anal. Chem. 2015, 43, 7–12. [Google Scholar] [CrossRef]
- Navarro-Reig, M.; Jaumot, J.; Pina, B.; Moyano, E.; Galceran, M.T.; Tauler, R. Metabolomic analysis of the effects of cadmium and copper treatment in Oryza sativa L. using untargeted liquid chromatography coupled to high resolution mass spectrometry and all-ion fragmentation. Metallomics 2017, 9, 660–675. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Analysis of young shoots of ‘Anji Baicha’ (Camellia sinensis) at three developmental stages using nontargeted LC-MS-based metabolomics. J. Food Sci. 2019, 84, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Kramer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. S. Afr. J. Bot. 2011, 77, 36–44. [Google Scholar]
- Motoda, H.; Kano, Y.; Hiragami, F.; Kawamura, K.; Matsumoto, H. Morphological changes in the apex of pea roots during and after recovery from aluminium treatment. Plant Soil. 2010, 333, 49–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, K.; Zhang, D.; Duan, H.; Liu, Y. Metabolite accumulation and metabolic network in developing roots of Rehmannia glutinosa reveals its root developmental mechanism and quality. Sci. Rep. 2018, 8, 14127. [Google Scholar] [CrossRef] [PubMed]
- He, X.M.; Zhang, J.; Ren, Y.N.; Sun, C.Y.; Deng, X.P.; Qian, M.; Hu, Z.B.; Li, R.; Chen, Y.H.; Shen, Z.G.; et al. Polyaspartate and liquid amino acid fertilizer are appropriate alternatives for promoting the phytoextraction of cadmium and lead in Solanum nigrum L. Chemosphere 2019, 237, 124483. [Google Scholar] [CrossRef]
- Samiksha, S.; Parul, P.; Rachana, S.; Vijay Pratap, S.; Sheo Mohan, P. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar]
- Costa, G.; Morel, J.L. Water relations, gas exchange and amino acid content in cd-treated lettuce. Plant Physiol. Bioch. 1994, 32, 561–570. [Google Scholar]
- Pedro, D.; Borsani, O.; Márquez, A.; Monza, J. Nitrogen metabolism in relation to drought stress responses in cultivated and model Lotus species. Lotus Newsletter. 2005, 35, 83–92. [Google Scholar]
- Liu, L.J.; Lin, L.D. Effect of heat stress on Sargassum fusiforme leaf metabolome. J. Plant Biol. 2020, 63, 229–241. [Google Scholar] [CrossRef]
- Kosová, K.; Urban, M.; Vítámvás, P.; Prasil, I. Drought stress response in common wheat, durum wheat, and barley: Transcriptomics, proteomics, metabolomics, physiology, and breeding for an enhanced drought tolerance. In Drought Stress Tolerance in Plants; Springer International Publishing: Cham, Germany, 2016; pp. 277–314. [Google Scholar]
- Asha, K.; Paromita, D.; Asish, P.; Pradeep, A. Proteomics, metabolomics and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar]
- Naidu, B.; Paleg, L.G.; Aspinall, D.; Jennings, A.C.; Jones, G. Amino acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry 1991, 30, 407–409. [Google Scholar] [CrossRef]
- Blunden, G.; Patel, A.V.; Adrian-Romero, M.; Meléndez, P. The accumulation of trans-4-hydroxy-N-methylproline and N-methylproline by some plant species. Biochem. Syst. Ecol. 2004, 32, 1153–1158. [Google Scholar] [CrossRef]
- Zhu, G.X.; Xiao, H.Y.; Guo, Q.J.; Zhang, Z.Y.; Zhao, J.J.; Yang, D. Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol. Environ. Saf. 2018, 158, 300–308. [Google Scholar] [CrossRef]
- Cosio, C.; Renault, D. Effects of cadmium, inorganic mercury and methyl-mercury on the physiology and metabolomic profiles of shoots of the macrophyte Elodea nuttallii. Environ. Pollut. 2020, 257, 113557. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.H.; Du, L.G.; Liu, X.J. Comparative transcriptome analysis of cadmium responses in Solanum nigrum and Solanum torvum. New Phytol. 2012, 196, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.D.; Chen, W.Q.; Lai, X.C.; Dai, H.B.; Sun, H.; Zhou, X.Y.; Chen, T.B. Metabolic responses and their correlations with phytochelatins in Amaranthus hypochondriacus under cadmium stress. Environ. Pollut. 2019, 252, 1791–1800. [Google Scholar] [CrossRef]
- Stepansky, A.; Leustek, T. Histidine biosynthesis in plants. Amino Acids 2006, 30, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.Y.; Song, S.Y.; Shu, Q.Y.; Huang, J.Z. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef]
- Slocum, R.D. Genes, enzymes and regulation of arginine biosynthesis in plants. Plant Physiol. Biochem. 2005, 43, 729–745. [Google Scholar] [CrossRef]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Chaudhary, H.J.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol. Environ. Saf. 2017, 141, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Sun, T.H.; Sun, L.N. Low molecular weight organic acids in root exudates and cadmium accumulation in cadmium hyperaccumulator Solanum nigrum L. and non-hyperaccumulator Solanum lycopersicum L. Afr. J. Biotechnol. 2011, 10, 17180–17185. [Google Scholar]
- Han, F.; Shan, X.; Zhang, S.; Wen, B.; Owens, G. Enhanced cadmium accumulation in maize roots—the impact of organic acids. Plant Soil 2006, 289, 355–368. [Google Scholar] [CrossRef]
- Fu, X.; Dou, C.; Hu, S.; Chen, X.; Shi, J.; Chen, Y. A review of progress in roles of organic acids on heavy metal resistance and detoxification in plants. Chin. J. Plant Ecol. 2010, 54, 960–961. [Google Scholar]
- Patton, A.J.; Cunningham, S.M.; Volenec, J.J.; Reicher, Z.J. Differences in freeze tolerance of zoysiagrasses: II. Carbohydrate and proline accumulation. Crop Sci. 2007, 47, 2170–2181. [Google Scholar] [CrossRef]
- Guy, C.L.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef]
- Whittaker, A.; Bochicchio, A.; Vazzana, C.; Lindsey, G.; Farrant, J. Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species Sporobolus stapfianus and Xerophyta viscosa. J. Exp. Bot. 2001, 52, 961–969. [Google Scholar] [CrossRef]
- Jouve, L.; Hoffmann, L.; Hausman, J.F. Polyamine, carbohydrate, and proline content changes during salt stress exposure of aspen (populus tremula L.): Involvement of oxidation and osmoregulation metabolism. Plant Biol. 2004, 6, 74–80. [Google Scholar]
- Wang, H.L.; Lee, P.D.; Chen, W.L.; Huang, D.J.; Su, J.C. Osmotic stress-induced changes of sucrose metabolism in cultured sweet potato cells. J. Exp. Bot. 2000, 51, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, L.X.; Du, Z.M.; Sun, X.Y.; Amombo, E.; Fan, J.B.; Fu, J.M. Effects of cadmium exposure on growth and metabolic profile of bermudagrass [Cynodon dactylon (L.) Pers.]. PLoS ONE 2014, 9, e115279. [Google Scholar] [CrossRef]
- Krasavina, M.; Burmistrova, N.A.; Raldugina, G. The role of carbohydrates in plant resistance to abiotic stresses. In Emerging Technologies and Management of Crop Stress Tolerance; Elsevier Inc.: San Diego, CA, USA, 2014; pp. 229–270. [Google Scholar]
- Zhang, G.; Slaski, J.J.; Archambault, D.J.; Taylor, G.J. Alternation of plasma membrane lipids in aluminum-resistant and aluminum-sensitive wheat genotypes in response to aluminum stress. Physiol. Plant. 1997, 99, 302–308. [Google Scholar] [CrossRef]
- Hernández, L.E.; Cooke, D.T. Modification of the root plasma membrane lipid composition of cadmium-treated Pisum sativum. J. Exp. Bot. 1997, 48, 1375–1381. [Google Scholar] [CrossRef]
- Jones, G.J.; Nichols, P.D.; Johns, R.B.; Smith, J.D. The effect of mercury and cadmium on the fatty acid and sterol composition of the marine diatom Asteronella glacialis. Phytochemistry 1987, 26, 1343–1348. [Google Scholar] [CrossRef]
- Huynh, V.B.; Repellin, A.; Zuily-Fodil, Y.; Pham-Thi, A.T. Aluminum stress response in rice: Effects on membrane lipid composition and expression of lipid biosynthesis genes. Physiol. Plant. 2012, 146, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cao, X.; Tan, C.; Deng, Y.; Bai, J. Analysis of the effect of cadmium stress on root exudates of Sedum plumbizincicola based on metabolomics. Ecotoxicol. Environ. Saf. 2020, 205, 111152. [Google Scholar] [CrossRef] [PubMed]
- Maleva, M.; Garmash, E.; Chukina, N.; Malec, P.; Waloszek, A.; Strzalka, K. Effect of the exogenous anthocyanin extract on key metabolic pathways and antioxidant status of Brazilian elodea (Egeria densa (Planch.) Casp.) exposed to cadmium and manganese. Ecotoxicol. Environ. Saf. 2018, 160, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Y.; Gu, C.X.; Sun, Y.H.; Li, G.Z.; Li, L.L.; Hao, L. Root defense in salicylic acid-altering Arabidopsis plants in responses to cadmium stress. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Li, C.J.; Zhang, X.X.; Johnson, R.; Bao, G.S.; Yao, X.; Chai, Q. Effects of cold shocked Epichloe infected Festuca sinensis on ergot alkaloid accumulation. Fungal. Ecol. 2015, 14, 99–104. [Google Scholar] [CrossRef]
- Srivastava, N.K.; Srivastava, A.K. Influence of some heavy metals on growth, alkaloid content and composition in Catharanthus roseus L. Indian J. Pharm. Sci. 2010, 72, 775–778. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Agami, R.A.; Mohamed, G.F. Exogenous treatment with indole-3-acetic acid and salicylic acid alleviates cadmium toxicity in wheat seedlings. Ecotoxicol. Environ. Saf. 2013, 94, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Glawischnig, E.; Hansen, B.G.; Olsen, C.E.; Halkier, B.A. Camalexin is synthesized from indole-3-acetaldoxime, a key branching point between primary and secondary metabolism in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 8245–8250. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Jha, M.; Okeola, O.G. Camalexin induces detoxification of the phytoalexin brassinin in the plant pathogen Leptosphaeria maculans. Phytochemistry 2005, 66, 2609–2616. [Google Scholar] [CrossRef]
- Campos, N.V.; Araujo, T.O.; Arcanjo-Silva, S.; Freitas-Silva, L.; Azevedo, A.A.; Nunes-Nesi, A. Arsenic hyperaccumulation induces metabolic reprogramming in Pityrogramma calomelanos to reduce oxidative stress. Physiol. Plant. 2016, 157, 135–146. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, L.; Shen, H.; Wang, J.; Liu, W.; Zhu, X.; Wang, R.; Sun, X.; Liu, L. Metabolomic analysis with GC-MS to reveal potential metabolites and biological pathways involved in Pb &Cd stress response of radish roots. Sci. Rep. 2015, 5, 18296. [Google Scholar] [PubMed]
- Liu, Q.; Zhang, Y.; Wang, Y.; Wang, W.; Gu, C.; Huang, S.; Yuan, H.; Dhankher, O.P. Quantitative proteomic analysis reveals complex regulatory and metabolic response of Iris lactea Pall. var. chinensis to cadmium toxicity. J. Hazard. Mater. 2020, 400, 123165. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Choudhary, S.; Dubey, P.; Bhardwaj, P. Comparative transcriptome profiling reveals the reprogramming of gene networks under arsenic stress in Indian mustard. Genome 2019, 62, 833–847. [Google Scholar] [CrossRef]
- Liu, D.W.; Tao, L.; Chen, J.S.; Zhang, Y.J. Analysis of early transcriptomes of huipizhiheidou and liaodou 15 infected by soybean cyst nematode (Heterodera glycines). Int. J. Agric. Biol. 2018, 20, 2169–2174. [Google Scholar]
- Zhang, Y.J.; Wei, M.Y.; Liu, A.L.; Zhou, R.; Li, D.H.; Dossa, K.; Wang, L.H.; Zhang, Y.X.; Gong, H.H.; Zhang, X.R.; et al. Comparative proteomic analysis of two sesame genotypes with contrasting salinity tolerance in response to salt stress. J. Proteom. 2019, 201, 73–83. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Peng, Z.; Li, J.; Huang, W.; Liu, Y.; Wang, X.; Xie, S.; Sun, L.; Han, E.; et al. Ectopic expression of poplar ABC transporter PtoABCG36 confers cd tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 3293. [Google Scholar] [CrossRef]
- Bovet, L.; Feller, U.; Martinoia, E. Possible involvement of plant ABC transporters in cadmium detoxification: A cDNA sub-microarray approach. Environ. Int. 2005, 31, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.S.; Benci, J.L.; Selote, D.S.; Sharma, A.K.; Chen, A.G.Y.; Dang, H.; Fares, H.; Vatamaniuk, O.K. Detoxification of multiple heavy metals by a half-molecule ABC transporter, HMT-1, and coelomocytes of Caenorhabditis elegans. PLoS ONE 2010, 5, e9564. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Sugimoto, E.; Shinozaki, K. Arabidopsis mutants of AtABCG22, an ABC transporter gene, increase water transpiration and drought susceptibility. Plant J. 2011, 67, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Choi, H.; Huh, S.U.; Bassin, B.; Kim, J.; Martinoia, E.; Sohn, K.H.; Paek, K.H.; Lee, Y. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin. Proc. Natl. Acad. Sci. USA 2017, 114, E5712–E5720. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Peng, Y.H.; Meckes, N.; Allen, S.; Stewart, C.N.; Traw, M.B. ATP-dependent binding cassette transporter G family member 16 increases plant tolerance to abscisic acid and assists in basal resistance against Pseudomonas syringae DC3000. Plant Physiol. 2014, 166, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, S.; He, D. Transcriptome analysis of Saliz matsudana under cadmium stress. Biotechnol. Bull. 2020, 36, 32–39. [Google Scholar]
- Wang, Y.; Wang, X.; Wang, C.; Peng, F.; Wang, R.J.; Xiao, X.; Zeng, J.; Kang, H.Y.; Fan, X.; Sha, L.N.; et al. Transcriptomic profiles reveal the interactions of Cd/Zn in dwarf polish wheat (Triticum polonicum L.) roots. Front. Physiol. 2017, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.S.; Singh, S.; Srivastava, S.; Singh, N.K.; Mani, A. Whole transcriptome expression profiling and biological network analysis of chickpea during heavy metal stress. J. Plant Biochem. Biotechnol. 2019, 28, 345–352. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Cui, P.; Shen, E.; Farooq, M.A.; Islam, F.; Ali, S.; Mao, B.; Zhou, W. Comparative transcriptome profiling of two Brassica napus cultivars under chromium toxicity and its alleviation by reduced glutathione. BMC Genom. 2016, 17, 885. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Ors, S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L.; Ma, J.; Ghasemimianaei, A.; Yang, C.H. Functional relationships between aboveground and belowground spinach (Spinacia oleracea L., cv. Racoon) microbiomes impacted by salinity and drought. Sci. Total Environ. 2020, 717, 137207. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, J.; Li, Q.; Gan, B.; Peng, W.; Zhang, X.; Tan, W.; Jiang, L.; Li, X. Manganese affects the growth and metabolism of Ganoderma lucidum based on LC-MS analysis. Peer J. 2019, 7, e6846. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, J.; Zhu, G.; Yao, R.; Chen, X.; Liu, L. CgHog1-mediated CgRds2 phosphorylation alters glycerophospholipid composition to coordinate osmotic stress in Candida glabrata. Appl. Environ. Microbiol. 2019, 85, e02822-18. [Google Scholar] [CrossRef]
- Xia, Z.; Zhou, X.; Li, J.; Li, L.; Ma, Y.; Wu, Y.; Huang, Z.; Li, X.; Xu, P.; Xue, M. Multiple-omics techniques reveal the role of glycerophospholipid metabolic pathway in the response of Saccharomyces cerevisiae against hypoxic stress. Front. Microbiol. 2019, 10, 1398. [Google Scholar] [CrossRef]
- Zeng, W.Y.; Sun, Z.D.; Cai, Z.Y.; Chen, H.Z.; Lai, Z.G.; Yang, S.Z.; Tang, X.M. Proteomic analysis by iTRAQ-MRM of soybean resistance to Lamprosema indicate. BMC Genom. 2017, 18, 444. [Google Scholar] [CrossRef]
- Haudenschild, C.; Schalk, M.; Karp, F.; Croteau, R. Functional expression of regiospecific cytochrome P450 limonene hydroxylases from mint (Mentha spp.) in Escherichia coli and saccharomyces cerevisiae. Arch. Biochem. Biophys. 2000, 379, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Weinblum, N.; Cna’ani, A.; Yaakov, B.; Sadeh, A.; Avraham, L.; Opatovsky, I.; Tzin, V. Tomato cultivars resistant or susceptible to spider mites differ in their biosynthesis and metabolic profile of the monoterpenoid pathway. Front. Plant Sci. 2021, 12, 630155. [Google Scholar] [CrossRef]
- Ling, F.L.; Su, Q.W.; Jiang, H.; Cui, J.J.; He, X.L.; Wu, Z.H.; Zhang, Z.A.; Liu, J.; Zhao, Y.J. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci. Rep. 2020, 10, 6183. [Google Scholar] [CrossRef] [PubMed]
- Matse, D.T.; Huang, C.H.; Huang, Y.M.; Yen, M.Y. Nitrogen uptake and growth of white clover inoculated with indigenous and exotic Rhizobium strains. J. Plant Nutr. 2020, 43, 2013–2027. [Google Scholar] [CrossRef]
- Liu, N.; Lin, Z. Use of evans blue for testing cell viability of intact leaves of plant. J. Plant Physiol. 2011, 47, 570–574. [Google Scholar]
- Xiong, Q.; Cao, C.; Shen, T.; Zhong, L.; He, H.; Chen, X. Comprehensive metabolomic and proteomic analysis in biochemical metabolic pathways of rice spikes under drought and submergence stress. BBA-Proteins Proteom. 2019, 1867, 237–247. [Google Scholar] [CrossRef]
- Cao, M.Y.; Liu, Y.Y.; Jiang, W.M.; Meng, X.X.; Zhang, W.; Chen, W.D.; Peng, D.Y.; Xing, S.H. UPLC/MS-based untargeted metabolomics reveals the changes of metabolites profile of Salvia miltiorrhiza bunge during Sweating processing. Sci. Rep. 2020, 10, 19524. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, Y.; Su, X.; Chu, H. mmeta: An R package for multivariate meta-analysis. J. Stat. Softw. 2014, 56, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Hu, O.; Fu, H.; Ouyang, L.; Gong, X. UPLC-Q-TOF/MS-based untargeted metabolomics coupled with chemometrics approach for Tieguanyin tea with seasonal and year variations. Food Chem. 2019, 15, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, C.F.; Ma, J.Q.; Huang, D.J.; Ma, C.L.; Jin, J.Q.; Yao, M.Z.; Chen, L. Comprehensive dissection of metabolic changes in albino and green tea cultivars. J. Agric. Food Chem. 2018, 66, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Minoru, K. KEGG bioinformatics resource for plant genomics and metabolomics. In Plant Bioinformatics; Humana Press: New York, NY, USA, 2016; pp. 55–70. [Google Scholar]

| Time (min) | Mobile Phase Composition | |
|---|---|---|
| A (%) | B (%) | |
| 0 | 95 | 5 |
| 1.5 | 95 | 5 |
| 3 | 70 | 30 |
| 7 | 40 | 60 |
| 9 | 10 | 90 |
| 11 | 0 | 100 |
| 13 | 0 | 100 |
| 13.2 | 95 | 5 |
| 16 | 95 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, P.; Zeng, C.; Wan, C.; Liu, Z.; Dong, X.; Peng, J.; Lin, H.; Li, M.; Liu, Z.; Yan, M. Metabolic Profiles of Brassica juncea Roots in Response to Cadmium Stress. Metabolites 2021, 11, 383. https://doi.org/10.3390/metabo11060383
Tan P, Zeng C, Wan C, Liu Z, Dong X, Peng J, Lin H, Li M, Liu Z, Yan M. Metabolic Profiles of Brassica juncea Roots in Response to Cadmium Stress. Metabolites. 2021; 11(6):383. https://doi.org/10.3390/metabo11060383
Chicago/Turabian StyleTan, Piaopiao, Chaozhen Zeng, Chang Wan, Zhe Liu, Xujie Dong, Jiqing Peng, Haiyan Lin, Mei Li, Zhixiang Liu, and Mingli Yan. 2021. "Metabolic Profiles of Brassica juncea Roots in Response to Cadmium Stress" Metabolites 11, no. 6: 383. https://doi.org/10.3390/metabo11060383
APA StyleTan, P., Zeng, C., Wan, C., Liu, Z., Dong, X., Peng, J., Lin, H., Li, M., Liu, Z., & Yan, M. (2021). Metabolic Profiles of Brassica juncea Roots in Response to Cadmium Stress. Metabolites, 11(6), 383. https://doi.org/10.3390/metabo11060383