Dried Blood Spot (DBS) Methodology Study for Biomarker Discovery in Lysosomal Storage Disease (LSD)
Abstract
1. Introduction
2. Results
2.1. Stability Study
2.1.1. Stability Study 1
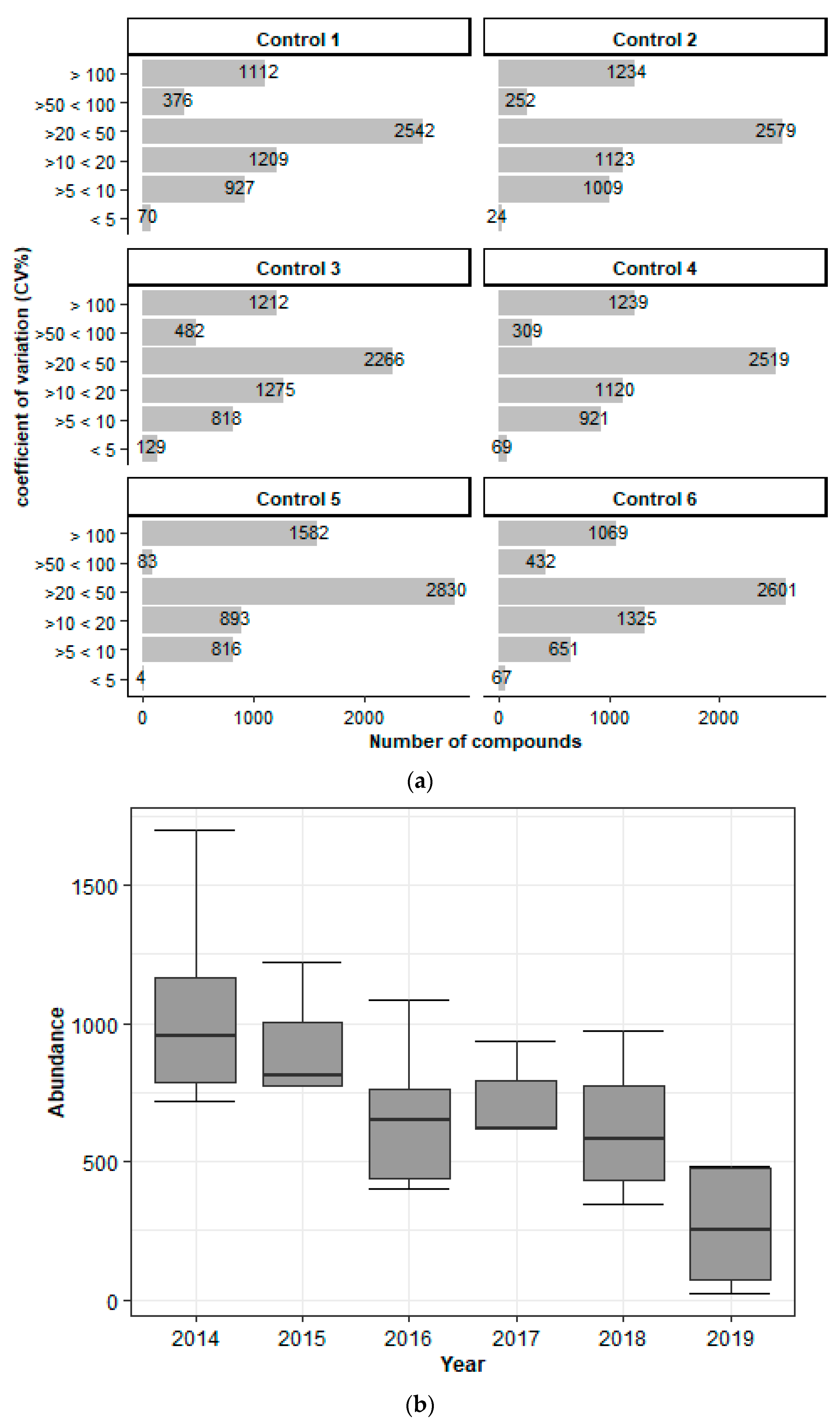
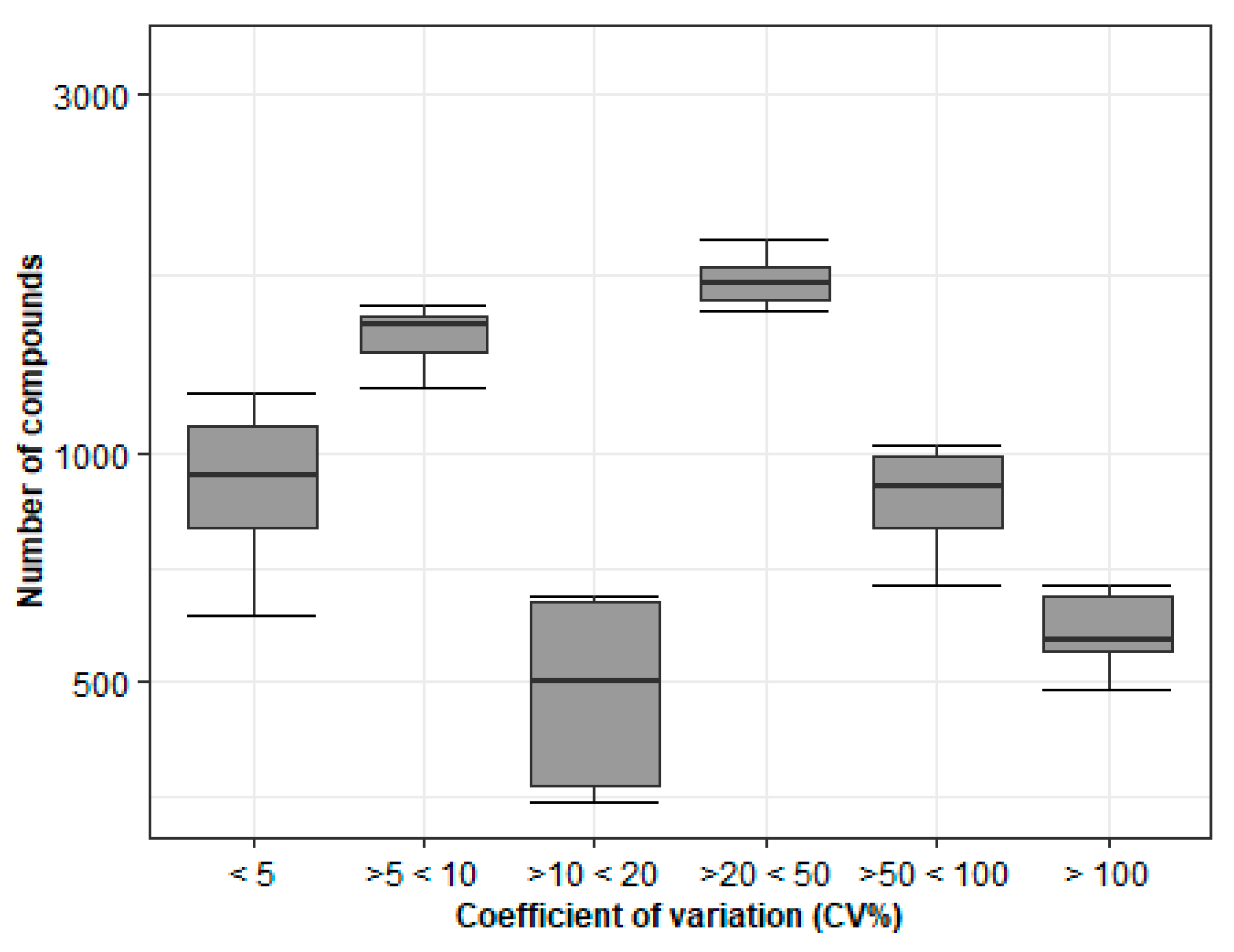
Storage at −20 °C over an Extended Period Has a Major Impact on Metabolite Stability
The Stability of the Metabolites Is Affected by Short-Term Storage in Various Conditions
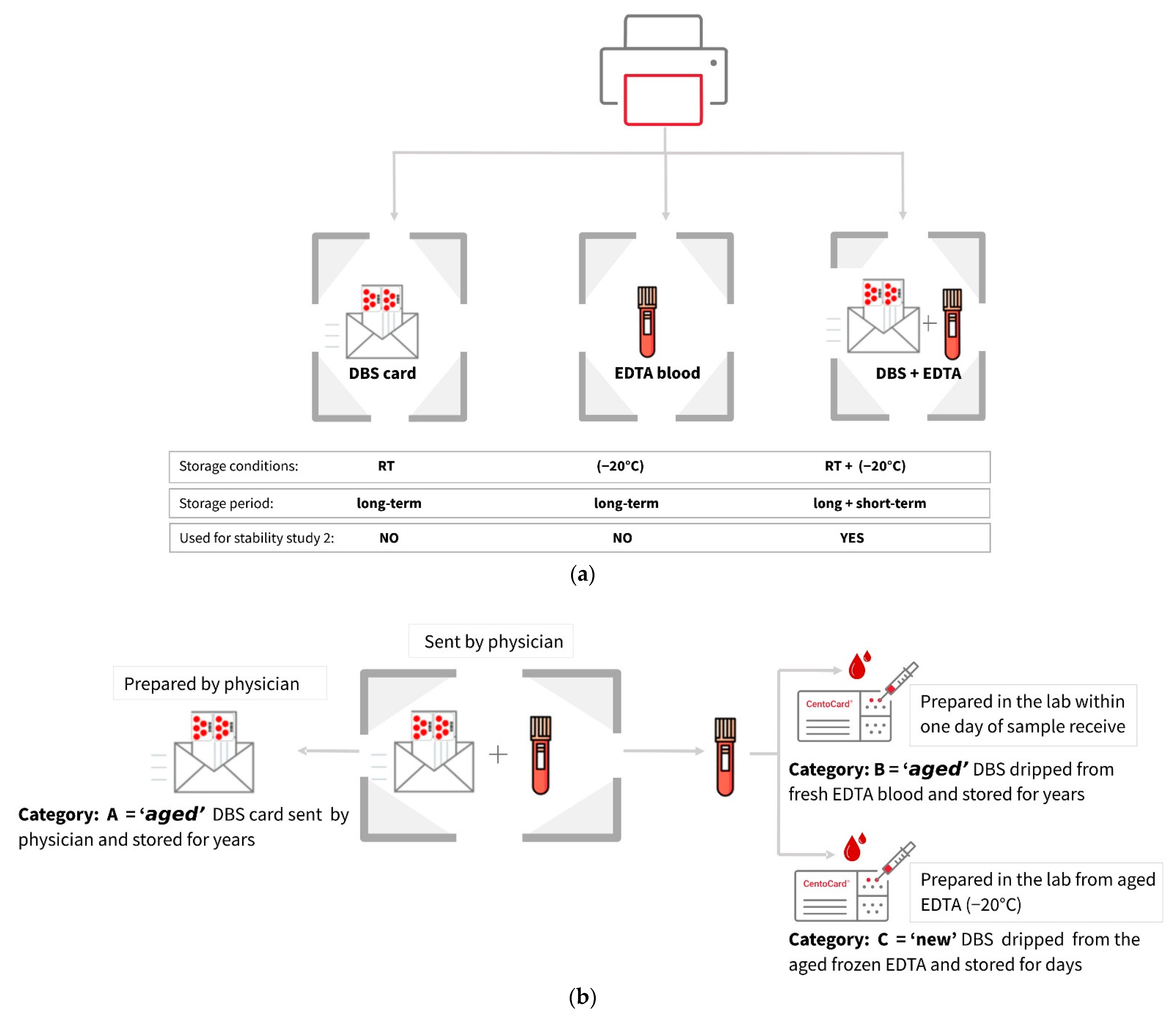
2.1.2. Stability Study 2
Metabolite Yield Is Influenced by Storage Conditions, Sample Types, and Card Age
2.2. Optimization of Extraction Solvent
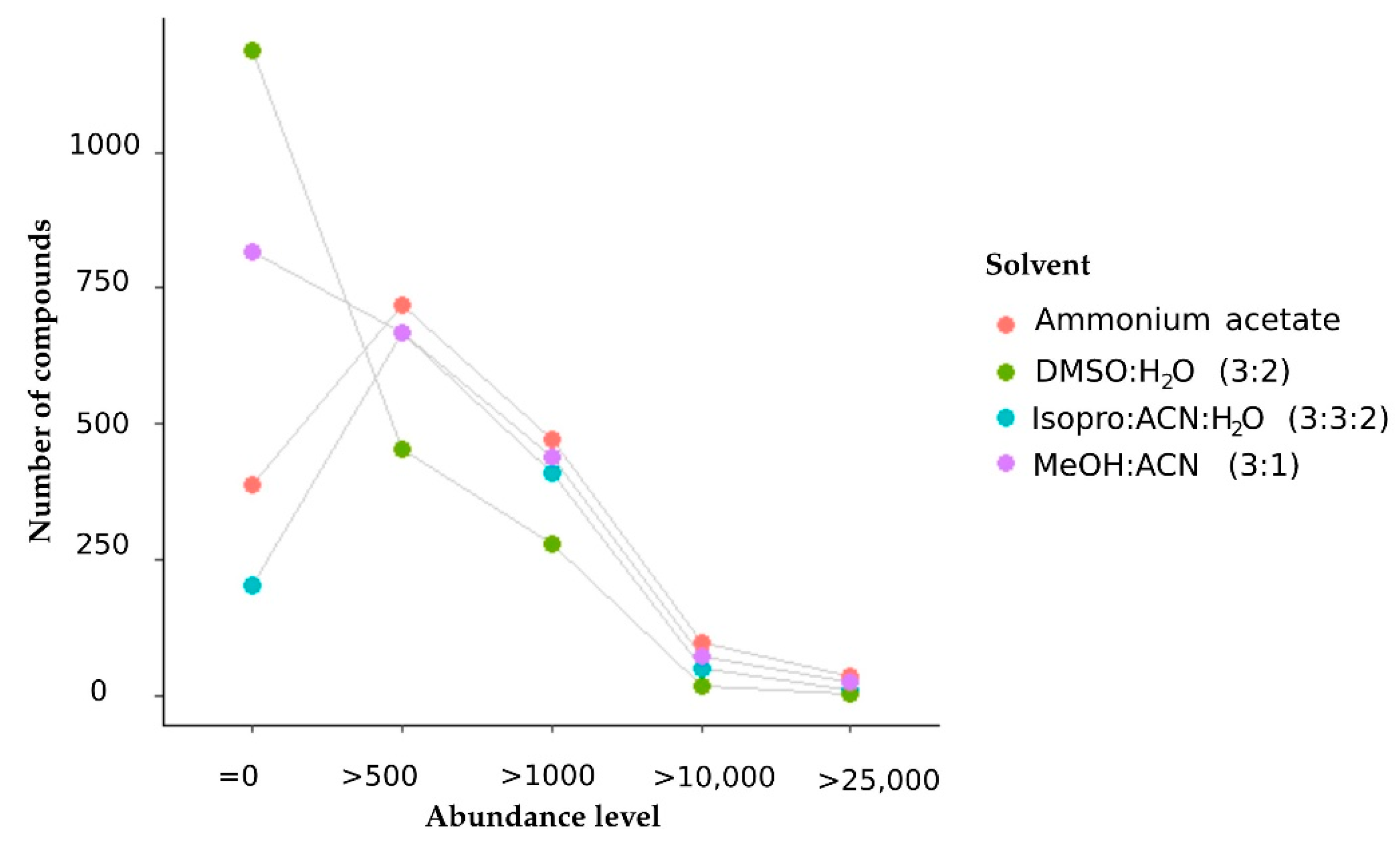
2.2.1. The Extraction Solvent Methanol: Acetonitrile Produced Metabolites with the Highest Peak Intensity
2.2.2. Methanol–Acetonitrile (3:1) Ranks Well in Terms of Metabolome Coverage and Metabolite Intensity
2.2.3. The Application of Learned Principles to the CLN6 Metabolomics Study Helps in the Discovery of Disease-Specific Metabolites
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mass Spectrometric Analysis
4.3. Data Acquisition and Analysis
4.4. Blood Sample Collection and Preparation
4.5. Dried Blood Spot Extraction
4.6. Patients Inclusion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faber, J.; Fonseca, L.M. How sample size influences research outcomes. Dent. Press J. Orthod. 2014, 19, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- Stevens, V.L.; Hoover, E.; Wang, Y.; Zanetti, K.A. Pre-Analytical Factors that Affect Metabolite Stability in Human Urine, Plasma, and Serum: A Review. Metabolites 2019, 9, 156. [Google Scholar] [CrossRef]
- Balashova, E.; Trifonova, O.P.; Maslov, D.L.; Lokhov, P.G. Application of dried blood spot for analysis of low molecular weight fraction (metabolome) of blood. Health Prim. Care 2018, 2, 1–11. [Google Scholar] [CrossRef][Green Version]
- Zakaria, R.; Allen, K.J.; Koplin, J.; Roche, P.; Greaves, R.F. Advantages and Challenges of Dried Blood Spot Analysis by Mass Spectrometry across the Total Testing Process. EJIFCC 2016, 27, 288–317. [Google Scholar]
- Lim, M.D. Dried Blood Spots for Global Health Diagnostics and Surveillance: Opportunities and Challenges. Am. J. Trop. Med. Hyg. 2018, 99, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.D.; Rosman, L.M.; Ratcliff, J.; Strickland, P.T.; Graham, D.R.; Silbergeld, E.K. State of the Science in Dried Blood Spots. Clin. Chem. 2018, 64, 656–679. [Google Scholar] [CrossRef]
- ORPHANET. Prevalence and Incidence of Rare Diseases: Bibliographic—Prevalence, Incidence or Number of Published Cases Listed by Diseases (in Alphabetical Order). Orphanet Reports Series N1. January 2021. Available online: https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf (accessed on 20 January 2021).
- Wakap, S.N.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef]
- Dawkins, H.J.; Draghia-Akli, R.; Lasko, P.; Lau, L.P.; Jonker, A.H.; Cutillo, C.M.; Rath, A.; Boycott, K.M.; Baynam, G.; Lochmüller, H.; et al. Progress in Rare Diseases Research 2010–2016: An IRDiRC Perspective. Clin. Transl. Sci. 2018, 11, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Meikle, P.J.; Hopwood, J.J. Epidemiology of Lysosomal Storage Diseases: An Overview; Oxford PharmaGenesis: Oxford, UK, 2006. [Google Scholar]
- Gagne, J.J.; Thompson, L.; O’Keefe, K.; Kesselheim, A.S. Innovative research methods for studying treatments for rare diseases: Methodological review. BMJ 2014, 349, g6802. [Google Scholar] [CrossRef]
- Southall, N.T.; Natarajan, M.; Lau, L.P.L.; Jonker, A.H.; Deprez, B.; Guilliams, T.; Hunter, L.; Rademaker, C.M.; Hivert, V.; Ardigò, D.; et al. The use or generation of biomedical data and existing medicines to discover and establish new treatments for patients with rare diseases—Recommendations of the IRDiRC Data Mining and Repurposing Task Force. Orphanet. J. Rare Dis. 2019, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Goldansaz, S.A.; Jaine, J. Translational Metabolomics: Current Challenges and Future Opportunities. Metabolites 2019, 9, 108. [Google Scholar] [CrossRef]
- Kohler, I.; Hankemeier, T.; van der Graaf, P.H.; Knibbe, C.A.; van Hasselt, J.C. Integrating clinical metabolomics-based biomarker discovery and clinical pharmacology to enable precision medicine. Eur. J. Pharm. Sci. 2017, 109, S15–S21. [Google Scholar] [CrossRef]
- Gülbakan, B.; Özgül, R.K.; Yüzbaşıoğlu, A.; Kohl, M.; Deigner, H.-P.; Özgüç, M. Discovery of biomarkers in rare diseases: Innovative approaches by predictive and personalized medicine. EPMA J. 2016, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; A Ward, P. Opportunities and challenges of disease biomarkers: A new section in the journal of translational medicine. J. Transl. Med. 2012, 10, 240. [Google Scholar] [CrossRef]
- Considine, E.C. The Search for Clinically Useful Biomarkers of Complex Disease: A Data Analysis Perspective. Metabolites 2019, 9, 126. [Google Scholar] [CrossRef]
- Paulovich, A.G.; Whiteaker, J.R.; Hoofnagle, A.N.; Wang, P. The interface between biomarker discovery and clinical validation: The tar pit of the protein biomarker pipeline. Proteom. Clin. Appl. 2008, 2, 1386–1402. [Google Scholar] [CrossRef]
- Marchand, C.R.; Farshidfar, F.; Rattner, J.; Bathe, O.F. A Framework for Development of Useful Metabolomic Biomarkers and Their Effective Knowledge Translation. Metabolites 2018, 8, 59. [Google Scholar] [CrossRef]
- Zhang, Z.; Chan, D.W. The Road from Discovery to Clinical Diagnostics: Lessons Learned from the First FDA-Cleared In Vitro Diagnostic Multivariate Index Assay of Proteomic Biomarkers. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2995–2999. [Google Scholar] [CrossRef]
- Drolet, J.; Tolstikov, V.; Williams, B.A.; Greenwood, B.P.; Hill, C.; Vishnudas, V.K.; Sarangarajan, R.; Narain, N.R.; Kiebish, M.A. Integrated Metabolomics Assessment of Human Dried Blood Spots and Urine Strips. Metabolites 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Bossuyt, P.M.M. Waste, Leaks, and Failures in the Biomarker Pipeline. Clin. Chem. 2017, 63, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Gika, H.; Theodoridis, G. Sample preparation prior to the LC–MS-based metabolomics/metabonomics of blood-derived samples. Bioanalysis 2011, 3, 1647–1661. [Google Scholar] [CrossRef]
- Barnes, S.; Benton, H.P.; Casazza, K.; Cooper, S.; Cui, X.; Du, X.; Engler, J.; Kabarowski, J.H.; Li, S.; Pathmasiri, W.; et al. Training in metabolomics research. I. Designing the experiment, collecting and extracting samples and generating metabolomics data. J. Mass Spectrom. 2016, 51, 461–475. [Google Scholar] [CrossRef]
- Surowiec, I.; Johansson, E.; Torell, F.; Idborg, H.; Gunnarsson, I.; Svenungsson, E.; Jakobsson, P.-J.; Trygg, J. Multivariate strategy for the sample selection and integration of multi-batch data in metabolomics. Metabolomics 2017, 13, 114. [Google Scholar] [CrossRef]
- Cozma, C.; Iurașcu, M.-I.; Eichler, S.; Hovakimyan, M.; Brandau, O.; Zielke, S.; Böttcher, T.; Giese, A.-K.; Lukas, J.; Rolfs, A. C26-Ceramide as highly sensitive biomarker for the diagnosis of Farber Disease. Sci. Rep. 2017, 7, 6149. [Google Scholar] [CrossRef]
- Wu, Z.E.; Kruger, M.C.; Cooper, G.J.; Poppitt, S.D.; Fraser, K. Tissue-Specific Sample Dilution: An Important Parameter to Optimise Prior to Untargeted LC-MS Metabolomics. Metabolites 2019, 9, 124. [Google Scholar] [CrossRef]
- Wu, D.-W.; Li, Y.-M.; Wang, F. How Long Can We Store Blood Samples: A Systematic Review and Meta-Analysis. EBioMedicine 2017, 24, 277–285. [Google Scholar] [CrossRef]
- Trifonova, O.P.; Maslov, D.L.; Balashova, E.E.; Lokhov, P.G. Evaluation of Dried Blood Spot Sampling for Clinical Metabolomics: Effects of Different Papers and Sample Storage Stability. Metabolites 2019, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Prentice, P.; Turner, C.; Wong, M.C.; Dalton, R.N. Stability of metabolites in dried blood spots stored at different temperatures over a 2-year period. Bioanalysis 2013, 5, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D. Improving metabolome coverage and data quality: Advancing metabolomics and lipidomics for biomarker discovery. Chem. Commun. 2018, 54, 6728–6749. [Google Scholar] [CrossRef] [PubMed]
- Sitnikov, D.G.; Monnin, C.S.; Vuckovic, D. Systematic Assessment of Seven Solvent and Solid-Phase Extraction Methods for Metabolomics Analysis of Human Plasma by LC-MS. Sci. Rep. 2016, 6, 38885. [Google Scholar] [CrossRef]
- Fiehn, O.; Kind, T. Metabolite Profiling in Blood Plasma. In Metabolomics: Methods and Protocols; Weckwerth, W., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 3–17. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, A.S.A.; Siuzdak, G. Solvent-Dependent Metabolite Distribution, Clustering, and Protein Extraction for Serum Profiling with Mass Spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, T.M.; Al-Hassan, A.A.; Ben Hadda, T.; Aljofan, M. Comparison of different serum sample extraction methods and their suitability for mass spectrometry analysis. Saudi Pharm. J. 2015, 23, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yin, Y.; Chen, X.; Chen, C.; Xia, Y.; Qi, H.; Baker, P.N.; Zhang, H.; Han, T.-L. Evaluating different extraction solvents for GC-MS based metabolomic analysis of the fecal metabolome of adult and baby giant pandas. Sci. Rep. 2019, 9, 12017–12019. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, A.; Sääf, S.; Lehtiö, J.; Nordström, A. Tuning Metabolome Coverage in Reversed Phase LC–MS Metabolomics of MeOH Extracted Samples Using the Reconstitution Solvent Composition. Anal. Chem. 2017, 89, 7356–7364. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, UY, USA, 2016. [Google Scholar]


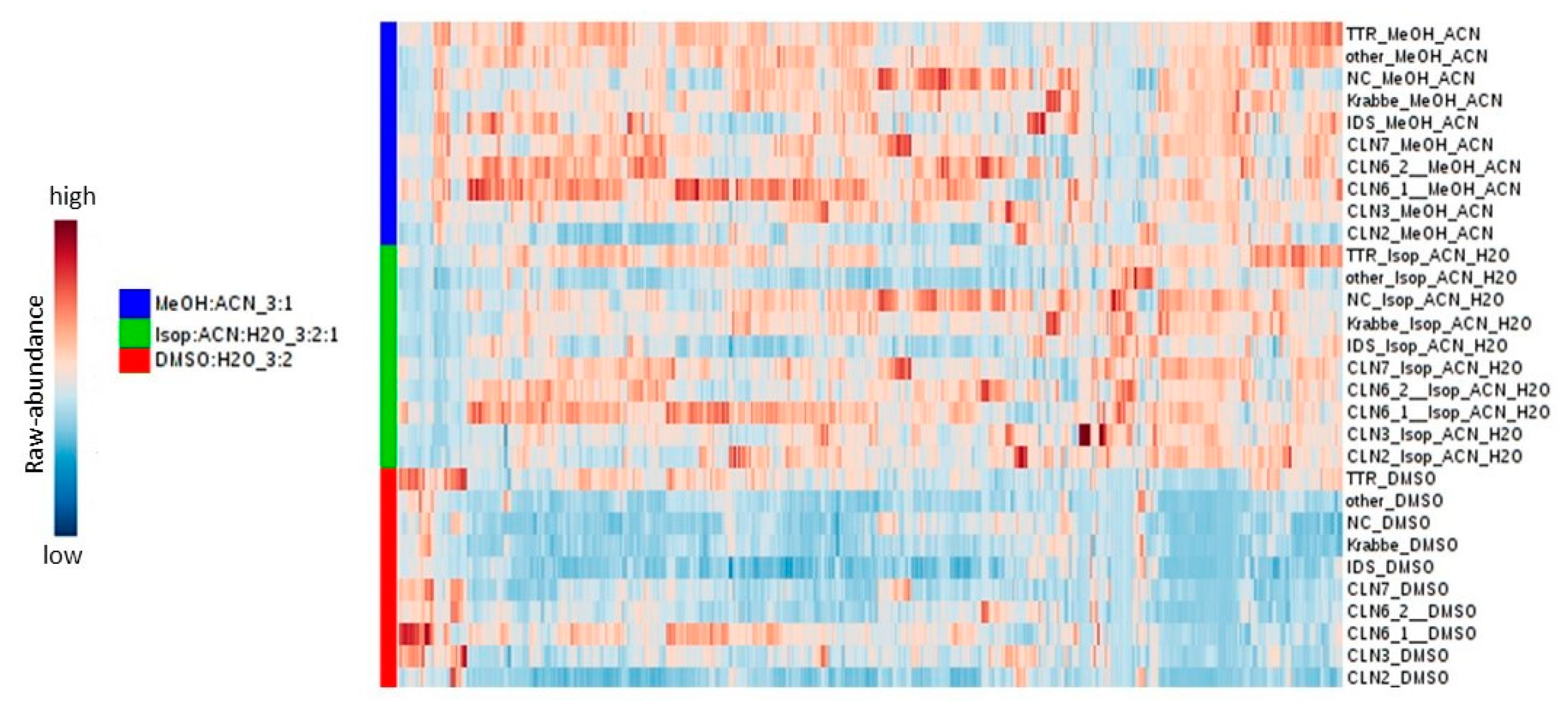

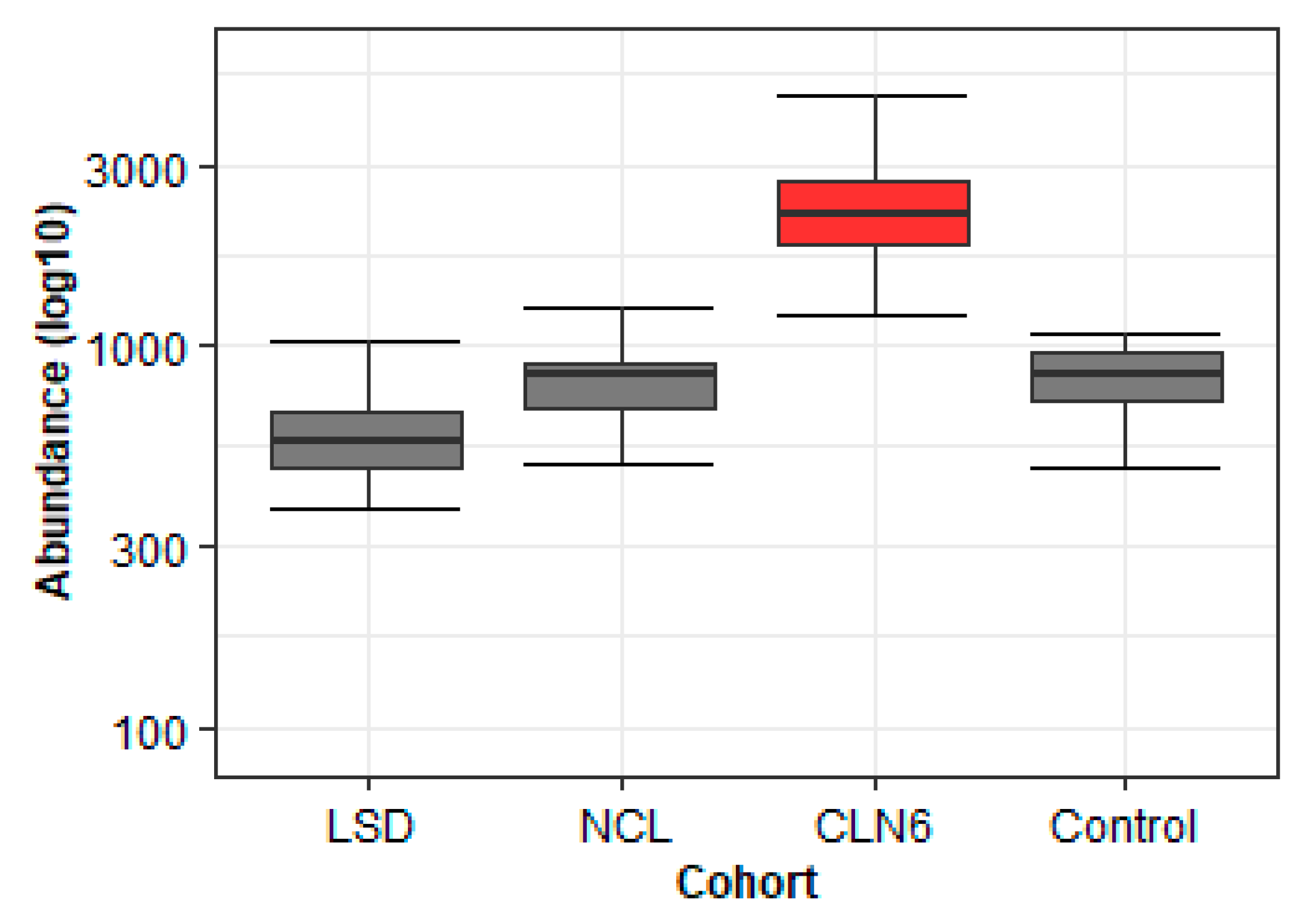
| (a) | |||||
| Extraction | Ratio v/v | Features Detected | Metabolites Abundance > 10.000 | Metabolome Coverage | Metabolites Abundance |
| dimethyl sulphoxide: water | 3:2 | 9867 | 16 | ++++ | + |
| isopropanol: acetonitrile: water | 3:3:2 | 9290 | 49 | +++ | ++ |
| methanol: acetonitrile | 3:1 | 7759 | 70 | ++ | +++ |
| ammonium acetate: water | 2 mM | 5970 | 95 | + | ++++ |
| (b) | |||||
| Methanol Mixtures | Ratio v/v | Features Detected | Metabolites Abundance > 10.000 | Metabolome Coverage | Metabolites Abundance |
| methanol: acetonitrile | 3:1 | 7759 | 70 | + | ++++ |
| methanol | 100% | 9120 | 39 | +++ | +++ |
| methanol: acetonitrile | 1:1 | 9964 | 29 | ++++ | ++ |
| methanol: water | 3:1 | 7833 | 25 | ++ | + |
| (1) Stability Study | (2) Extraction Study | (3) Cumulative Study | ||||||
|---|---|---|---|---|---|---|---|---|
| Subjects | Control | CLN6 | LSD | Control | LSD | Control | NCL | LSD |
| male/female | 3/3 | 2/8 | 6/5 | 10/11 | 4/7 | 10/11 | 19/30 | 10/15 |
| age (mean ± SD) | 36.5 | 6.5 | 15.8 | 32 | 6 | 33 | 6 | 6.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rus, C.-M.; Di Bucchianico, S.; Cozma, C.; Zimmermann, R.; Bauer, P. Dried Blood Spot (DBS) Methodology Study for Biomarker Discovery in Lysosomal Storage Disease (LSD). Metabolites 2021, 11, 382. https://doi.org/10.3390/metabo11060382
Rus C-M, Di Bucchianico S, Cozma C, Zimmermann R, Bauer P. Dried Blood Spot (DBS) Methodology Study for Biomarker Discovery in Lysosomal Storage Disease (LSD). Metabolites. 2021; 11(6):382. https://doi.org/10.3390/metabo11060382
Chicago/Turabian StyleRus, Corina-Marcela, Sebastiano Di Bucchianico, Claudia Cozma, Ralf Zimmermann, and Peter Bauer. 2021. "Dried Blood Spot (DBS) Methodology Study for Biomarker Discovery in Lysosomal Storage Disease (LSD)" Metabolites 11, no. 6: 382. https://doi.org/10.3390/metabo11060382
APA StyleRus, C.-M., Di Bucchianico, S., Cozma, C., Zimmermann, R., & Bauer, P. (2021). Dried Blood Spot (DBS) Methodology Study for Biomarker Discovery in Lysosomal Storage Disease (LSD). Metabolites, 11(6), 382. https://doi.org/10.3390/metabo11060382






