Abstract
Severe Acute Respiratory Syndrome (SARS) Coronavirus (CoV)-2 is a recently identified positive sense single-strand RNA (ssRNA) β-coronavirus. The viral spike proteins infect human hosts by binding to the cellular receptor angiotensin-converting enzyme 2 (ACE2). The infection causes a systemic illness involving cell metabolism. This widespread involvement is implicated in the pathophysiology of the illness which ranges from mild to severe, requiring multi organ support, ranging from oxygen supplementation to full cardiovascular and respiratory support. Patients with multiple co-existing comorbidities are also at a higher risk. The aim of this review is to explore the exact mechanisms by which COVID-19 affects patients systemically with a primary focus on the bleeding and thrombotic complications linked with the disease. Issues surrounding the thrombotic complications following administration of the ChAdOx1 nCoV-19 (Astra-Zeneca-Oxford) vaccine have also been illustrated. Risk stratification and treatment options in these patients should be tailored according to clinical severity with input from a multidisciplinary team.
1. SARS-CoV-2 Pathogenesis, Hemostatic Alterations and Metabolism Interference of Therapeutical Agents
1.1. SARS-CoV-2 Infection Strategy and Host Immune Response
Severe Acute Respiratory Syndrome (SARS) Coronavirus (CoV)-2 is a recently identified positive sense single-strand RNA (ssRNA) β-coronavirus. The viral spike proteins infect human hosts by binding to the cellular receptor angiotensin-converting enzyme 2 (ACE2) [1]. It is highly expressed on alveolar epithelial type 2 (AT2) cells, tubular epithelium of the kidney, cardiac myocytes, enterocytes, endothelial cells and other human tissues as well [2,3].
The consecutio temporum between transmission of SARS-CoV-2 infection and involvement of pulmonary, cardiac, endothelial, and blood cell metabolism assumes important relevance in preventing the devastating effects of the Covid-19 disease. After its transmission, which occurs primarily from inhalation of viral particles, the second stage involves localization in the cells of the respiratory tract [2]. Since the virus can survive for 24–72 h on surfaces, with differences depending on the type of surface and where it is located, viral transmission can be greatly amplified [4]. Infected people experience symptoms comparable to many viral infections with the onset of systemic spreading of COVID-19 leading to fever, fatigue, headache, cough, shortness of breath, diarrhea, and myalgia [5,6,7]. Covid-19 has the potential to cause severe damage to many tissues, including systemic inflammatory response syndrome (SIRS), acute respiratory disease syndrome (ARDS), multiorgan involvement, and shock [8]. One of the most feared complications is thromboembolism development which leads to severe clinical phenotypes: worsening of pulmonary conditions, oxygen desaturation, and acute respiratory distress. Older patients with multiple comorbidities, predominantly of cardiovascular interest, have a higher risk of developing severe disease compared to younger patients who, even if in good health conditions, are at risk of complications [9]. This trend has now been reversed by the presence of genetic variants due to the viral mutation which has increased its contagion capacity [10,11,12].
1.2. Alteration of Hematological Parameters from Clinical Experience of Thromboembolic Events
Laboratory tests performed on patients with Covid-19 have shown lymphopenia [5], mild thrombocytopenia and increased lactate dehydrogenase. In addition, the markers of inflammation such as C-reactive protein, D-dimer, ferritin, and interleukin-6 (IL-6) were altered [13]. High levels of IL-6 have been linked with disease severity and a procoagulant profile with an evolution towards more severe complications in frail patients [14].
Among patients with Covid-19 who required hospitalization due to their critical condtion, the most common haemostatic anomalies were mild thrombocytopenia [15] and increased levels of D-dimer [16]. Cui et al. noted that Covid-19 patients presenting with venous thromboembolism (VTE) had higher D-dimer levels compared to non-VTE patients. A large series of patients studied in the first phase of the SARS Cov 2 epidemic in China, who were hospitalized in severe clinical condition, showed higher D-dimer levels than those in whom the disease was less severe [2,6,13,17,18,19]. The evidence that D-dimer was a negative prognostic factor in non-survivors compared to survivors also emerged from other studies assessing patients hospitalized in intensive care units. Zhang L et al. [20] used a cutoff value of D-dimer of 2.0 μg/mL and reported a mortality rate of 0.37% for patients with values <2.0 μg/mL (1 of 267) compared to 17.9% for patients with values ≥2.0 μg/mL (12 of 67). However, using the same cutoff for D-dimer, there were differences in mortality rate with a lower range between survivors (10.4%) and non-survivors (18%) (<2.0 μg/mL; 8 out of 77 patients vs. ≥2.0 μg; 17 of 93) suggesting a potential selection bias [21].
Changes in blood coagulation status led to an increased risk of receiving mechanical ventilation, requiring hospitalization in an intensive care unit (ICU), or incurring death. A combination of a paucity of data alongside unclear and conflicting data supports the validity of other blood coagulation tests [22,23]. Evidence has shown that the variability in disease severity is due to a prolongation of prothrombin time (PT), a difficulty in normalizing the international normalized ratio (INR) [2,13,24] and the thrombin time (TT) [25]. Patients who remain in critical condition tend to maintain a reduced activated partial thromboplastin time (aPTT) [2,6,9] (Table 1).

Table 1.
2020 case-control retrospective studies comparing risk factors for thrombosis development in hospitalized patients with severe Covid-19 (controls) versus hospitalized patients with both severe infection and DVT or ATE (cases). VTE: venous thromboembolism, ATE: arterial thromboembolism, WBCs: white blood cells, INR: international normalized ratio, aPTT: activated partial thromboplastin time, CRP: C reactive protein, ICU: intensive care unit, CTPA: CT pulmonary angiography, IL-6: interleukin-6, DVT: deep vein thrombosis, IMV: invasive mechanical ventilation.
Evidence suggesting a role for platelets in viral infections has long been proven. For example, the presence of influenza virus type A (IAV) particles was noted in the platelets of patients with acute influenza infection. Once the IAV was incorporated into the platelets, TLR7-dependent C3 was released with the subsequent activation of neutrophils and neutrophil extracellular traps (NETs) release [34]. Platelets play an essential role in maintaining vascular integrity but can trigger thrombogenic mechanisms. More recently, the platelets role in viral infections has been studied highlighting its active participation in the host’s immune response [35]. The enormous amount of data available on the pathophysiological mechanisms that support SARS-CoV-2 infection have clearly shown that during viral infections, the risk of thrombosis is higher. A recent review discussed the potential role of platelets in thrombosis from COVID-19 infection. It confirmed the Chinese study investigating the close correlation between thrombocytopenia and risk of in-hospital mortality [36,37].
1.3. Interference of Antiviral Drugs with Antiplatelet and Anticoagulant Medications
The optimal therapy to be recommended for patients with severe Covid-19 disease has been tested and several drugs have been used to prevent the progression of the disease. The interactions that some of these drugs have with antiplatelet and anticoagulant agents have been clinically documented and considered important. The administration of experimental drugs has often been associated with excessive risk, as well as a reduced risk, of thrombotic events or thrombocytopenia in previous studies in non-Covid-19 populations and more recently in patients with Covid-19.
Bevacizumab has been associated with the occurrence of cardiovascular adverse events including MI, stroke and VTE. Bevacizumab is a monoclonal antibody that interferes with vascular endothelial growth factor (VEGF) and is being investigated for COVID-19 [38,39]. Another substance being tested is fingolimod. It is an immunomodulating agent that has been tested in patients with Covid-19 and is able to reduce reperfusion damage after neurological complications from stroke by improving outcomes [40].
Hydroxychloroquine, which has been the subject of open debates supported by opposing points of view, has since obtained FDA (food and drug administration) clearance for the treatment of COVID-19; however, it can potentially exert the antithrombotic action through interference with antiphospholipid antibodies [41].
In this last year, researchers have directed a big commitment towards the study of drugs for the treatment of COVID-19 that may have interfered with oral antiplatelet drugs in oxidative processes at the level of hepatic cytochrome P450 (CYP) enzymes. The consequence is that the action of many antiviral drugs affects platelet metabolism. For example, the administration of lopinavir or ritonavir, protease inhibitors acting on CYP3A4 metabolism, may result in the inhibition of platelet activity at the level of CYP3A4 metabolism. As the active metabolite of clopidogrel is formed mainly by oxidation in CYP2C19, it leads to a reduction in the effective dose of clopidogrel. Instead, the oxidative process leading to the active ticagrelor metabolite occurs in CYP3A4 which is inhibited by lopinavir and ritonavir. The result is an increase in the effect of ticagrelor [42,43,44,45] (Figure 1).
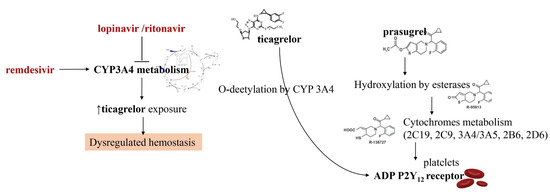
Figure 1.
Interaction between antiviral agents and antiplatelet drugs on CYP3A4 metabolism. In red, antiviral agents are depicted. Lopinavir and ritonavir exert an inhibitory action on the cytochrome. This increases the exposure of ticagrelor leading to a dysregulation of hemostasis (highlighted in the picture being it the only depicted potential effect). Remdesivir is instead an inducer of CYP3A4 function. Differently from ticagrelor, prasugrel is metabolized by several cytochromes (2C19, 2C9, 3A4/3A5, 2B6, 2D6), thus its effects seem to be unmodified by ritonavir or lopinavir interaction. CYP3A4: Cytochrome P450 3A4, ADP P2Y12 receptor: adenosine 5′diphosphate P2Y12 receptor.
The direct consequence of the combined use of these drugs is dysregulation of the haemostatic process which must be properly evaluated. Although the solution to this drawback is the use of P2Y12 platelet function tests to guide the use of clopidogrel or ticagrelor, there is limited clinical data available to support this strategy. A drug that is not affected by the action of lopinavir/ritonavir is prasugrel which may be a viable option in the absence of contraindications [42,43,44,45]. The effects of remdesivir appear to be different. This antiviral, which is a nucleotide analog inhibitor of RNA-dependent RNA polymerase, induces the function of CYP3A4. This may cause enzyme induction necessitating dose adjustments for oral antiplatelet drug agents that are currently not recommended. It is important to point out that significant drug interactions between different investigational therapies for Covid-19 and parenteral antiplatelet drugs such as cangrelor and glycoprotein IIb/IIIa inhibitors have not been reported.
We are aware of interactions between experimental drugs used for COVID-19 and therapies with oral anticoagulants administered for various cardiovascular diseases. Ritonavir use has also been shown to affect the choice and dosage of many anticoagulant agents. Careful surveillance after administration with vitamin K antagonists (VKAs), or apixaban and betrixaban, is required to make a dosage adjustment. The combination of drugs such as edoxaban and rivaroxaban and lopinavir/ritonavir administered contemporary is not recommended [46]. Although administration of tocilizumab which inhibits IL-6, increases the expression of CYP3A4; however, specific indications are lacking to adjust anticoagulant therapy when this anti-inflammatory is pharmacologically coupled in the treatment of Covid-19. To the best of our knowledge, we cannot affirm the evidence of major pharmacological interferences between the experimental COVID-19 therapies and the administration of anticoagulants for parenteral access.
2. Venous Thromboembolism Diagnosed in Covid-19 Patients and Management
2.1. Clinical Diagnoses of Venous Thromboembolism
We are aware of a small number of studies that have reported the incidence of VTE in patients with Covid-19 [47,48]. A Chinese retrospective study worked in this direction by reporting a percentage of 25% (20 out of 81) of patients admitted to the ICU in whom an accident of VTE occurred. Of note, none of the patients had been managed with the use of VTE prophylaxis drugs [49]. Klok et al. [50], in a multicenter study that included 184 patients with severe Covid-19, recorded the percentage of 31% (95% confidence interval: 20% to 41%) of patients who developed an accident of VTE. All patients had been treated with VTE prophylaxis drugs, although the authors noted underdosing in 2 of the 3 recruiting centers (81). It cannot be ruled out that VTE may go undiagnosed and unrecognized in patients with severe Covid-19. This is an important aspect during the clinical evolution of Covid-19, as ARDS in those patients is potentially the cause of a vicious circle involving hypoxia, pulmonary vasoconstriction, pulmonary hypertension and right ventricular failure. The onset of pulmonary embolism is an additional clinical event that often cannot be resolved.
2.2. Medical Treatment in the Acute Setting and on Discharge
The use of drugs for systemic anticoagulation has been a crucial point for the treatment of VTE. The choice of the most suitable drug requires clinical considerations factoring in comorbidities and possible impairment of renal or hepatic function, haematological disorders such as thrombocytopenia or that of the gastrointestinal system. Multiple therapies may be considered including a change in the anticoagulant pharmacological treatment in progress during the hospitalization period, due to the critical state at hand to a more suitable regiment at the time of discharge to adapt to the convalescence period.
This pharmacological management used in critically ill hospitalized patients with VTE, in whom the administration of parenteral anticoagulants such as, for example, UFH may be preferred because it has good pharmacodynamic and pharmacokinetic requirements without known drug interactions with experimental Covid-19 therapies. It is important to underline that the effects of the therapy are obtained after a longer period with the administration of UFH (unfractionated heparin) because they depend on the achievement of a therapeutic aPTT. In addition, healthcare workers are more exposed to the risk of contamination due to frequent blood draws. Therefore, these aspects argue for the preferential choice in the administration of LMWH in patients in whom invasive procedures are not planned.
The use of oral anticoagulation with DOAC is certainly advantageous because it does not involve the need for monitoring, easing the planning of discharge, and for the outpatient management of the patient. It is possible that the worsening of the patient’s clinical condition including, for example, the deterioration of the respiratory function in patients with Covid-19, may necessitate a switch between these classes of medications. Patients who are expected to be discharged are advised to use DOAC or LMWH to limit contact with healthcare personnel because controls for INR monitoring as for vitamin K antagonists (VKA) are not required.
2.3. Pulmonary Embolism in Covid-19 Patients: Stratification and Choice of Therapy
The vast majority of patients with Covid-19 and symptoms referable to acute DVT should be treated at home with anticoagulant therapy whenever possible. However, there are a number of patients for whom hospitalization is necessary to initiate acute endovascular techniques such as local fibrinolysis or embolectomy and patients with refractory symptoms [51]. Patients at medium and high risk for VTE may need the input of a multidisciplinary team to manage their PE [52,53,54,55]. The available data are limited but report a lower mortality with the use of advanced therapies for the treatment of VTE. The caveat here is the limited data which marginally demonstrates lower mortality from routine use of advanced VTE therapies. Therefore, severe respiratory failure sustained by a PE treated with the use of catheter-guided therapies during the pandemic should be limited to the most critical patients. Likewise, the indiscriminate use of filters positioned in the inferior vena cava should be avoided [56]. The placement of a filter is recommended in restricted cases for patients experiencing recurrent PE despite optimal anticoagulant treatment or with clinically significant VTE in the context of absolute contraindications to anticoagulant therapy [57]. IVC filters do not exonerate patients from the use of anticoagulation which must be restored immediately, with the dosage of the drug gradually increased whilst considering the potential to bleed. Patients with intermediate-risk and hemodynamically stable [52,58,59,60] should be closely monitored and managed with anticoagulant therapy.
Rescue systemic fibrinolysis should be considered in patients with progressive deterioration, either systemically or by transcatheter approach. Instead, for patients with evident haemodynamic instability and high-risk PE [52,58,59,60] systemic fibrinolysis is indicated with two possible options, a percutaneous catheter approach or if this is contraindicated, systemic fibrinolysis.
2.4. Management of Antithrombotic Drugs in Patients with SARS-CoV-2 Infection and Critical Illness
Patients with severe SARSCoV-2 infection and critical illness have a higher risk of VTE. Many factors contribute to the development of VTE which is often associated with worsening clinical condition and rapid deterioration of the patient. First, they have haemostatic imbalance coupled with a systemic inflammatory state and are forced into long immobility because they are assisted with mechanical ventilation. Second, the placement of central venous catheters contributes to increasing the risk of VTE in the ICU and infections [61,62,63]. Third, nutritional shortage and liver dysfunction can interfere with the production of clotting factors [64]. A modification of the pharmacokinetics is to be considered in critically ill patients who require dosage adjustment of anticoagulant drugs [65], caused by factors related to the absorption, metabolism, and renal or hepatic elimination of these drugs to be considered with the possible presence of organ dysfunction.
Administration of anticoagulant therapy by systemic parenteral infusion is recommended in majority of patients whereby Covid-19 with acute thrombosis occurs. In these cases, the use of UFH can be used based on expected protocols, or in patients with deterioration of renal function. In the absence of an application for an urgent procedure, the use of LMWH is a reasonable alternative [66]. Anticoagulation is of particular importance in patients undergoing ECMO (extracorporeal membrane oxygenation) for whom continuous monitoring of anticoagulant drugs is required to maintain the patency of the circuit especially when hemodynamic stability is ensured by lower blood flow.
Available data are scarce to establish complication rates in SARS-CoV-2 patients, but thrombosis and haemorrhage rates can be high reaching the rate of 53% and 16% respectively as reported in other populations with respiratory failure [67]. Even more limited are the data from the surveillance of ECMO patients in critical condition for SARSCoV-2 infection. Two studies reported results with very high mortality which was five out of six patients in one series and three out of three in another [13,24]. To date concerns related to recommend target anticoagulation therapy for critically patients with overt disease from severe SARS Cov 2 infection requiring ECMO exists due to lack of insufficient data [68].
2.5. Risk Stratification Scores to Drive Pharmacological Prophylactic Treatment
Patients with Covid-19 and a lung infection who required hospitalization following clinical complications have an increased risk of VTE [52,69]. The use of prophylactic anticoagulation and the dosage are recommended by current guidelines and the position papers of professional societies, predominantly based on large randomized clinical trials that have reported a benefit concerning outcomes after hospitalization [52,70,71,72].
Several studies have reported that early initiation of appropriate prophylactic anticoagulation reduces the risk of VTE in critically ill patients [73,74,75]. The Caprini score, the International Registry of Medical Prevention on Venous Thromboembolism (IMPROVE) model and the Padua model are risk stratification tools useful for VTE risk assessment [76,77,78,79,80,81]. For example, the use of the Padua model was reported by Wang et al. highlighting that 40% of patients admitted to hospital for Covid-19 and in critical condition were at high risk of VTE. However, the study lacks data on the use either of prophylaxis for VTE or about the incidence of VTE [82]. VTE drug prophylaxis is indicated for patients admitted to the hospital for COVID-19 who have respiratory failure or comorbidities such as active cancer and heart failure [83]. This therapy should also be extended to patients with prolonged bed rest and who require a prolonged admission to the intensive care unit unless patients have specific contraindications.
In January 2020 a WHO report recommended interim guidance for daily prophylaxis with low molecular weight heparin (LMWH) or unfractionated subcutaneous heparin (UFH) to be administered twice daily [84]. In patients forced into prolonged immobilization, in whom prophylactic pharmacological treatment is contraindicated, the use of mechanical prophylaxis with intermittent pneumatic compression of the VTE should be considered [84,85]. The administration of pharmacological prophylaxis for VTE should be very rigorous to avoid episodes of failed doses which has been a common occurrence leading to worse outcomes [86]. Pregnant patients with Covid-19 deserve particular consideration and must be meticulously evaluated. This population of individuals have a higher risk of VTE that also extends to the postpartum period [87,88]. To date, there are insufficient data to draw definitive conclusions on pregnant women who have been admitted to hospital with Covid-19 even if a greater risk of VTE is conceivable. VTE risk stratification for this patient population helps consider the use of pharmacological thromboprophylaxis, especially if they have other risk factors for VTE. Further research is needed to establish the appropriate prophylactic dosage of anticoagulants based on the weight of pregnant patients [89].
It is important to point out that the problem of extended prophylaxis with LMWH [90] or direct oral anticoagulants (DOAC) involves patients once discharged from the hospital after the resolution of the acute episode of Covid-19 [91,92,93,94]. In discharged patients, although the risk of VTE is reduced, the occurrence of bleeding events is increased [95,96]. There is no detailed information that clarifies this aspect of Covid patient management. However, it would be useful to employ individualized risk stratification models for thrombotic and haemorrhagic episodes when considering prolonged, 45-day prophylactic anticoagulant therapy. The latter is indicated in discharged patients with a high risk of VTE, with reduced mobility or with active neoplastic disease, who may have a high D-dimer > 2 times the upper limit, but who are at low risk of bleeding [93,97,98].
3. Hypotheses of Thrombosis Generation and Pathophysiological Mechanisms
3.1. Covid-19 and Disseminated Intravascular Coagulation
Patients with clinically critical Covid-19 have a greater chance of developing disseminated intravascular coagulation (DIC) [99,100] which is common to many diseases that evolve with severity [101]. It has not yet been demonstrated whether Covid-19 can lead to the development of a DIC with mechanisms intrinsic to SARS Cov 2 that cause direct activation of coagulation cascades. The hypercoagulable state caused by DIC leads to an activation of the tissue factor pathway with consequent platelet consumption and a consistent bleeding diathesis. It is characterized by raised fibrin degradation products (FDPs) which are commonly elevated in Covid-19 patients suffering from thrombosis.
The ISTH DIC score calculator is commonly used to establish the diagnosis of DIC [102]. The worsening of blood coagulation in patients with a critical picture of Covid-19 is carried out by monitoring platelet counts, PT, D-dimer, and fibrinogen. The continuous monitoring of the parameters listed above is the first step for correct identification of the DIC and starting its management. The appearance of bacterial superinfections is not uncommon and must be promptly treated with aggressive antibiotic therapy. The sudden prophylaxis with LMWH can decrease the formation of thrombin and reduce its consumption, to be useful in modulating the evolution of DIC. Preliminary results are based on limited data which, however, have shown an effective benefit with the prophylactic administration of LMWH [93,96]. Regarding the discontinuation of long-acting single or dual antiplatelet therapy (DAPT), it is recommended in most patients when the diagnosis of DIC is suspected, administration of antiplatelet medications in patients who have suffered from a recent ACS or who have implanted stents are left alone. For patients with a moderate or severe clinical condition of COVID-19 in whom dual antiplatelet therapy is indicated because they have received PCI in the past 3 months or have suffered from recent MI, even in the presence of a suspected or confirmed diagnosis of DIC but without noticeable bleeding, therapy must be individually tailored. In the absence of strong evidence confirming a DIC it is reasonable to continue the DAPT, if the patient has a platelet count of at least 50,000. The administration of a single antiplatelet drug is indicated when the platelet count is between 25,000 and 50,000 while an interruption of antiplatelet therapy is recommended for a platelet count of less than 25,000. However, rigidity in the application of these indications is not necessary and therapy must be adapted according to the patient’s condition, increasing or decreasing the antiplatelet dose and paying close attention to the possible onset of thrombotic complications or bleeding. Parenteral systemic fibrinolysis with the consequent elimination of thrombi scattered in the organs favors the resolution of DIC.
Patients with Covid-19 rarely suffer from clinically evident bleeding. However, significant blood loss is common in patients who develop DIC often when septic coagulopathy is associated [103]. If bleeding occurs, treatment consists of transfusion of blood products.
First, the use of platelet concentrate is useful in patients who have DIC with active bleeding to maintain platelet counts greater than 50 × 109/L or in patients who have a high risk of bleeding because they require more invasive procedures to maintain a platelet count greater than 20 × 109/L. Second, the administration of fresh frozen plasma (15 to 25 mL/kg) in patients who experience active bleeding and whose PT or aPTT values are altered (PTT> 1.5 times normal) or who have a decrease in fibrinogen (<1.5 g/L). Third, the administration of concentrated fibrinogen or cryoprecipitate for patients in whom the values of fibrinogen are low and persistent over time (<1.5 g/L) and the administration of prothrombin complex concentrate where transfusion of fresh frozen plasma is not possible. There are currently no indications for the routine use of tranexamic acid in patients with DIC associated with COVID-19 [104].
3.2. Direct Viral Damage and Endothelitis-Driven Inflammatory Reaction
Concerns about the triggering of changes in the hemostatic process may be also related to the direct effect of SARS-CoV-2 viral particles. Varga et al. demonstrated the presence of viral elements within endothelial cells due to the expression of ACE2 receptors. Also, inflammatory cells were demonstrated, corroborating evidence of a direct viral involvement towards endothelitis development, also noted in several organs [105].
Preclinical analyses have also demonstrated the successful infection of organoids by viruses confirming their tropism for endothelial cells. Investigators used clinical-grade soluble human ACE2 to achieve the infection [106,107]. Endothelial damage further contributes to inflammatory cell infiltrate in the lungs worsening the respiratory parameters [108].
3.3. Covid-19-Associated Hyperinflammatory Syndrome (cHIS)
A further hypothesis for thrombosis generation is related to cytokine storm precipitating an initially controlled inflammation in the more complicated SIRS [101], as emerged from the observation of similar cases of individuals infected with other viral diseases such as HIV, Zika, Chikungunya, and Ebola [109,110,111].
As a consequence of dysregulated immune reactions, hypercoagulable states are established due to continued inflammation. Macrophages activation syndrome (MAS) and cytokines released play major roles by activating a pro-inflammatory cascade [112,113] (Figure 2).
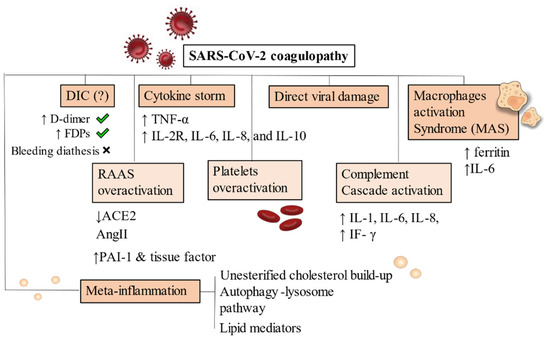
Figure 2.
Pathophysiology of SARS-CoV-2 coagulopathy. DIC has been frequently noticed in Covid-19 severe patients but bleeding diathesis was a less present feature. Cytokine storm and inflammatory-driven thrombogenesis is the most known hypothesis, due to IL-2R, IL-6, IL-8, and IL-10 cascade generation. A direct viral damage has also been acknowledged to start endothelitis and endothelial damage. MAS is instead an added mechanism present on an already compromised immune condition where hyperferritinaemia and increase in IL-6 production are pathognomonic of macrophages overactivation. RAAS system, complement and platelets also drive uncontrollable responses by generating hypercytokinemia and dysregulating fibrinolysis. Increased levels of PAI-1 and tissue factor have been demonstrated. Meta-inflammation is another possible trigger for coagulopathy. Obesity, hyperinsulinemia and metabolic syndrome are strong risk factors for severity of infection in hospitalized patients. Primary conditions developing after viral infection are depicted with a darker background. RAAS overactivation, platelets and complement activation are mainly secondary mechanisms, thus they appear in a lighter background. Abbreviations: DIC: disseminated intravascular coagulation, FDPs: fibrin degradation products, ACE: angiotensin converting enzyme, AngII: angiotensin II, PAI-1: plasminogen activator inhibitor, IF-γ: interferon- γ, TNF-α: tumor necrosis factor-α, IL-6, IL-8, IL-10: interleukin 6, interleukin 8, interleukin 10. IL-2R: interleukin 2 receptor.
Recently, Webb et al. [114] proposed several criteria to diagnose the Covid-19-associated hyperinflammatory syndrome (cHIS) which has been associated with coagulopathy and hypercytokinemia leading, among the other complications, to mechanical ventilation and death in most severe cases. Criteria for the diagnosis have not been established at the time of writing. Thus, the authors proposed six of them: fever, hyperferritinaemia from macrophages activation syndrome, haematological dysfunction (N/L, neutrophil to lymphocyte ratio), hepatic injury (lactate dehydrogenase or aspartate aminotransferase), D-dimer to diagnose coagulopathy and cytokinaemia (C-reactive protein, interleukin-6, or triglycerides levels).
4. Inflammatory Cascade: The Bridge between Metabolic Derangements and Thrombogenesis
The Immunologic Dialogue of Cytokines, T Cells, and Checkpoint Proteins Accelerating Thrombosis in Atherosclerotic Lesions
Endothelial dysfunction, which is the first recognizable step of Covid-19 thrombogenesis from an inflammatory cause, is first seen in atherosclerosis development. Metabolic derangements play main roles as atherogenic stimuli such as dyslipidemias, hypertension, and obesity which stimulate a series of changes in lesion-prone vascular endothelium, towards a vasoconstrictor, prothrombotic, proliferative, inflammatory phenotype. Pro-inflammatory cytokines, oxidized lipoproteins (ox-LDL), and advanced glycation end products (AGE), as well as disturbed blood flow associated with reciprocating, low shear stress [115] lead to endothelial activation (EA). These signals transduce mainly via the pleiotropic transcription factor TF nuclear factor-κB (NF-κB), resulting in a coordinated program of genetic regulation within the endothelial cell, among which chemokines, surface expression of adhesion molecules (e.g., vascular cell adhesion molecule-1 [VCAM-1), and prothrombotic agents such as tissue factor, von Willebrand Factor and plasminogen activator inhibitor (PAI-1) [116,117].
According to the Virmani classification, the intimal xanthoma is the initial detectable small lesion constituted by foam cells with intracellular- and not extracellular- lipid accumulation.
The endothelial expression of adhesion molecules VCAM-1 and ICAM-1 drives leukocytes and platelet recruitment. Also, smooth muscle cells coming from the tunica media, migrate to the plaque attracted by the PDGF stimulus and start to produce other inflammatory cytokines, such as IL-1 and IL-6.
An interesting role is one of the activated T cells, mainly CD4+: the recognition of proteic antigens presented by macrophages on MHC type II molecules triggers a response for which Th1 cells produce IFN-ɣ, in turn activating macrophages and overly stimulating the synthesis of pro-atherogenic inflammatory cytokines. In this process, costimulatory and coinhibitory receptors on T cells direct T-cell function and determine T-cell fate. These co-signaling molecules are divided into pro-atherogenic and anti-atherogenic ones. It is known, in fact, at least in the pre-clinical setting, that CTLA-4 blocking accelerates atherosclerosis development and decreases luminal patency [118,119].
The evolution of the plaque sequentially favours the formation of pathological intimal thickening (PIT) lesions consisting of macrophage foam cells and small extracellular lipid pools. The lesions eventually progress to fibrous cap atheroma (FCA) presenting with a fibro-adipous cap and necrotic cores from extracellular lipids, cholesterol crystals, and calcifications due to the accumulation of osteocalcin, osteopontin, and Bone Morphogenetic Protein (BMP). The last stages of atherogenesis involve mainly complicated lesions with fissuration and erosion resulting in thrombus which provokes a critical reduction of blood flow, thus occluding the vessel.
During plaque progression, regulators of immune cells divide into pro-atherogenic immune checkpoint proteins CD28–CD80/86, OX40–OX40L, CD137–CD137L and CD30–CD30L and anti-atherogenic ones CTLA-4–CD80/CD86, PD-1–PD-L1/2, ICOS–ICOSL, GITR–GITRL, CD27–CD70 and TIM proteins [120]. CTLA-4 is mainly expressed on Tregs and activated CD4+ and CD8+ T-cells but also on monocytes and activated B cells. Their anti-atherogenic role has been demonstrated by preclinical and clinical studies, without a clear elucidation of the mechanism of pathogenesis.
5. Meta-Inflammation: Alterations of Metabolism Leading to Thrombus Generation
5.1. Obesity, Metabolic Syndrome and Dysregulated Lipid Metabolism
Obesity, metabolic syndrome and type 2 diabetes mellitus (T2DM) are among the best-known risk factors for both thrombosis and severe Covid-19 infection since they promote a state of chronic inflammation and impaired fibrinolysis. Meta-inflammation, i.e., chronic inflammation driven by chronically altered metabolic pathways presents with increased release of cytokines from adipocytes. Consequently, a contribution comes from macrophages (recruited in inflamed sites) and transient hypoxia which is proper of visceral fat rather than subcutaneous one.
Macrophages in particular, extend the secretion of cytokines ensuring the vascular endothelium is stimulated towards a pro-thrombotic state with upregulation of adhesion molecules and downregulation of coagulation inhibitory proteins.
Consequently, thrombin generation is increased, platelets are further activated and TNF-α and IL-6 increase the expression of tissue factor, promoting the hypercoagulable state.
Chronic inflammation is also associated with dysregulation of endogenous anticoagulant mechanisms, including tissue factor pathway inhibitor, antithrombin, and the protein C anticoagulation system.
The second important mechanism is impaired fibrinolysis. Expression of PAI-1 is markedly upregulated in visceral adipose tissue and elevated levels of PAI-1, together with tissue plasminogen activator (tPA), have been found in severe Covid-19 patients [121]. 118 hospitalized patients and 30 healthy controls were evaluated through laboratory, imaging, and clot-lysis assays. Elevated levels of those markers were associated with worse respiratory status, enhancement of ex-vivo clot lysis, and mortality. Also, strong correlations were demonstrated with neutrophil counts and circulating calprotectin, a neutrophil activation marker. Plasma levels of PAI-1 are also elevated in patients with obesity or metabolic syndrome. TNF-α is one of the key regulators of PAI-1 expression since it acts by stimulating its expression [122]. This mechanism suggests a clear link between antifibrinolytic activity and obesity-associated chronic inflammatory state.
Recent meta-analyses and case-control studies underlined the association between obesity and severe COVID-19. Also, they showed that obese patients had worse prognoses than non-obese patients (OR, 2.31; 95% CI, 1.3–4.12) [123]. In general, dyslipidemias were also associated with severe COVID-19 (relative risk (RR), 1.39; 95% CI, 1.03–1.87; p = 0.03) [124].
5.2. Hyperinsulinemia Contributes to Both Impaired Fibrinolysis and Hypercoagulable States
As a causative factor, hyperinsulinemia is related to all the metabolic conditions associated with poor outcomes from Covid-19 infection. Again, the primary mechanism is impaired fibrinolysis, due to increased PAI-1 expression [125]. It, therefore, produces a decrease in plasminogen activator activity.
The impairment of fibrinolysis by insulin is completely independent of glycemia while hyperglycemia produces effects on coagulation which is not related to insulinemia. Impaired fibrinolysis is consistently found in T2DM patients [126] (Figure 3).
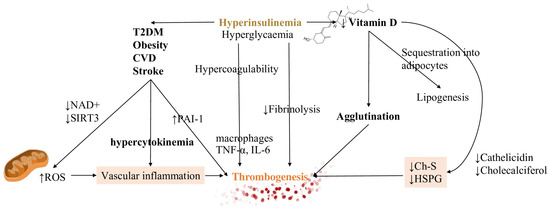
Figure 3.
Hyperinsulinemia, CVD and vitamin D have a strong impact on homeostatic equilibrium. Both hyperinsulinemia (depicted in bold and colored font, in order to emphasize it) and hyperglycemia generate states of increased coagulation and decreased fibrinolysis. By driving the development of CVD, diabetes mellitus and obesity, they contribute to the inflammatory substrate of cytokines. They increase the ROS production due to the damage in decreasing both NAD+ and reduced glutathione (GSH). A reduction in vitamin D, due to sequestration into the adipocytes, leads to decreased levels of ChS and HSPG, regulators of RBCs deformation, increasing cells agglutination. These mechanisms are all responsible for thrombosis initiation. Vascular inflammation and decreasing levels of Ch-S and HSPG are represented with colored backgrounds being the main actors of thrombogenesis trigger. Also, thrombogenesis, the main effect, is outlined with a different color too. Abbreviations: Ch-S: cholesterol sulfate, HSPG: heparan sulfate proteoglycans, NAD+: nicotinamide adenine dinucleotide, PAI-1 plasminogen activator inhibitor type 1, ROS: reactive oxygen species, T2DM: type 2 diabetes mellitus, SIRT3: sirtuin 3, TNF-α: tumor necrosis factor-α, IL-6: interleukin 6.
Hyperglycemia instead stimulates clotting factors synthesis from the liver and inflammatory reaction by increasing the secretion of IL-6. Biochemical effects of hyperinsulinemia are several and entail β-oxidation and ketolysis inhibition [127].
Due to the major depletion of nicotinamide adenine nucleotides (NAD+) by glucose oxidation rather than beta-oxidation and ketolysis, NAD+ availability for mitochondrial deacetylase sirtuin 3 (SIRT3) activity decreases [121]. This process, which is NAD+ dependent, normally increases the production of NADPH reduce oxidised glutathione (GSSG) to reduced glutathione (GSH) [128]. Therefore, the final result is that insulin increases mitochondrial production of reactive oxygen species (ROS), also via generation of ceramides [129]. ROS production is then responsible for further vascular inflammation. (Figure 2).
5.3. Vitamin D Metabolism and Its Interference in the Inflammatory Pathway
Vitamin D hydroxylation requires magnesium which is notably depleted in hyperinsulinemic conditions. Other mechanisms responsible for decreased Vitamin D levels are increased renal excretion, reduced intracellular levels, and sequestration into adipocytes. The last process is related to lipogenesis which is already increased in hyperinsulinemic patients [128]. Consequences of reduced activation are decreased levels of cholesterol sulfate (Ch-S), heparan sulfate proteoglycans (HSPG), and cathelicidin synthesis. The final result is the promotion of agglutination and, subsequently, of thrombosis. An explanation for this can be found in the action of HSPGs as potent anticoagulant molecules which also buffer glycation damage. Ch-S is instead implicated in RBCs shape deformation for traveling through vascular spaces of reduced caliber [128,129,130].
5.4. Extrahepatic Vitamin K Insufficiency and Dependency of Coagulation Factors
Evidence of altered laboratory values of vitamin K have been demonstrated in severe Covid-19 cases. Other than coagulation factors, several other molecules, among which matrix Gla protein (MGP), a potent inhibitor of soft tissue calcification and elastic fibers degradation, are dependent on vitamin K.
The proposed mechanism by Janssen et al. [131], implicates that following elastic fibers degradation due to SARS-CoV-2 proteolysis, partially degraded elastic fibers show increased polarity which drives an increase in the calcium content of elastic fibers.
With an increase in MGP synthesis, vitamin K is utilized for further molecule processing (MGP carboxylation) which decreases circulating vitamin K levels and increases damage to lung tissues. As a consequence, endothelial protein S is not sufficiently carboxylated and shifts the coagulation equilibrium towards thrombogenesis [132].
An important proof that this mechanism may be deranged in Covid-19 thromboembolic events, is that diabetes, hypertension, and CVD, which are correlated with worse outcomes, are conditions related to chronic elastic fibers pathology.
5.5. Hormonal Factors Contributing to Covid-19 Thrombotic Complications
Gender differences in Covid-19 outcomes and cardiovascular diseases have raised many issues regarding the hormonal asset of patients and its interference with coagulopathy development.
Endothelial dysfunction is found in the elderly, smokers, and patients with metabolic alterations such as dyslipidemias, T2DM and arterial hypertension [133,134].
An interesting mechanism is the presence of receptors on megakaryocytes and platelet membranes for both estrogen and androgens. Also, testosterone has been observed to increase the secretion of endothelial nitric oxide eNO, a potent inhibitor of platelet activation [135,136].
The function of estrogens, in women, is also directed to regulate and enhance platelet function according to the ovarian cycle release of hormones. On the contrary, lack of testosterone i.e., hypogonadism in the elderly produces a less protective effect on hypercoagulation [137]. Consequently, this aspect should be taken into account when evaluating older patients for possible thrombosis.
7. Future Directions and Perspectives
Despite the efforts research has made in the past year to formulate optimal therapies for the treatment of SARS-CoV-2 infection, the results have been disappointing in both patients with mild COVID-19 and those hospitalized in the critical phase of the disease. Two very recent RCTs depict a frustrating scenario.
The use of ivermectin for treatment was evaluated in a very recent RCT [157]. Mildly symptomatic Covid-19 patients receiving 5 days of ivermectin therapy compared to placebo population did not experience significant improvements in symptom resolution time. Although the results of using ivermectin have not shown a treatment benefit in recipients with mild Covid-19, larger studies may be needed to understand the effects of ivermectin on candidates with more severe forms of the disease to evaluate whether the drug has clinically relevant effects [156].
Thrombotic events are commonly reported in critically ill patients with Covid-19. As previously illustrated, there is limited data to guide antithrombotic prophylaxis. In the INSPIRATION investigation trial [158], authors studied patients for a 30-day follow-up. One population of patients received intermediate-dose (enoxaparin, 1 mg/kg daily) (n = 276) and was compared to those who were managed with standard prophylactic anticoagulation (enoxaparin, 40 mg daily) (n = 286). The results did not record a significant difference considering the primary outcome set on composite of venous or arterial thrombosis, on treatment with extracorporeal membrane oxygenation, or mortality within 30 days among patients admitted to intensive care with COVID-19 between the groups. Regarding unselected patients admitted to intensive care with COVID-19, the evidence does not support the routine empirical use of prophylactic anticoagulation at intermediate doses [158].
Another concern has been for many critically ill hospitalized patients who required immediate surgery [151,152,155]. and in which Sars-Cov-2 was not even considered at presentation. Therefore, a vacancy was created in the predefined guidelines raised doubts about the timing in which patients acquired the COVID-19 disease, fueling the suspicion that the infection had occurred in the preoperative period which was therefore not negligible [159,160,161].
Mass vaccination however raises the possibility of preventing death and avoiding hospitalization in critical conditions. In this regard, data from Israel, which is currently leading the world in terms of percentage of the vaccinated population, are comforting [162]. Covid-19 cases and hospitalizations recorded a significant decrease in mid-January. The greatest effects were related to the massive administration of the vaccine among older individuals, who were prioritized for vaccination. The Israeli health authority reported a reduction in Covid-19 hospitalizations by 36% and 29% with a significant decrease in patients experiencing clinically more severe Covid-19 compared to 3 weeks earlier. The main concern is variants of the original Wuhan-Hu-1 spike protein. Variant B.1.1.7, first identified in the UK, is now the dominant genetic variant of SARS-CoV-2 in both Israel and the UK. This variant does not appear to reduce neutralizing antibodies to the same extent as the South African variant B.1.351. We must be persuaded only the real-world experience can currently provide answers to the efficacy of Covid-19 vaccines against SARS-CoV-2 disease and death as well as its variants [163]. International cooperation has acquired a fundamental role in sharing and distributing large-scale data through verification of official sources (Table 5).

Table 5.
Total administrations of anti-SARS-CoV-2 vaccines according to selected countries and type of vaccine. Data are only available for countries which provided official reports. Time span is 24 December 2020–26 April 2021.
8. Conclusions
The systemic complications of the COVID-19 infection is due to a combination of factors as elaborated above. Treatment should be tailored to the patient with an escalating plan dictated by clinical severity. There are several mechanisms to explain the potential thrombotic complications by both the COVID-19 infection and the vaccines which should be considered. Despite the thrombotic complications, vaccines have been shown to reduce hospital admissions and the severity of illness in robust studies. Long term effects of these however remain uncertain.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
TLR7: Toll-like receptor 7, MI: Myocardial infarction, VTE: venous thromboembolism, FDA: Food and Drug Administration, UFH: Unfractionated Heparin, LMWH: low molecular weight heparin, DOAC: direct oral anticoagulants, aPTT: activated partial thromboplastin time, INR: international normalized ratio, PE: pulmonary embolism, ECMO: extracorporeal membrane oxygenation, PT: prothrombin time, ACS: acute coronary syndromes, PCI: percutaneous coronary intervention, SIRS: systemic inflammatory response syndrome, HIV: human immunodeficiency virus, PDGF: platelet-derived growth factor, MHC: major histocompatibility complex, CD: cluster of differentiation, CTLA: cytotoxic T-lymphocyte antigen, PD: programmed cell death protein, PD-L: programmed cell death ligand, TNF-α: tumor necrosis factor-α, PAI-1: plasminogen activator inhibitor-1, OR: odds ratio, CI: confidence interval, CVD: cardiovascular disease, eNO: endothelial nitric oxide, EMA: European Medicines Agency, ELISA: enzyme-linked immunosorbent assay, RCT: randomized controlled trial.
References
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 re-ceptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneu-monia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Volz, E.; Mishra, S.; Chand, M.; Barrett, J.C.; Johnson, R.; Geidelberg, L.; Hinsley, W.R.; Laydon, D.J.; Dabrera, G.; O’Toole, Á.; et al. Transmission of SARS-CoV-2 lineage B.1.1.7 in England: Insights from linking epidemio-logical and genetic data. medRxiv 2021. [Google Scholar] [CrossRef]
- Faria, N.R.; Claro, I.M.; Candido, D.; Franco, L.A.M.; Andrade, P.S.; Coletti, T.M.; Silva, C.A.M.; Sales, F.C.; Manuli, E.R.; Aguiar, R.S.; et al. Genomic characterization of an emergent SARS-CoV-2 lineage in Manaus: Prelimi-nary findings. Virological 2021. [Google Scholar] [CrossRef]
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Emergence and rapid spread of a new severe acute respiratory syn-drome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. MedRxiv 2020. [Google Scholar] [CrossRef]
- Yang, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar]
- Bester, J.; Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M.; Henry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infec-tions: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Favaloro, E.J. D-dimer is associated with severity of coronavirus disease 2019: A pooled analysis. Thromb. Haemost. 2020, 120, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological fea-tures of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Gris, J.C.; Quéré, I.; Pérez-Martin, A.; Lefrant, J.Y.; Sotto, A. Uncertainties on the prognostic value of D-dimers in COVID-19 patients. J. Thromb. Haemost. 2020, 18, 2066–2067. [Google Scholar] [CrossRef]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Wu, K.L.; Li, J.; Liu, X.H.; Zhu, C.L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020, 58, 1116–1120. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020, 58, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Stoneham, S.M.; Milne, K.M.; Nuttall, E.; Frew, G.H.; Sturrock, B.R.; Sivaloganathan, H.; Ladikou, E.E.; Drage, S.; Phillips, B.; Chevassut, T.J.; et al. Thrombotic risk in COVID-19: A case series and case-control study. Clin. Med. 2020, 20, e76–e81. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zuo, M.; Yalavarthi, S.; Gockman, K.; Madison, J.A.; Shi, H.; Woodard, W.; Lezak, S.P.; Lugogo, N.L.; Knight, J.S.; et al. Neutrophil extracellular traps and thrombosis in COVID-19. J. Thromb. Thrombolysis 2021, 51, 446–453. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, X.; Zhang, D.; Jiang, C.; Mei, H.; Wang, J.; Zhang, C.; Li, H.; Xia, X.; Kong, S.; et al. Deep Vein Thrombosis in Hospitalized Patients With COVID-19 in Wuhan, China: Prevalence, Risk Factors, and Outcome. Circulation 2020, 142, 114–128. [Google Scholar] [CrossRef]
- Planquette, B.; Le Berre, A.; Khider, L.; Yannoutsos, A.; Gendron, N.; de Torcy, M.; Mohamedi, N.; Jouveshomme, S.; Smadja, D.M.; Lazareth, I.; et al. Prevalence and characteristics of pulmonary embolism in 1042 COVID-19 patients with respiratory symptoms: A nested case-control study. Thromb. Res. 2021, 197, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Trimaille, A.; Curtiaud, A.; Marchandot, B.; Matsushita, K.; Sato, C.; Leonard-Lorant, I.; Sattler, L.; Grunebaum, L.; Ohana, M.; Von Hunolstein, J.J.; et al. Venous thromboembolism in non-critically ill patients with COVID-19 infection. Thromb. Res. 2020, 193, 166–169. [Google Scholar] [CrossRef]
- Shah, A.; Donovan, K.; McHugh, A.; Pandey, M.; Aaron, L.; Bradbury, C.A.; Stanworth, S.J.; Alikhan, R.; Von Kier, S.; Maher, K.; et al. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: A multicentre observational study. Crit Care 2020, 24, 561. [Google Scholar] [CrossRef]
- Koleilat, I.; Galen, B.; Choinski, K.; Hatch, A.N.; Jones, D.B.; Billett, H.; Indes, J.; Lipsitz, E. Clinical characteristics of acute lower extremity deep venous thrombosis diagnosed by duplex in patients hospitalized for coronavirus disease 2019. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 36–46. [Google Scholar] [CrossRef]
- Kampouri, E.; Filippidis, P.; Viala, B.; Méan, M.; Pantet, O.; Desgranges, F.; Tschopp, J.; Regina, J.; Karachalias, E.; Bianchi, C.; et al. Predicting Venous Thromboembolic Events in Patients with Coronavirus Disease 2019 Requiring Hospitalization: An Observational Retrospective Study by the COVIDIC Initiative in a Swiss University Hospital. Biomed. Res. Int. 2020, 2020, 9126148. [Google Scholar] [CrossRef]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Manni, G.; Pang, C.J.; Clancy, L.; Yao, C.; Rade, J.; Levy, D.; Wang, J.P.; et al. The role of platelets in mediating a response to human influenza infection. Nat. Commun. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M. Potential role of platelets in COVID-19: Implications for thrombosis. Res. Pr. Thromb. Haemost. 2020, 4, 737–740. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1469–1472. [Google Scholar] [CrossRef]
- Pang, J.; Xu, F.; Aondio, G.; Li, Y.; Fumagalli, A.; Lu, M.; Valmadre, G.; Wei, J.; Bian, Y.; Canesi, M.; et al. Efficacy and tolerability of bevacizumab in patients with severe Covid-19. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular adverse events in patients with cancer treated with bevacizumab: A meta-analysis of more than 20,000 patients. J. Am. Heart Assoc. 2017, 6, e006278. [Google Scholar] [CrossRef] [PubMed]
- Tasat, D.R.; Yakisich, J.S. Rationale for the use of sphingosine analogues in COVID-19 patients. Clin. Med. 2021, 21, e84–e87. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Tanguy, A.; Voswinkel, J.; Henrion, D.; Subra, J.F.; Loufrani, L.; Rohmer, V.; Ifrah, N.; Belizna, C. Antithrombotic effects of hydroxychloroquine in primary antiphospholipid syndrome patients. J. Thromb. Haemost. 2013, 11, 1927–1929. [Google Scholar] [CrossRef] [PubMed]
- Prescribing Information. Brilinta (Ticagrelor); AstraZeneca LP: Wilmington, DE, USA, 2011. [Google Scholar]
- Product Monograph. Brilinta (Ticagrelor); AstraZeneca Canada: Mississauga, ON, Canada, 2011. [Google Scholar]
- Itkonen, M.K.; Tornio, A.; Lapatto-Reiniluoto, O.; Neuvonen, M.; Neuvonen, P.J.; Niemi, M.; Backman, J.T. Clopidogrel Increases Dasabuvir Exposure With or Without Ritonavir, and Ritonavir Inhibits the Bioactivation of Clopidogrel. Clin. Pharm. Ther. 2019, 105, 219–228. [Google Scholar] [CrossRef]
- Marsousi, N.; Daali, Y.; Fontana, P.; Reny, J.-L.; Ancrenaz-Sirot, V.; Calmy, A.; Rudaz, S.; Desmeules, J.A.; Samer, C.F. Impact of Boosted Antiretroviral Therapy on the Pharmacokinetics and Efficacy of Clopidogrel and Prasugrel Active Metabolites. Clin. Pharmacokinet. 2018, 57, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X.; Yang, P.; Zhang, S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiol. Cardiothorac. Imaging 2020, 2, e200067. [Google Scholar] [CrossRef] [PubMed]
- Danzi, G.B.; Loffi, M.; Galeazzi, G.; Gherbesi, E. Acute pulmonary embolism and COVID-19 pneumonia: A random association? Eur. Hear. J. 2020, 41, 1858. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Kruip, M.J.; Van der Meer, N.J.; Arbous, M.S.; Gommers, D.A.; Kant, K.M.; Kaptein, F.H.; van Paassen, J.; Stals, M.A.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–1457. [Google Scholar] [CrossRef]
- Vedantham, S.; Goldhaber, S.Z.; Julian, J.A.; Kahn, S.R.; Jaff, M.R.; Cohen, D.J.; Magnuson, E.; Razavi, M.K.; Comerota, A.J.; Gornik, H.L.; et al. ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein throm-bosis. N. Engl. J. Med. 2017, 377, 2240–2252. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Chodakowski, J.D.; Courtney, D.M. Pulmonary embolism critical care update: Prognosis, treatment, and research gaps. Curr. Opin. Crit. Care 2018, 24, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Barnes, G.D.; Kabrhel, C.; Courtney, D.M.; Naydenov, S.; Wood, T.; Rosovsky, R.; Rosenfield, K.; Giri, J.; National PERT Consortium Research Committee. Diversity in the pulmonary embolism response team model: An organizational survey of the National PERT Consortium Members. Chest 2016, 150, 1414–1417. [Google Scholar] [CrossRef]
- Rosovsky, R.; Zhao, K.; Sista, A.; Rivera-Lebron, B.; Kabrhel, C. Pulmonary embolism response teams: Purpose, evidence for efficacy, and future research directions. Res. Pr. Thromb. Haemost. 2019, 3, 315–330. [Google Scholar] [CrossRef]
- Bikdeli, B.; Chatterjee, S.; Desai, N.R.; Kirtane, A.J.; Desai, M.M.; Bracken, M.B.; Spencer, F.A.; Monreal, M.; Goldhaber, S.Z.; Krumholz, H.M. Inferior vena cava filters to prevent pulmonary embolism: Systematic review and meta-analysis. J. Am. Coll. Cardiol. 2017, 70, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef]
- Piazza, G. Advanced Management of Intermediate- and High-Risk Pulmonary Embolism: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2117–2127. [Google Scholar] [CrossRef]
- Giri, J.; Sista, A.K.; Weinberg, I.; Kearon, C.; Kumbhani, D.J.; Desai, N.D.; Piazza, G.; Gladwin, M.T.; Chatterjee, S.; Kobayashi, T.; et al. Interventional Therapies for Acute Pulmonary Embolism: Current Status and Principles for the Development of Novel Evidence: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e774–e801. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, D.; Bikdeli, B.; Marshall, P.S.; Tapson, V. Aggressive Treatment of Intermediate-Risk Patients with Acute Symptomatic Pulmonary Embolism. Clin. Chest Med. 2018, 39, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, J.A.S.; Rech, T.H.; Schwarz, P.; de Oliveira, A.C.T.; Vieceli, T.; Moraes, R.B.; Sekine, L.; Viana, M.V. Incidence of venous thromboembolism among patients with severe COVID-19 requiring mechanical ventilation compared to other causes of respiratory failure: A prospective cohort study. J. Thromb. Thrombolysis 2021, 1–11. [Google Scholar] [CrossRef]
- Moll, M.; Zon, R.L.; Sylvester, K.W.; Chen, E.C.; Cheng, V.; Connell, N.T.; Fredenburgh, L.E.; Baron, R.M.; Cho, M.H.; Woolley, A.E.; et al. VTE in ICU Patients With COVID-19. Chest 2020, 158, 2130–2135. [Google Scholar] [CrossRef]
- Di Minno, A.; Ambrosino, P.; Calcaterra, I.; Di Minno, M.N.D. COVID-19 and Venous Thromboembolism: A Meta-analysis of Literature Studies. Semin. Thromb. Hemost. 2020, 46, 763–771. [Google Scholar] [CrossRef]
- Mansfield, A.; Tafur, A.; Vulih, D.; Smith, G.; Harris, P.; Ivy, S. Severe hepatic dysfunction is associated with venous thromboembolic events in phase 1 clinical trials. Thromb. Res. 2015, 136, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S.; Yogaratnam, D.; Levasseur-Franklin, K.E.; Forni, A.; Fong, J. Introduction to Drug Pharmacokinetics in the Critically III Patient. Chest 2012, 141, 1327–1336. [Google Scholar] [CrossRef]
- Aryal, M.R.; Gosain, R.; Donato, A.; Pathak, R.; Bhatt, V.R.; Katel, A.; Kouides, P. Venous Thromboembolism in COVID-19: Towards an Ideal Approach to Thrombo-prophylaxis, Screening, and Treatment. Curr. Cardiol. Rep. 2020, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Sklar, M.C.; Sy, E.; Lequier, L.; Fan, E.; Kanji, H.D. Anticoagulation practices during venovenous extracorporeal membrane oxy-genation for respiratory failure. A systematic review. Ann. Am. Thorac. Soc. 2016, 13, 2242–2250. [Google Scholar] [CrossRef]
- Extracorporeal Life Support Organization. ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support. v1.4. 2017. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/jth.12155 (accessed on 1 April 2020).
- Dobesh, P.P.; Trujillo, T.C. Coagulopathy, Venous Thromboembolism, and Anticoagulation in Patients with COVID-19. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 1130–1151. [Google Scholar] [CrossRef] [PubMed]
- Schunemann, H.J.; Cushman, M.; Burnett, A.E.; Kahn, S.R.; Beyer-Westendorf, J.; Spencer, F.A.; Rezende, S.M.; Zakai, N.A.; Bauer, K.A.; Dentali, F.; et al. American Society of Hematology 2018 guidelines for management of ve-nous thromboembolism: Prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018, 2, 3198–3225. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.D.; de Barros ESilva, P.G.M.; Furtado, R.H.M.; Macedo, A.V.S.; Ramacciotti, E.; Damini, L.P.; Bronhara, B.; Cavalcanti, A.B.; Rosa, R.G.; Azevedo, L.C.P.; et al. Randomized Clinical Trial to Evaluate a Routine Full Anticoagulation Strategy in Patients with Coronavirus Infection (SARS-CoV2) Admitted to Hospital: Rationale and Design of the ACTION (AntiCoagulaTlon cOroNavirus)-Coalition IV Trial. Am. Heart J. 2021. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence. NICE Clinical Guideline 92: Venous Thromboembolism: Reducing the Risk. Available online: http://www.1000livesplus.wales.nhs.uk/sitesplus/documents/1011/CG92NICEGuidelinePDF.pdf (accessed on 30 March 2020).
- Lenchus, J.D.; Biehl, M.; Cabrera, J.; De Moraes, A.G.; Dezfulian, C. In-Hospital Management and Follow-Up Treatment of Venous Thromboembolism: Focus on New and Emerging Treatments. J. Intensiv. Care Med. 2017, 32, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Encke, A.; Haas, S.; Kopp, I. The Prophylaxis of Venous Thromboembolism. Dtsch. Aerzteblatt Online 2016, 113, 532–538. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Levy, J.H.; Ageno, W.; Connors, J.M.; Hunt, B.J.; Iba, T.; Levi, M.; Samama, C.M.; Thachil, J.; Giannis, D.; et al. Scientific and Stand-ardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous throm-boembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, I.E.; Nielsen, P.B. Searching for High-Risk Venous Thromboembolism Patients Using Risk Scores: Adding to the Heap or Closing a Gap? Thromb. Haemost. 2018, 118, 1686–1687. [Google Scholar] [CrossRef]
- Zhou, H.; Hu, Y.; Li, X.; Wang, L.; Wang, M.; Xiao, J.; Yi, Q. Assessment of the Risk of Venous Thromboembolism in Medical Inpatients using the Padua Prediction Score and Caprini Risk Assessment Model. J. Atheroscler. Thromb. 2018, 25, 1091–1104. [Google Scholar] [CrossRef]
- Stuck, A.; Spirk, D.; Schaudt, J.; Kucher, N. Risk Assessment Models for Venous Thromboembolism in Acutely Ill Medical Patients: A Systematic Review. Thromb. Haemost. 2017, 5, 769–770. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Chen, X.; Wu, W.; Lu, G. Comparison between Caprini and Padua risk assessment models for hospitalized medi-cal patients at risk for venous thromboembolism: A retrospective study. Interact. Cardiovasc. Thorac. Surg. 2016, 23, 538–543. [Google Scholar] [CrossRef]
- Moumneh, T.; Riou, J.; Douillet, D.; Henni, S.; Mottier, D.; Tritschler, T.; Le Gal, G.; Roy, P. Validation of risk assessment models predicting venous thromboembolism in acutely ill medical inpatients: A cohort study. J. Thromb. Haemost. 2020, 18, 1398–1407. [Google Scholar] [CrossRef]
- Mlaver, E.; Lynde, G.C.; Gallion, C.; Sweeney, J.F.; Sharma, J. Development of a Novel Preoperative Venous Thromboembolism Risk Assessment Model. Am. Surg. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, R.; Liu, C.; Liang, W.; Guan, W.; Tang, R.; Tang, C.; Zhang, N.; Zhong, N.; Li, S. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020, 7, e362–e363. [Google Scholar] [CrossRef]
- Hunt, B.J. Hemostasis at Extremes of Body Weight. Semin. Thromb. Hemost. 2018, 44, 632–639. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected. INTERIM Guidance 28 January 2020. Available online: https://www.who.int/docs/defaultsource/coronaviruse/clinical-management-ofnovel-cov.pdf (accessed on 7 April 2020).
- Liew, N.C.; Alemany, G.V.; Angchaisuksiri, P.; Bang, S.M.; Choi, G.; De Silva, D.; Hong, J.M.; Lee, L.; Li, Y.J.; Rajamoney, G.N.; et al. Asian venous thromboembolism guidelines: Updated recommendations for the prevention of venous thromboembolism. Int. Angiol. 2016, 36, 1–20. [Google Scholar] [PubMed]
- Popoola, V.O.; Tavakoli, F.; Lau, B.D.; Lankiewicz, M.; Ross, P.; Kraus, P.; Shaffer, D.; Hobson, D.B.; Aboagye, J.K.; Farrow, N.A.; et al. Exploring the impact of route of administration on medication acceptance in hospi-talized patients: Implications for venous thromboembolism prevention. Thromb. Res. 2017, 160, 109–113. [Google Scholar] [CrossRef]
- Bates, S.M.; Rajasekhar, A.; Middeldorp, S.; McLintock, C.; Rodger, M.A.; James, A.H.; Vazquez, S.R.; Greer, I.A.; Riva, J.J.; Bhatt, M.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Venous thromboembolism in the context of pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Reducing the Risk of Venous Thromboembolism during Pregnancy and the Puerperium. Green-Top Guideline No. 37a. 2015. Available online: https://www.rcog.org.uk/globalassets/documents/guide-lines/gtg%5f37a.pdf (accessed on 1 April 2020).
- Rybstein, M.D.; DeSancho, M.T. Risk factors for and clinical management of venous thromboembolism during pregnancy. Clin. Adv. Hematol. Oncol. 2019, 17, 396–404. [Google Scholar]
- Akel, T.; Qaqa, F.; Abuarqoub, A.; Shamoon, F. Pulmonary embolism: A complication of COVID 19 infection. Thromb. Res. 2020, 193, 79–82. [Google Scholar] [CrossRef]
- Cohen, A.T.; Harrington, R.A.; Goldhaber, S.Z.; Hull, R.D.; Wiens, B.L.; Gold, A.; Hernandez, A.F.; Gibson, C.M.; APEX Investigators. Extended thromboprophylaxis with betrixaban in acutely ill medical pa-tients. N. Engl. J. Med. 2016, 375, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Arigondam, A.K.; Hakeem, A.R.; Reddy, M.S.; Rela, M. An Evidence-based Protocol for Minimizing Thromboembolic Events in SARS-CoV-2 Infection. Arch. Med. Res. 2021, 52, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Ageno, W.; Albers, G.W.; Elliott, C.G.; Halperin, J.L.; Hiatt, W.R.; Maynard, G.A.; Steg, P.G.; Weitz, J.I.; Suh, E.; et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical ill-ness. N. Engl. J. Med. 2018, 379, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Lipardi, C.; Xu, J.; Peluso, C.; Spiro, T.E.; De Sanctis, Y.; Barnathan, E.S.; Raskob, G.E. Modified IMPROVE VTE Risk Score and Elevated D-Dimer Identify a High Venous Thromboembolism Risk in Acutely Ill Medical Population for Extended Thromboprophylaxis. TH Open 2020, 4, e59–e65. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Mumoli, N.; Prisco, D.; Fontanella, A.; Di Minno, M.N. Efficacy and safety of extended thromboprophylaxis for medi-cally ill patients. A meta-analysis of randomised controlled trials. Thromb. Haemost. 2017, 117, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Schindewolf, M.; Weitz, J.I. Broadening the Categories of Patients Eligible for Extended Venous Thromboembolism Treatment. Thromb. Haemost. 2019, 120, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, A.C.; Lipardi, C.; Xu, J.; Lu, W.; Suh, E.; Yuan, Z.; Levitan, B.; Sugarmann, C.; De Sanctis, Y.; Spiro, T.E.; et al. Improved Benefit Risk Profile of Rivaroxaban in a Subpopulation of the MAGELLAN Study. Clin. Appl. Thromb. Hemost. 2019, 25. [Google Scholar] [CrossRef]
- Chi, G.; Goldhaber, S.Z.; Kittelson, J.M.; Turpie, A.G.G.; Hernandez, A.F.; Hull, R.D.; Gold, A.; Curnutte, J.T.; Cohen, A.T.; Harrington, R.A.; et al. Effect of extended-duration thromboprophylaxis on venous thromboembolism and major bleeding among acutely ill hospitalized medical patients: A bivariate analysis. J. Thromb. Haemost. 2017, 15, 1913–1922. [Google Scholar] [CrossRef]
- Zhang, A.; Leng, Y.; Zhang, Y.; Wu, K.; Ji, Y.; Lei, S.; Xia, Z. Meta-analysis of coagulation parameters associated with disease severity and poor prognosis of COVID-19. Int. J. Infect. Dis. 2020, 100, 441–448. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020, 18, 2103–2109. [Google Scholar] [CrossRef]
- Gando, S.; Shiraishi, A.; Yamakawa, K.; Ogura, H.; Saitoh, D.; Fujishima, S.; Mayumi, T.; Kushimoto, S.; Abe, T.; Shiino, Y.; et al. Japanese Association for Acute Medicine (JAAM) Focused Outcomes Research in Emergency Care in Acute Respiratory Distress Syndrome, Sepsis and Trauma (FORECAST) Study Group. Role of disseminated intravascular coagulation in severe sepsis. Thromb Res. 2019, 178, 182–188. [Google Scholar]
- Jackson Chornenki, N.L.; Dwivedi, D.J.; Kwong, A.C.; Zamir, N.; Fox-Robichaud, A.E.; Liaw, P.C.; Canadian Critical Care Translational Biology Group. Canadian Critical Care Translational Biology Group. Identification of hemostatic markers that define the pre-DIC state: A multi-center observational study. J. Thromb. Haemost. 2020, 18, 2524–2531. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coro-navirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Wada, H.; Thachil, J.; Di Nisio, M.; Mathew, P.; Kurosawa, S.; Gando, S.; Kim, H.K.; Nielsen, J.D.; Dempfle, C.E.; Levi, M.; et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommenda-tions from 3 guidelines. J. Thromb. Haemost. 2013. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Perico, L.; Benigni, A.; Casiraghi, F.; Ng, L.F.P.; Renia, L.; Remuzzi, G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021, 17, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Jackson, B.S.; Pretorius, E. Pathological Clotting and Deep Vein Thrombosis in Patients with HIV. Semin. Thromb. Hemost. 2019, 45, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ramacciotti, E.; Agati, L.B.; Aguiar, V.C.R.; Wolosker, N.; Guerra, J.C.; De Almeida, R.P.; Alves, J.C.; Lopes, R.D.; Wakefield, T.W.; Comerota, A.J.; et al. Zika and Chikungunya Virus and Risk for Venous Thromboembolism. Clin. Appl. Thromb. 2019, 25. [Google Scholar] [CrossRef]
- Smither, S.J.; O’Brien, L.M.; Eastaugh, L.; Woolley, T.; Lever, S.; Fletcher, T.; Parmar, K.; Hunt, B.J.; Watts, S.; Kirkman, E. Haemostatic Changes in Five Patients Infected with Ebola Virus. Viruses 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Karakike, E.; Giamarellos-Bourboulis, E.J. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis. Front. Immunol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Webb, B.J.; Peltan, I.D.; Jensen, P.; Hoda, D.; Hunter, B.; Silver, A.; Starr, N.; Buckel, W.; Grisel, N.; Hummel, E.; et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: A co-hort study. Lancet Rheumatol. 2020, 2, e754–e763. [Google Scholar] [CrossRef]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial Response to Pathophysiological Stress. Arter. Thromb. Vasc. Biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, J.; Milojkovic, M.; Stoimenov, T.J.; Djindjic, B.; Miljkovic, E. Mechanisms of plasminogen activator inhibitor 1 action in stromal remodeling and related diseases. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2017, 161, 339–347. [Google Scholar] [CrossRef]
- Poels, K.; van Leent, M.M.; Reiche, M.E.; Kusters, P.J.; Huveneers, S.; de Winther, M.P.; Mulder, W.J.; Lutgens, E.; Seijkens, T.T. Antibody-Mediated Inhibition of CTLA4 Aggravates Atherosclerotic Plaque In-flammation and Progression in Hyperlipidemic Mice. Cells 2020, 9, 1987. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sasaki, N.; Yamashita, T.; Emoto, T.; Kasahara, K.; Mizoguchi, T.; Hayashi, T.; Yodoi, K.; Kitano, N.; Saito, T.; et al. Overexpression of Cytotoxic T-Lymphocyte-Associated Antigen-4 Prevents Ath-erosclerosis in Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Foks, A.; Kuiper, J. Immune checkpoint proteins: Exploring their therapeutic potential to regulate atherosclerosis. Br. J. Pharmacol. 2017, 174, 3940–3955. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Warnock, M.; Harbaugh, A.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Knight, J.S.; Kanthi, Y.; Lawrence, D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Engin, A. Endothelial Dysfunction in Obesity. Adv. Exp. Med. Biol. 2017, 960, 345–379. [Google Scholar] [CrossRef]
- Yang, J.; Hu, J.; Zhu, C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2020, 93, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Kurniawan, A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1463–1465. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Adjedj, J.; Varenne, O. Diabetes Mellitus, a prothrombotic disease. Ann. Cardiol. D’angeiologie 2017, 66, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Bryk, A.H.; Satała, D.; Natorska, J.; Rąpała-Kozik, M.; Undas, A. Interaction of glycated and acetylated human α2-antiplasmin with fibrin clots. Blood Coagul. Fibrinolysis 2020, 31, 393–396. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.D.; Crofts, C.A.P.; DiNicolantonio, J.J.; Malhotra, A.; Elliott, B.; Kyriakidou, Y.; Brookler, K.H. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: Rationale for clinical management. Open Hear. 2020, 7, e001356. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.E.; Tippetts, T.S.; Anderson, M.C.; Holub, Z.E.; Moulton, E.R.; Swensen, A.C.; Prince, J.T.; Bikman, B.T. Insulin Increases Ceramide Synthesis in Skeletal Muscle. J. Diabetes Res. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Dias, I.H.; Ferreira, R.; Gruber, F.; Vitorino, R.; Rivas-Urbina, A.; Sanchez-Quesada, J.L.; Silva, J.V.; Fardilha, M.; de Freitas, V.; Reis, A. Sulfate-based lipids: Analysis of healthy human fluids and cell extracts. Chem. Phys. Lipids 2019, 221, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.; Visser, M.P.J.; Dofferhoff, A.S.M.; Vermeer, C.; Janssens, W.; Walk, J. Vitamin K metabolism as the potential missing link between lung damage and thromboembolism in Coronavirus disease 2019. Br. J. Nutr. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levy, J.H. Sepsis-induced Coagulopathy and Disseminated Intravascular Coagulation. Anesthesiology 2020, 132, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J. Int. Med. Res. 2020, 48. [Google Scholar] [CrossRef]
- Hotta, Y.; Kataoka, T.; Kimura, K. Testosterone Deficiency and Endothelial Dysfunction: Nitric Oxide, Asymmetric Dimethylarginine, and Endothelial Progenitor Cells. Sex. Med. Rev. 2019, 7, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Guastamacchia, E.; Magrone, T.; Jirillo, E.; Lisco, G.; De Pergola, G.; Triggiani, V. Worse progression of COVID-19 in men: Is testosterone a key factor? Andrology 2021, 9, 53–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: WHO says rollout of AstraZeneca vaccine should continue, as Europe divides over safety. BMJ 2021, 372, n728. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: AstraZeneca vaccine is not linked to increased risk of blood clots, finds European Medicine Agency. BMJ 2021, 372, n774. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. COVID-19 Vaccine AstraZeneca: Benefits Still Outweigh the Risks Despite Possible Link to Rare Blood Clots with Low Blood Platelets. Available online: https://www.ema.europa.eu/en/news/covid-19-vac-cine-astrazeneca-benefits-still-outweigh-risks-despite-possible-linkrare-blood-clots (accessed on 18 March 2021).
- European Medicines Agency. Covid-19 Vaccine AstraZeneca: PRAC Investigating Cases of Thromboembolic Events—Vaccine’s Benefits Currently Still Outweigh Risks: Update. Mar. 2021. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-investigating-casesthromboembolic-events-vaccines-benefits (accessed on 19 April 2021).
- WHO’s Advisory Committee on Vaccine Safety. Available online: https://www.who.int/news/item/19-03-2021-statement-of-the-who-global-advisory-committee-on-vaccine-safety-(gacvs)-covid-19-subcommittee-on-safety-signals-related-to-the-astrazeneca-covid-19-vaccine (accessed on 20 April 2021).
- AstraZeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets Share. Available online: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (accessed on 20 April 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChA-dOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, random-ised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- VID-19: Latest Updates Share. The Latest Updates on the COVID-19 Pandemic from the European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-latest-updates (accessed on 15 April 2021).
- WHO Coronavirus (COVID-19) Dashboard. Geneva: World Health Organization. Available online: https://covid19.who.int/ (accessed on 5 April 2021).
- European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker. Available online: https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed on 15 April 2021).
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus ma-caques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Vasileiou, E.; Simpson, C.R.; Robertson, C.; Shi, T.; Kerr, S.; Agrawal, U.; Akbari, A.; Bedston, S.; Beggs, J.; Bradley, D.; et al. Effectiveness of First Dose of COVID-19 Vaccines Against Hospital Admissions in Scotland: National Prospective Cohort Study of 5.4 Million People. Lancet 2021. [Google Scholar] [CrossRef]
- López-Medina, E.; López, P.; Hurtado, I.C.; Dávalos, D.M.; Ramirez, O.; Martínez, E.; Díazgranados, J.A.; Oñate, J.M.; Chavarriaga, H.; Herrera, S.; et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B. Effect of Intermediate-Dose vs. Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit The INSPIRATION Randomized Clinical Trial. JAMA 2021, 325, 1620–1630. [Google Scholar]
- COVIDSurg Collaborative; GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: An international prospective cohort study. Anaesthesia 2021, 76, 748–758. [Google Scholar] [CrossRef]
- Nappi, F.; Avatar Singh, S.S.; Santana, O.; Mihos, C.G. Functional mitral regurgitation: An overview for surgical management framework. J. Thorac. Dis. 2018, 10, 4540–4555. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Chello, M.; Acar, C. The Ross procedure: Underuse or under-comprehension? J. Thorac. Cardiovasc. Surg. 2015, 149, 1463–1464. [Google Scholar] [CrossRef][Green Version]
- COVIDSurg Collaborative; GlobalSurg Collaborative. SARS-CoV-2 vaccination modelling for safe surgery to save lives: Data from an international prospective cohort study. Br. J. Surg. 2021. [Google Scholar] [CrossRef]
- Rubin, R. COVID-19 Vaccines vs. Variants—Determining How Much Immunity Is Enough. JAMA 2021, 325, 1241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).