Dietary Macronutrient Composition Differentially Modulates the Remodeling of Mitochondrial Oxidative Metabolism during NAFLD
Abstract
1. Introduction
2. Results
2.1. Phenotypic Characteristics for the Mice Reared on Low-Fat (LF), High-Fat (HF), and High-Fructose/High-Fat (HFr/HF) Diets
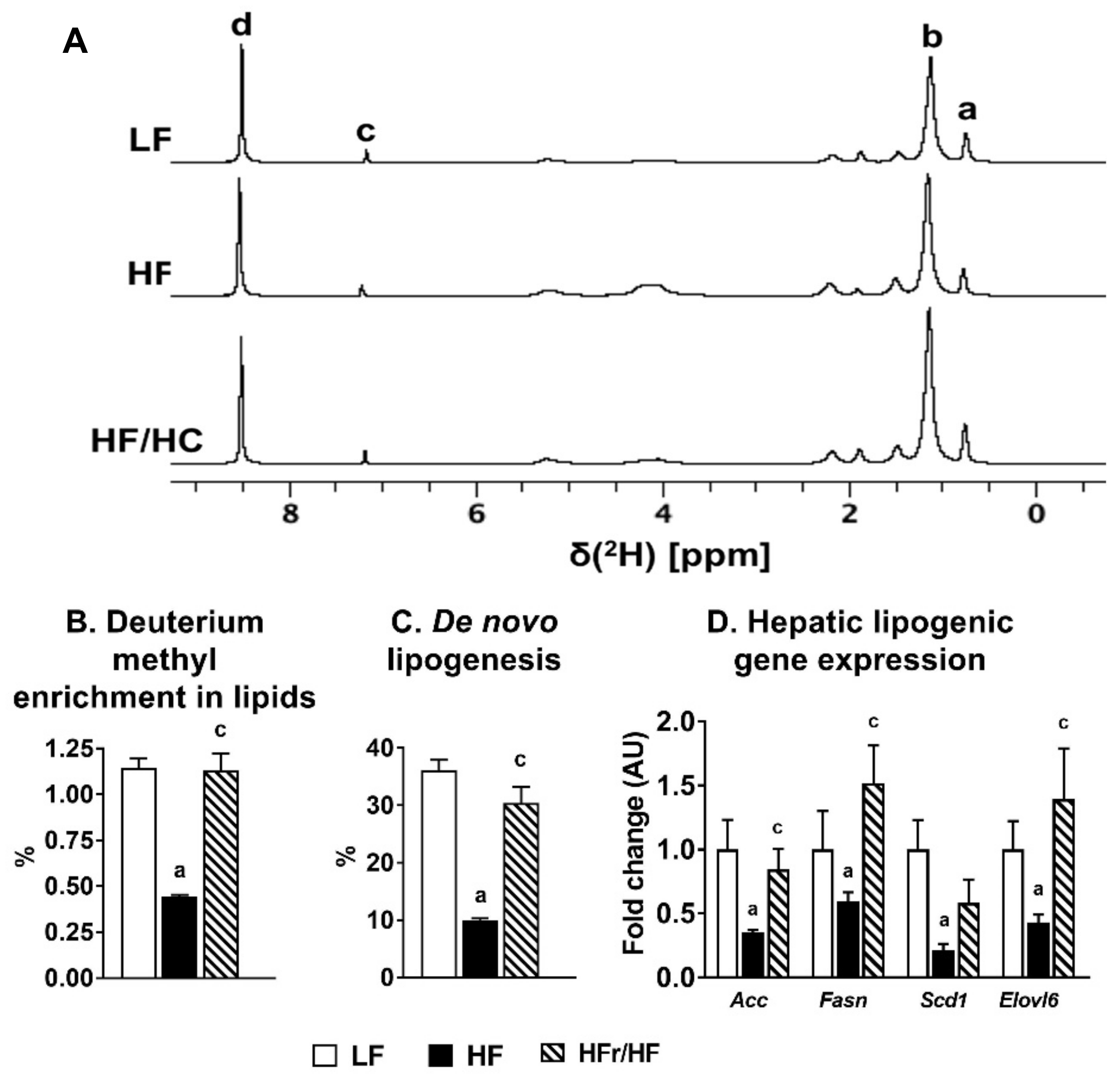
2.2. Hepatic De Novo Lipogenesis Was Higher in Mice Fed High-Fructose/High-Fat Diet Compared to those Fed a High-Fat Diet.
2.3. Differences in Diet Composition Alter AKT Phosphorylation and Hepatic Mitochondrial Protein Expression during Fed and Overnight Fasting Conditions
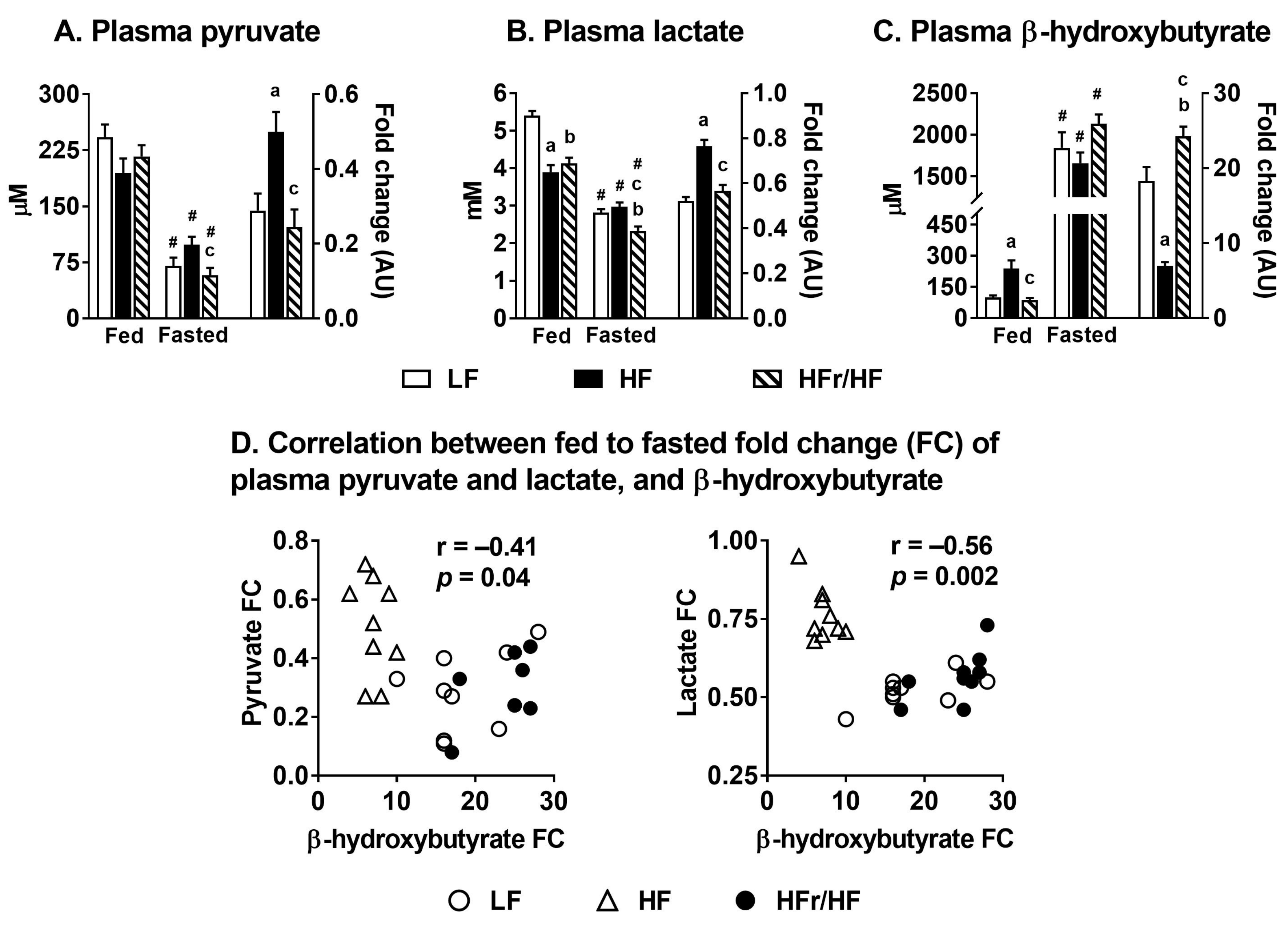
2.4. Switch from Carbohydrate to Free Fatty Acid Utilization during Feeding to Fasting Transition in HF and HFr/HF Mice
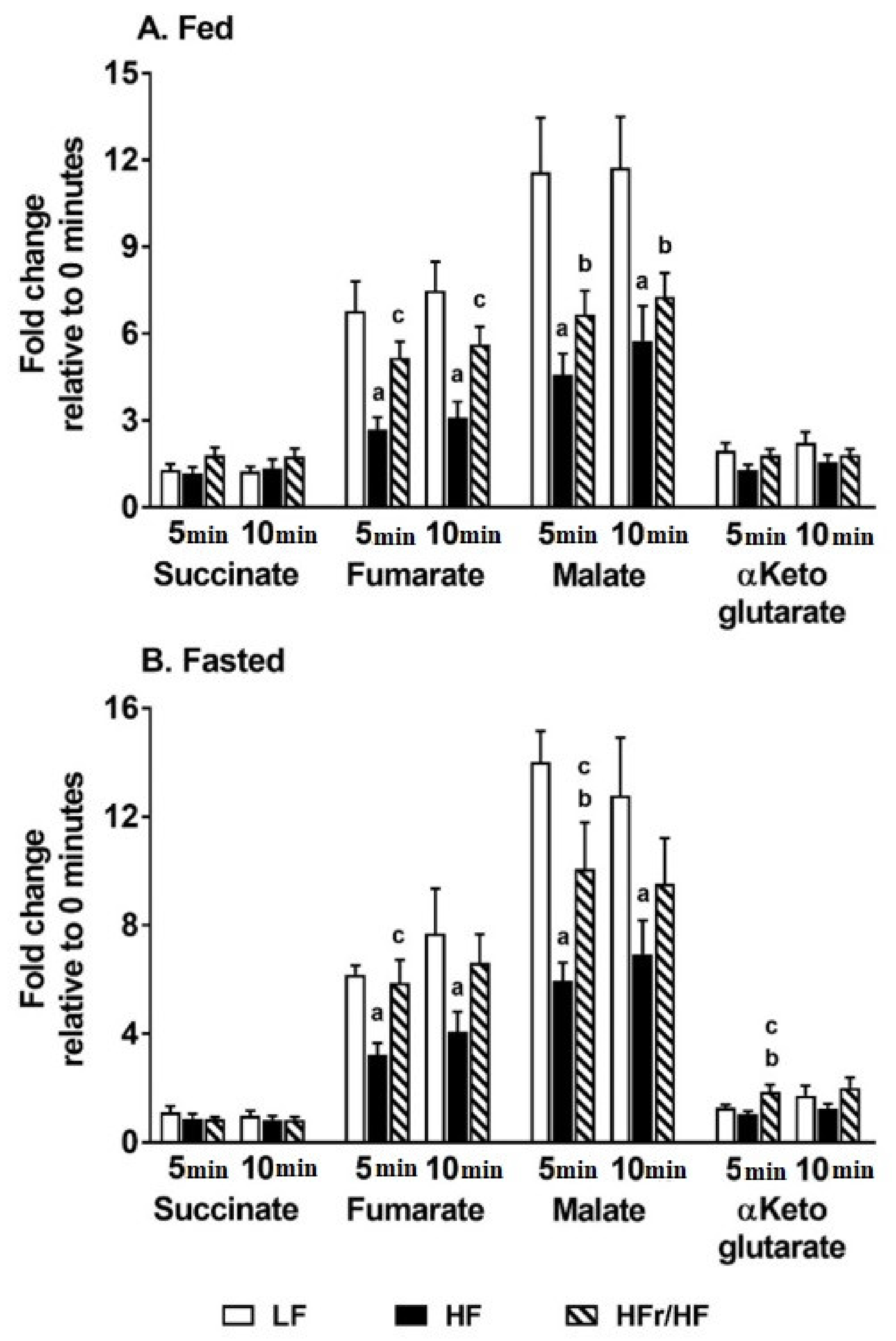
2.5. Profiles of Metabolic Intermediates in the Liver Tissue Point to Differences in Mitochondrial Metabolism between Mice Reared on HF and HFr/HF Diets
2.6. Alteration in the Pool Sizes of Hepatic TCA Cycle Intermediates Following Incubation of Isolated Mitochondria in a Respiration Buffer
2.7. Incorporation of 13C into Mitochondrial TCA Cycle Intermediates from [13C3]Pyruvate
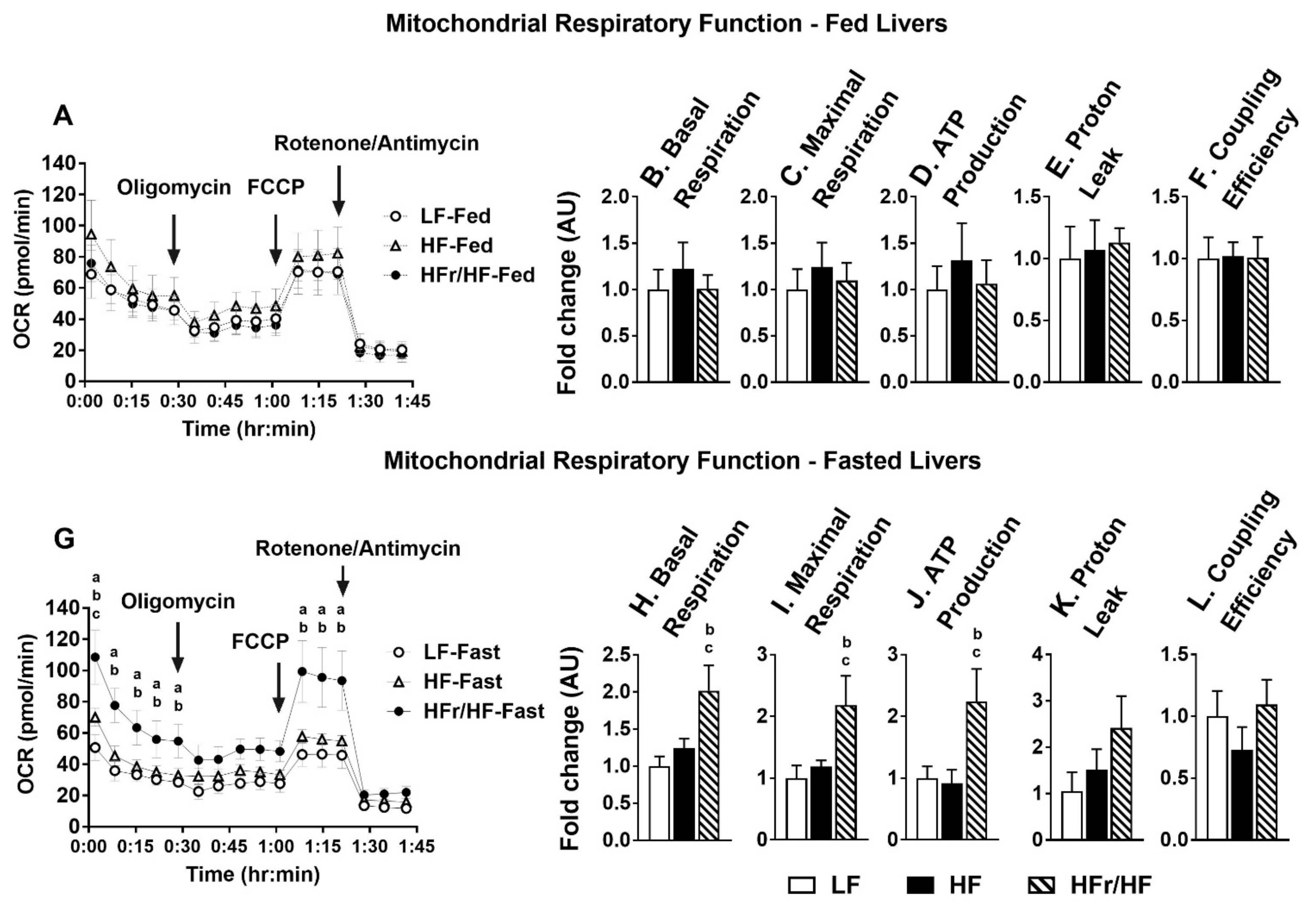
2.8. Rates of Hepatic Mitochondrial Respiration Were Higher in Overnight Fasted HFr/HF Mice
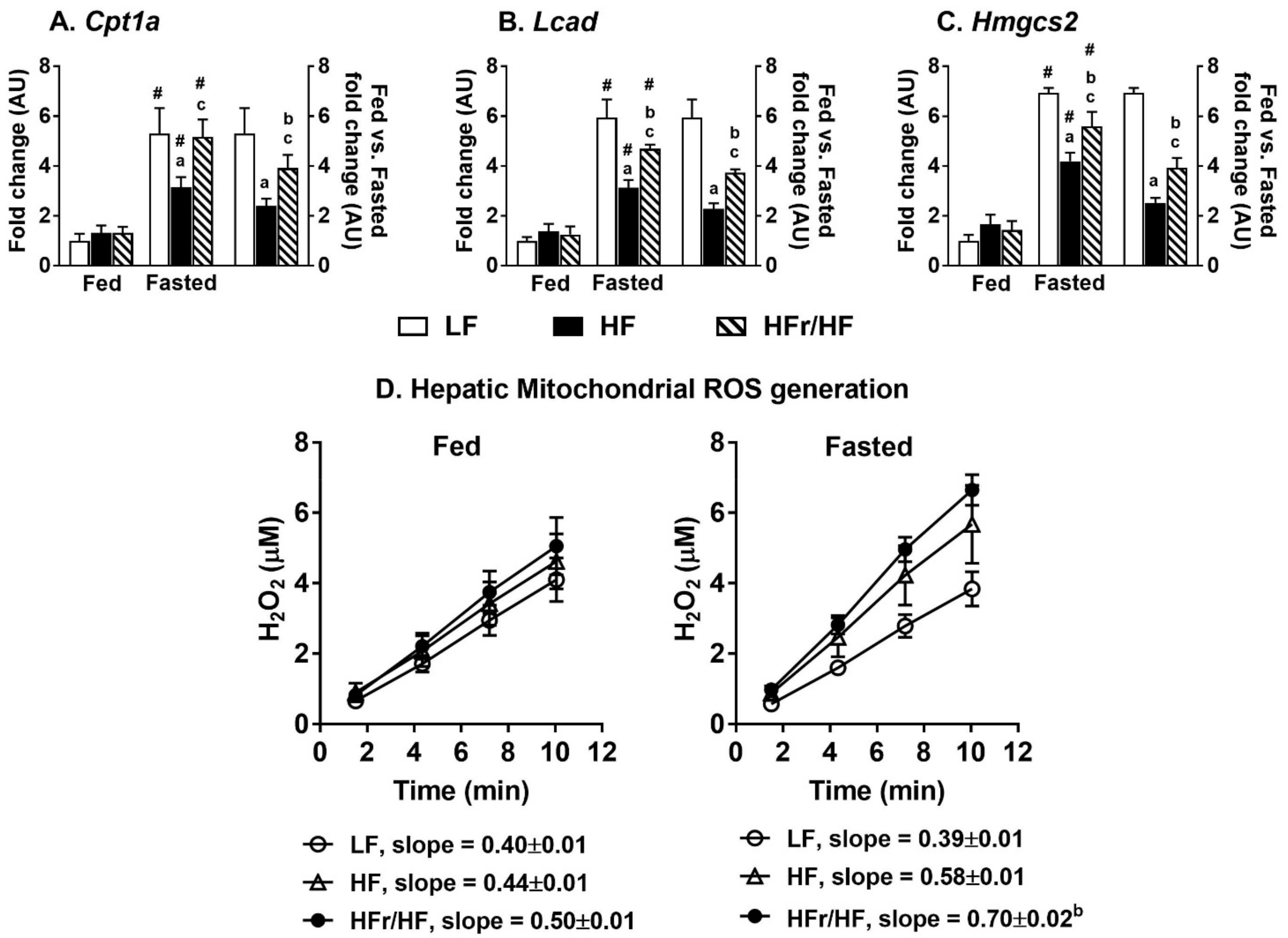
2.9. Expression of Genes Involved in Hepatic Lipid Oxidation and Mitochondrial Reactive Oxygen Species (ROS) Generation Were Higher in HFr/HF Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Hepatic Mitochondrial Isolation
4.3. Incubation of Isolated Mitochondria to Determine Changes in TCA Cycle Metabolism
4.4. Metabolite Profiling by GC-MS
4.5. Biochemical Assays
4.6. Determination of ROS Production by Isolated Mitochondria
4.7. Respiration by Isolated Liver Mitochondria
4.8. Gene Expression Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E. Nonalcoholic Fatty Liver Disease. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef]
- Sunny, N.E.; Parks, E.J.; Browning, J.D.; Burgess, S.C. Excessive Hepatic Mitochondrial TCA Cycle and Gluconeogenesis in Humans with Nonalcoholic Fatty Liver Disease. Cell Metab. 2011, 14, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Kalavalapalli, S.; Williams, C.M.; Nautiyal, M.; Mathew, J.T.; Martinez, J.; Reinhard, M.K.; McDougall, D.J.; Rocca, J.R.; Yost, R.A.; et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am. J. Physiol. Metab. 2016, 310, E484–E494. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- García-Berumen, C.I.; Ortiz-Avila, O.; Vargas-Vargas, M.A.; Del Rosario-Tamayo, B.A.; Guajardo-López, C.; Saavedra-Molina, A.; Rodríguez-Orozco, A.R.; Cortés-Rojo, C. The severity of rat liver injury by fructose and high fat depends on the degree of respiratory dysfunction and oxidative stress induced in mitochondria. Lipids Health Dis. 2019, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Bronson, F.H. Susceptibility of the fat reserves of mice to natural challenges. J. Comp. Physiol. B 1987, 157, 551–554. [Google Scholar] [CrossRef]
- Bronson, F.H.; Heideman, P.D.; Kerbeshian, M.C. Lability of fat stores in peripubertal wild house mice. J. Comp. Physiol. B 1991, 161, 15–18. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Selen, E.S.; Choi, J.; Wolfgang, M.J. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight 2021, 6. [Google Scholar] [CrossRef]
- Shannon, C.E.; Ragavan, M.; Palavicini, J.P.; Fourcaudot, M.; Bakewell, T.M.; Valdez, I.A.; Ayala, I.; Jin, E.S.; Madesh, M.; Han, X.; et al. Insulin resistance is mechanistically linked to hepatic mitochondrial remodeling in non-alcoholic fatty liver disease. Mol. Metab. 2021, 45, 101154. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos–Roman, M.A.; Browning, J.D.; Parks, E.J. Increased De Novo Lipogenesis Is a Distinct Characteristic of Individuals with Nonalcoholic Fatty Liver Disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, K.W.; Vatner, D.F.; Zhang, D.; Cline, G.W.; Ackermans, M.T.; Nederveen, A.J.; Verheij, J.; Demirkiran, A.; Van Wagensveld, B.A.; Dallinga-Thie, G.M.; et al. Hepatic Insulin Resistance Is Not Pathway Selective in Humans With Nonalcoholic Fatty Liver Disease. Diabetes Care 2021, 44, 489–498. [Google Scholar] [CrossRef]
- Todoric, J.; Di Caro, G.; Reibe, S.; Henstridge, D.C.; Green, C.R.; Vrbanac, A.; Ceteci, F.; Conche, C.; McNulty, R.; Shalapour, S.; et al. Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2020, 2, 1034–1045. [Google Scholar] [CrossRef]
- Satapati, S.; Kucejova, B.; Duarte, J.A.; Fletcher, J.A.; Reynolds, L.; Sunny, N.E.; He, T.; Nair, L.A.; Livingston, K.A.; Fu, X.; et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J. Clin. Investig. 2016, 126, 1605. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Gupta, M.K.; Wang, G.-X.; Fujisaka, S.; O’Neill, B.T.; Rao, T.N.; Willoughby, J.; Harbison, C.; Fitzgerald, K.; Ilkayeva, O.; et al. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Investig. 2017, 127, 4059–4074. [Google Scholar] [CrossRef]
- Satapati, S.; Sunny, N.E.; Kucejova, B.; Fu, X.; He, T.T.; Méndez-Lucas, A.; Shelton, J.M.; Perales, J.C.; Browning, J.D.; Burgess, S.C. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012, 53, 1080–1092. [Google Scholar] [CrossRef]
- Duarte, J.A.; Carvalho, F.; Pearson, M.; Horton, J.D.; Browning, J.D.; Jones, J.G.; Burgess, S.C. A high-fat diet suppresses de novo lipogenesis and desaturation but not elongation and triglyceride synthesis in mice. J. Lipid Res. 2014, 55, 2541–2553. [Google Scholar] [CrossRef]
- Green, C.J.; Pramfalk, C.; A Charlton, C.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calvo, R.; Barroso, E.; Serrano, L.; Coll, T.; Sánchez, R.M.; Merlos, M.; Palomer, X.; Laguna, J.C.; Vázquez-Carrera, M. Atorvastatin prevents carbohydrate response element binding protein activation in the fructose-fed rat by activating protein kinase A. Hepatology 2008, 49, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Iozzo, P.; Bucci, M.; Roivainen, A.; Någren, K.; Järvisalo, M.J.; Kiss, J.; Guiducci, L.; Fielding, B.; Naum, A.G.; Borra, R.; et al. Fatty Acid Metabolism in the Liver, Measured by Positron Emission Tomography, Is Increased in Obese Individuals. Gastroenterology 2010, 139, 846–856. [Google Scholar] [CrossRef]
- Sunny, N.E.; Satapati, S.; Fu, X.; He, T.; Mehdibeigi, R.; Spring-Robinson, C.; Duarte, J.; Potthoff, M.J.; Browning, J.D.; Burgess, S.C. Progressive adaptation of hepatic ketogenesis in mice fed a high-fat diet. Am. J. Physiol. Metab. 2010, 298, E1226–E1235. [Google Scholar] [CrossRef]
- Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Roncal-Jimenez, C.A.; Orlicky, D.J.; Cicerchi, C.; Mcmahan, R.H.; Abdelmalek, M.F.; Rosen, H.R.; Jackman, M.R.; et al. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatology 2013, 58, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.S.M.; Basford, J.E.; Kuhel, D.G.; Konaniah, E.S.; Cash, J.G.; Lima, V.L.M.; Hui, D.Y. Distinct Influence of Hypercaloric Diets Predominant with Fat or Fat and Sucrose on Adipose Tissue and Liver Inflammation in Mice. Molecules 2020, 25, 4369. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; A Watkins, B.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Boland, M.L.; Oldham, S.; Boland, B.B.; Will, S.; Lapointe, J.-M.; Guionaud, S.; Rhodes, C.J.; Trevaskis, J.L. Nonalcoholic steatohepatitis severity is defined by a failure in compensatory antioxidant capacity in the setting of mitochondrial dysfunction. World J. Gastroenterol. 2018, 24, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Chacko, V.P.; Arnold, C.; Diehl, A.M. Hepatic ATP reserve and efficiency of replenishing: Comparison between obese and nonobese normal individuals. Am. J. Gastroenterol. 2003, 98, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Cortez–Pinto, H.; Lin, H.Z.; Yang, S.Q.; da Costa‡, S.O.; Diehl, A.M. Lipids up-regulate uncoupling protein 2 expression in rat hepatocytes. Gastroenterology 1999, 116, 1184–1193. [Google Scholar] [CrossRef]
- Perez-Carreras, M.; Del Hoyo, P.; Martin, M.A.; Rubio, J.C.; Martin, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef]
- Einer, C.; Hohenester, S.; Wimmer, R.; Wottke, L.; Artmann, R.; Schulz, S.; Gosmann, C.; Simmons, A.; Leitzinger, C.; Eberhagen, C.; et al. Mitochondrial adaptation in steatotic mice. Mitochondrion 2018, 40, 1–12. [Google Scholar] [CrossRef]
- Simoes, I.C.M.; Karkucinska-Wieckowska, A.; Janikiewicz, J.; Szymanska, S.; Pronicki, M.; Dobrzyn, P.; Dabrowski, M.; Dobrzyn, A.; Oliveira, P.J.; Zischka, H.; et al. Western Diet Causes Obesity-Induced Nonalcoholic Fatty Liver Disease Development by Differentially Compromising the Autophagic Response. Antioxidants 2020, 9, 995. [Google Scholar] [CrossRef] [PubMed]
- Aydos, L.R.; Amaral, L.A.D.; De Souza, R.S.; Jacobowski, A.C.; Dos Santos, E.F.; Macedo, M.L.R. Nonalcoholic Fatty Liver Disease Induced by High-Fat Diet in C57bl/6 Models. Nutrients 2019, 11, 3067. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, P.A.; Kowaltowski, A.J. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol. 2016, 8, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, M.; Estall, J.L. Mitochondrial Dysfunction in the Transition from NASH to HCC. Metabolites 2019, 9, 233. [Google Scholar] [CrossRef]
- Sunny, N.E.; Bril, F.; Cusi, K. Mitochondrial Adaptation in Nonalcoholic Fatty Liver Disease: Novel Mechanisms and Treatment Strategies. Trends Endocrinol. Metab. 2017, 28, 250–260. [Google Scholar] [CrossRef]
- Liu, M.; Lampi, A.-M.; Ertbjerg, P. Unsaturated fat fraction from lard increases the oxidative stability of minced pork. Meat Sci. 2018, 143, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Muyyarikkandy, M.S.; McLeod, M.; Maguire, M.; Mahar, R.; Kattapuram, N.; Zhang, C.; Surugihalli, C.; Muralidaran, V.; Vavilikolanu, K.; Mathews, C.E.; et al. Branched chain amino acids and carbohydrate restriction exacerbate ketogenesis and hepatic mitochondrial oxidative dysfunction during NAFLD. FASEB J. 2020, 34, 14832–14849. [Google Scholar] [CrossRef]
- Surugihalli, C.; Porter, T.E.; Chan, A.; Farley, L.S.; Maguire, M.; Zhang, C.; Kattapuram, N.; Muyyarikkandy, M.S.; Liu, H.-C.; Sunny, N.E. Hepatic Mitochondrial Oxidative Metabolism and Lipogenesis Synergistically Adapt to Mediate Healthy Embryonic-to-Neonatal Transition in Chicken. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]



| Feeding Status | LF | HF | HFr/HF | |
|---|---|---|---|---|
| Body Weight (g) | Fed | 29.0 ± 0.6 | 42.9 ± 1.1 a | 33.4 ± 1.1 bc |
| Fasted | 26.2 ± 0.8 | 35.1 ± 1.4 a | 31.7 ± 1.2 b | |
| Food Intake (kcal/day) | 9.97 ± 0.14 | 12.95 ± 0.19 a | 12.30 ± 0.18 bc | |
| Liver Weight (g) | Fed | 1.43 ± 0.05 | 1.84 ± 0.18 a | 1.74 ± 0.08 b |
| Fasted | 1.10 ± 0.05 | 1.15 ± 0.05 | 1.21 ± 0.06 | |
| Plasma Glucose (mM) | Fed | 7.38 ± 0.24 | 7.70 ± 0.29 | 7.31 ± 0.24 |
| Fasted | 4.76 ± 0.36 | 5.99 ± 0.33 a | 5.20 ± 0.36 | |
| Liver Glycogen (mg/g liver) | Fed | 52.7 ± 4.6 | 50.5 ± 4.5 | 64.3 ± 3.4 c |
| Fasted | 5.0 ± 1.9 | 8.6 ± 1.7 | 2.4 ± 0.6c | |
| Plasma Insulin (ng/mL) | Fed | 1.24 ± 0.40 | 3.83 ± 1.13 | 2.92 ± 1.20 |
| Fasted | 0.42 ± 0.20 | 0.59 ± 0.18 | 0.78 ± 0.25 | |
| Plasma NEFA (mM) | Fed | 0.34 ± 0.05 | 0.52 ± 0.03 a | 0.37 ± 0.04 c |
| Fasted | 0.92 ± 0.04 | 0.78 ± 0.04 a | 0.78 ± 0.05 | |
| Liver TG (mg/g liver) | Fed | 23.1 ± 2.0 | 31.1 ± 2.7 a | 23.5 ± 1.7 c |
| Fasted | 41.2 ± 3.8 | 35.3 ± 2.2 | 34.9 ± 2.3 | |
| Total Liver TG (mg) | Fed | 33.4 ± 3.6 | 60.3 ± 12.3 | 40.9 ± 3.7 |
| Fasted | 46.2 ± 5.7 | 40.6 ± 3.0 | 43.5 ± 4.5 | |
| Inguinal Adipose Tissue Weight (g) | Fed | 0.92 ± 0.14 | 2.74 ± 0.25 a | 1.56 ± 0.15 bc |
| Fasted | 0.78 ± 0.22 | 2.00 ± 0.42 a | 1.58 ± 0.20 b | |
| HOMA-IR | 2.45 ± 1.32 | 4.07 ± 1.41 | 5.29 ± 2.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kattapuram, N.; Zhang, C.; Muyyarikkandy, M.S.; Surugihalli, C.; Muralidaran, V.; Gregory, T.; Sunny, N.E. Dietary Macronutrient Composition Differentially Modulates the Remodeling of Mitochondrial Oxidative Metabolism during NAFLD. Metabolites 2021, 11, 272. https://doi.org/10.3390/metabo11050272
Kattapuram N, Zhang C, Muyyarikkandy MS, Surugihalli C, Muralidaran V, Gregory T, Sunny NE. Dietary Macronutrient Composition Differentially Modulates the Remodeling of Mitochondrial Oxidative Metabolism during NAFLD. Metabolites. 2021; 11(5):272. https://doi.org/10.3390/metabo11050272
Chicago/Turabian StyleKattapuram, Nathan, Christine Zhang, Muhammed S. Muyyarikkandy, Chaitra Surugihalli, Vaishna Muralidaran, Tabitha Gregory, and Nishanth E. Sunny. 2021. "Dietary Macronutrient Composition Differentially Modulates the Remodeling of Mitochondrial Oxidative Metabolism during NAFLD" Metabolites 11, no. 5: 272. https://doi.org/10.3390/metabo11050272
APA StyleKattapuram, N., Zhang, C., Muyyarikkandy, M. S., Surugihalli, C., Muralidaran, V., Gregory, T., & Sunny, N. E. (2021). Dietary Macronutrient Composition Differentially Modulates the Remodeling of Mitochondrial Oxidative Metabolism during NAFLD. Metabolites, 11(5), 272. https://doi.org/10.3390/metabo11050272






