Association between Urinary Metabolites and the Exposure of Intensive Care Newborns to Plasticizers of Medical Devices Used for Their Care Management
Abstract
1. Introduction
2. Results
2.1. Study Population
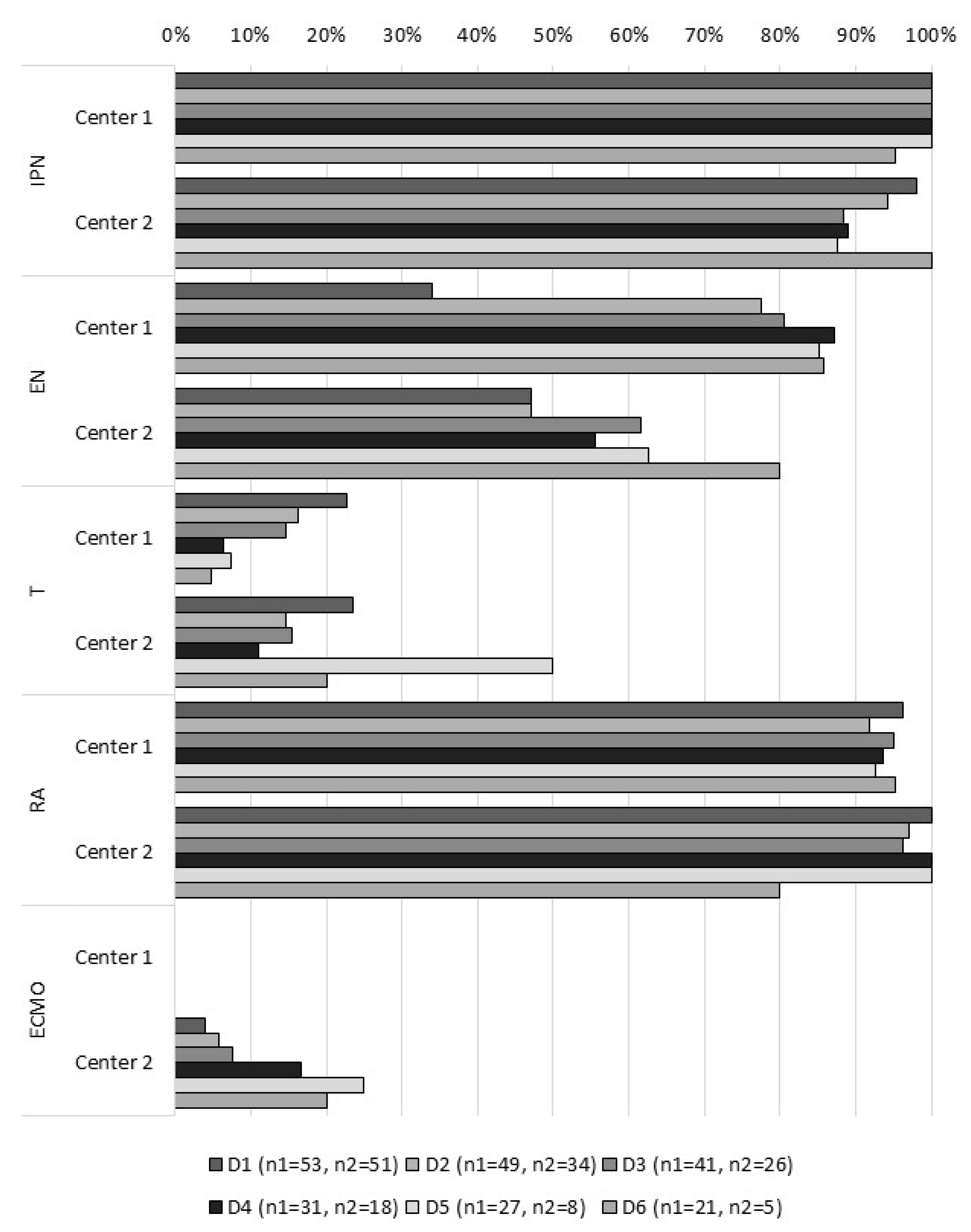
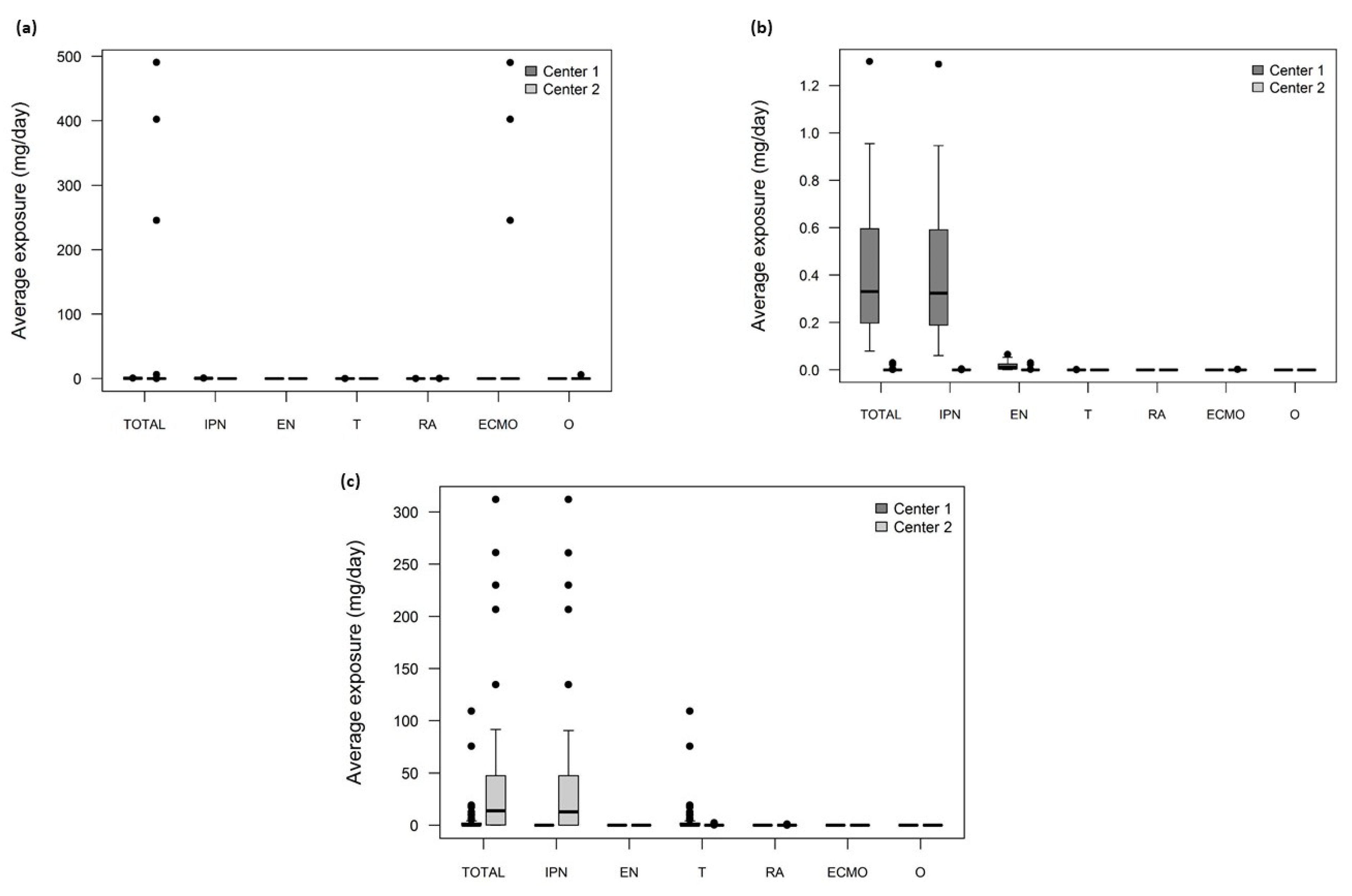
2.2. Assessment of Plasticizers’ Exposure
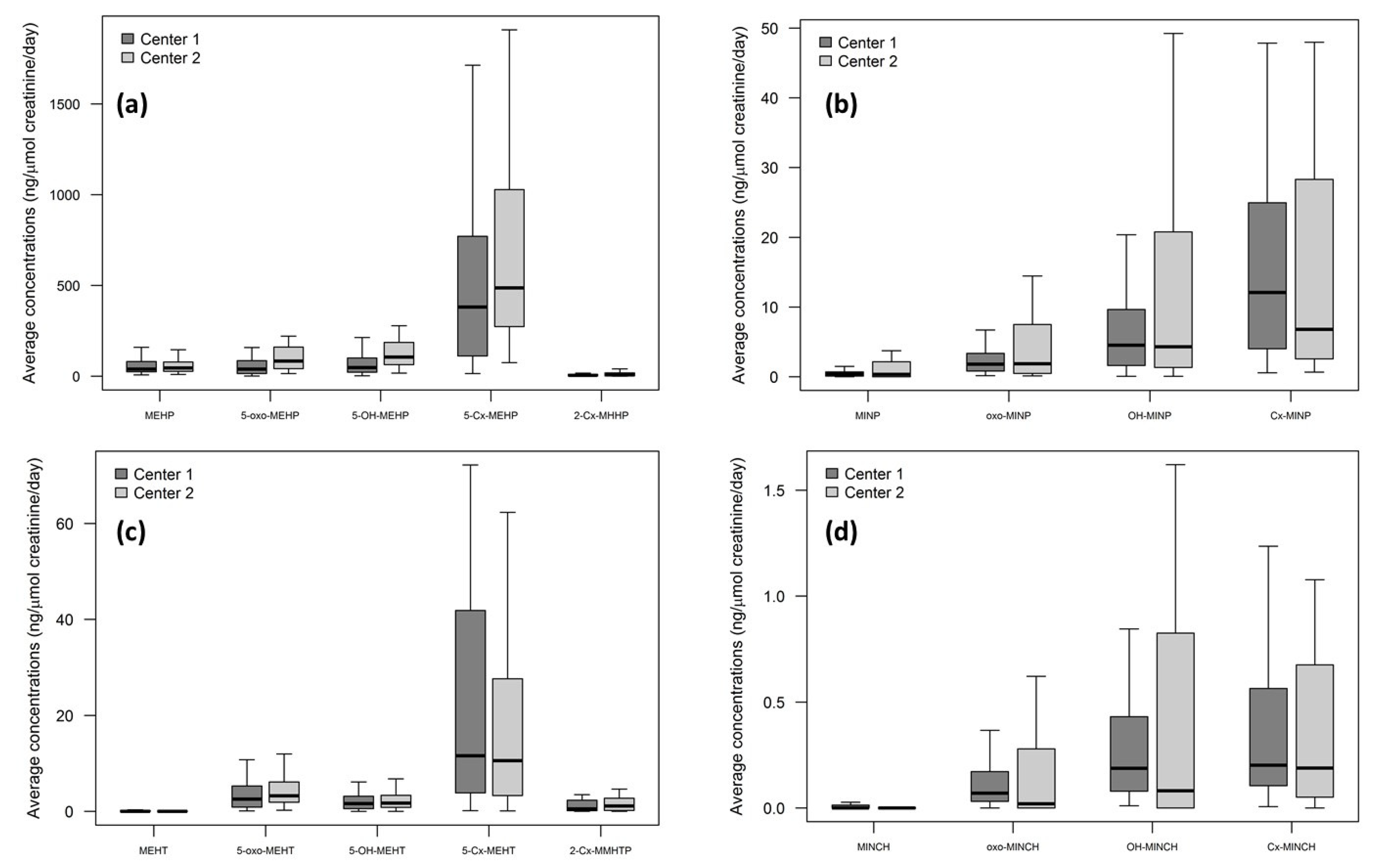
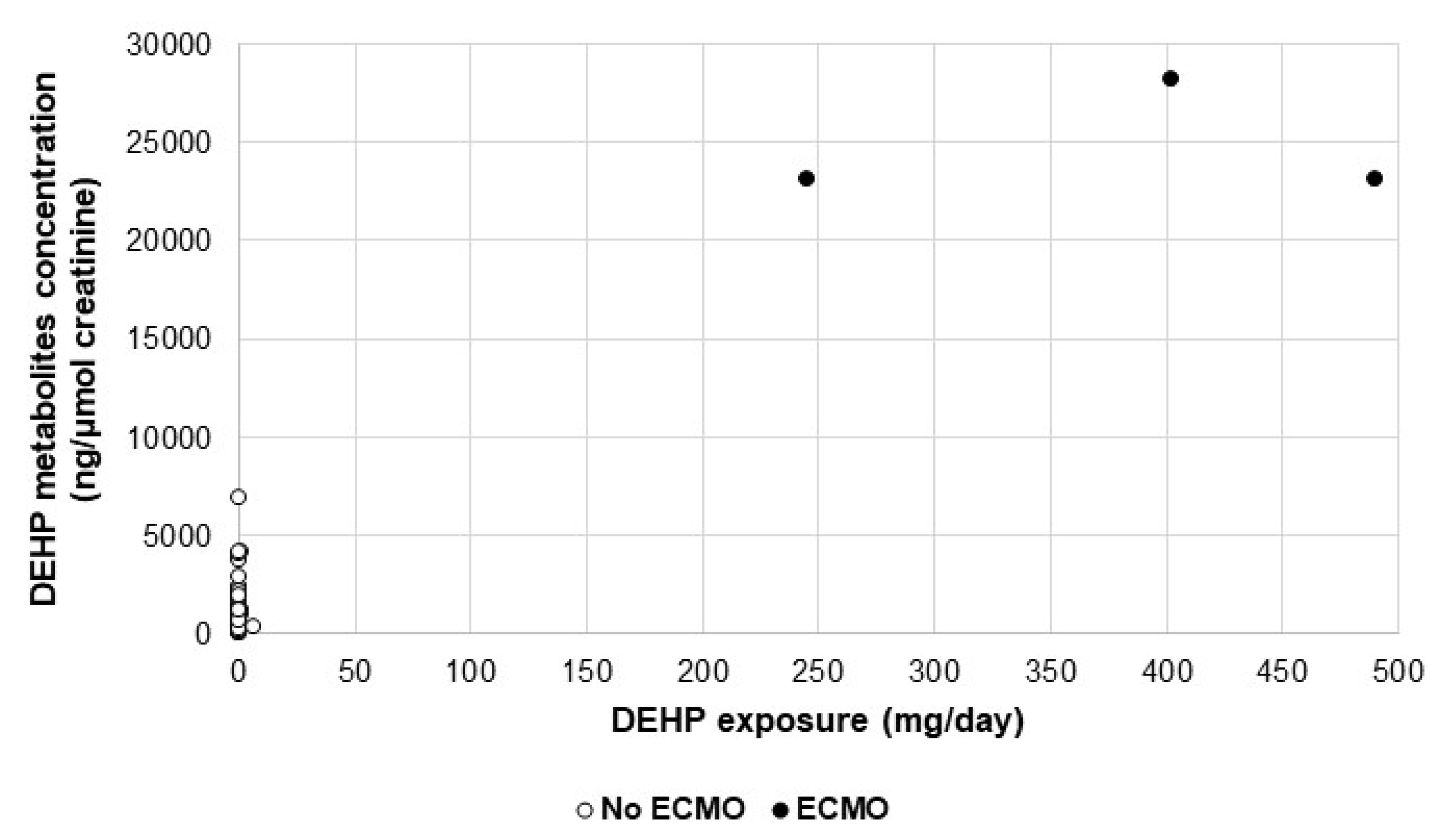
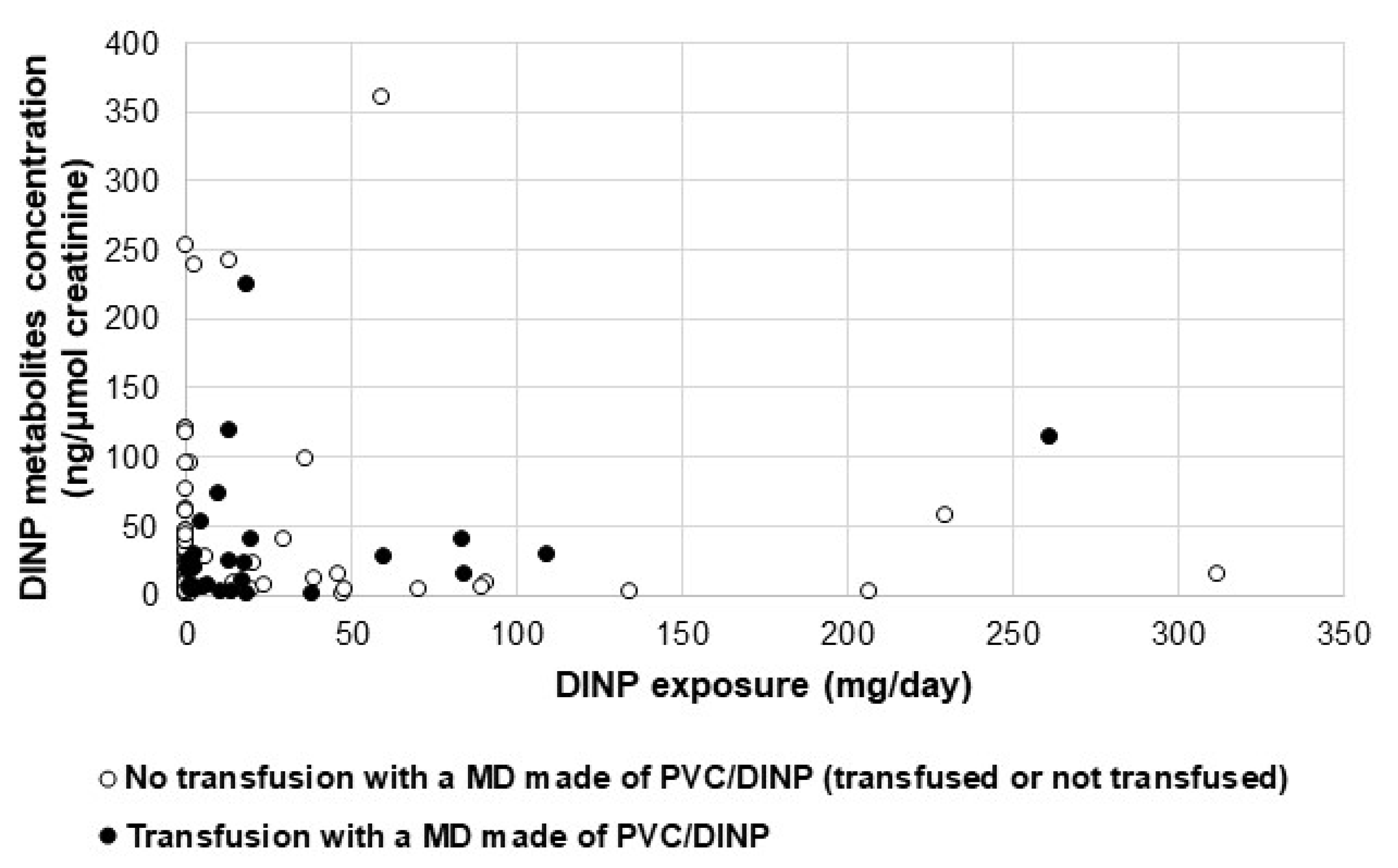
2.3. Assessment of Biomarkers in Urine
3. Discussion
4. Materials and Methods
4.1. Study Population
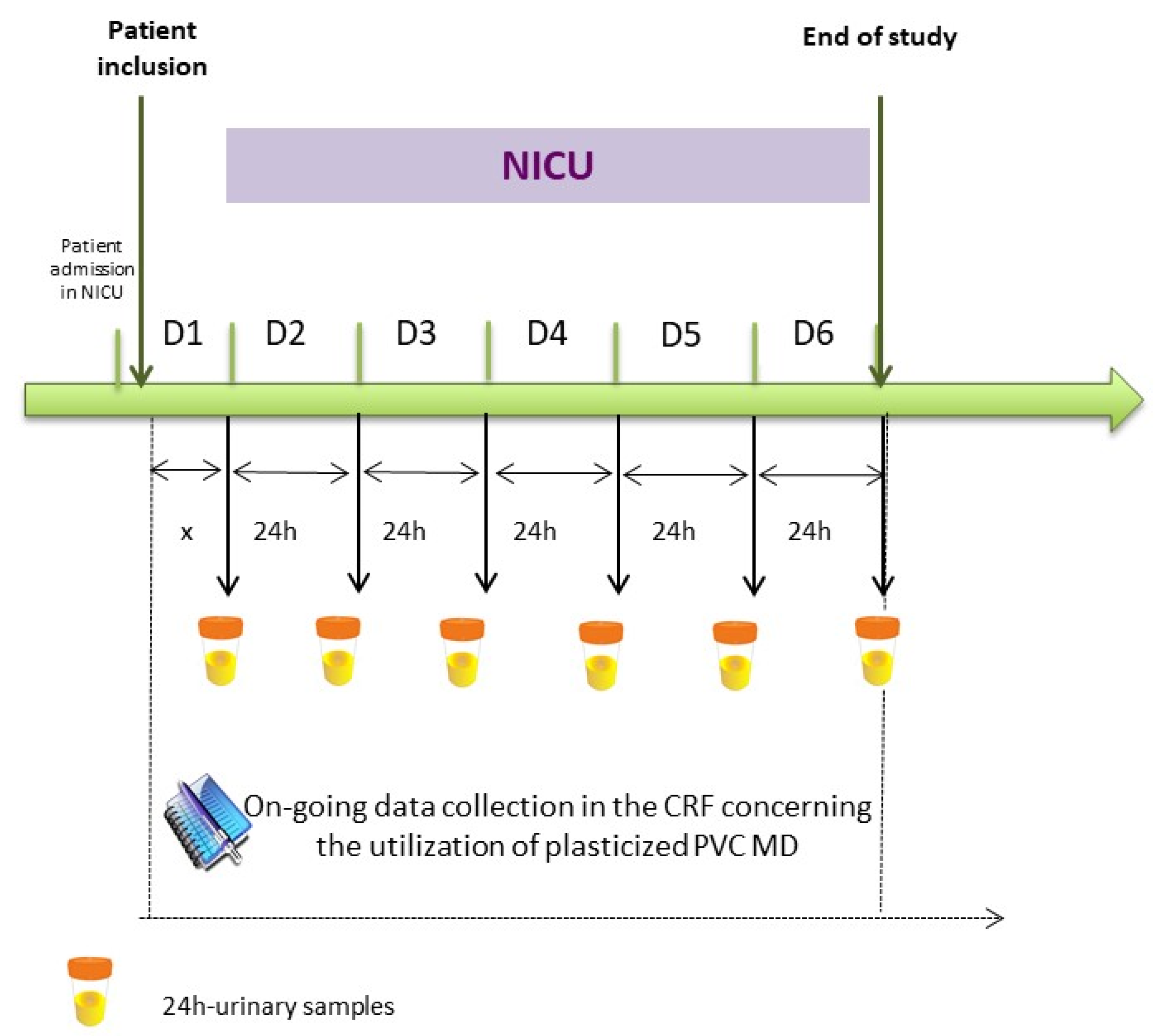
4.2. Study design
4.3. Assessment of Plasticizers Exposure
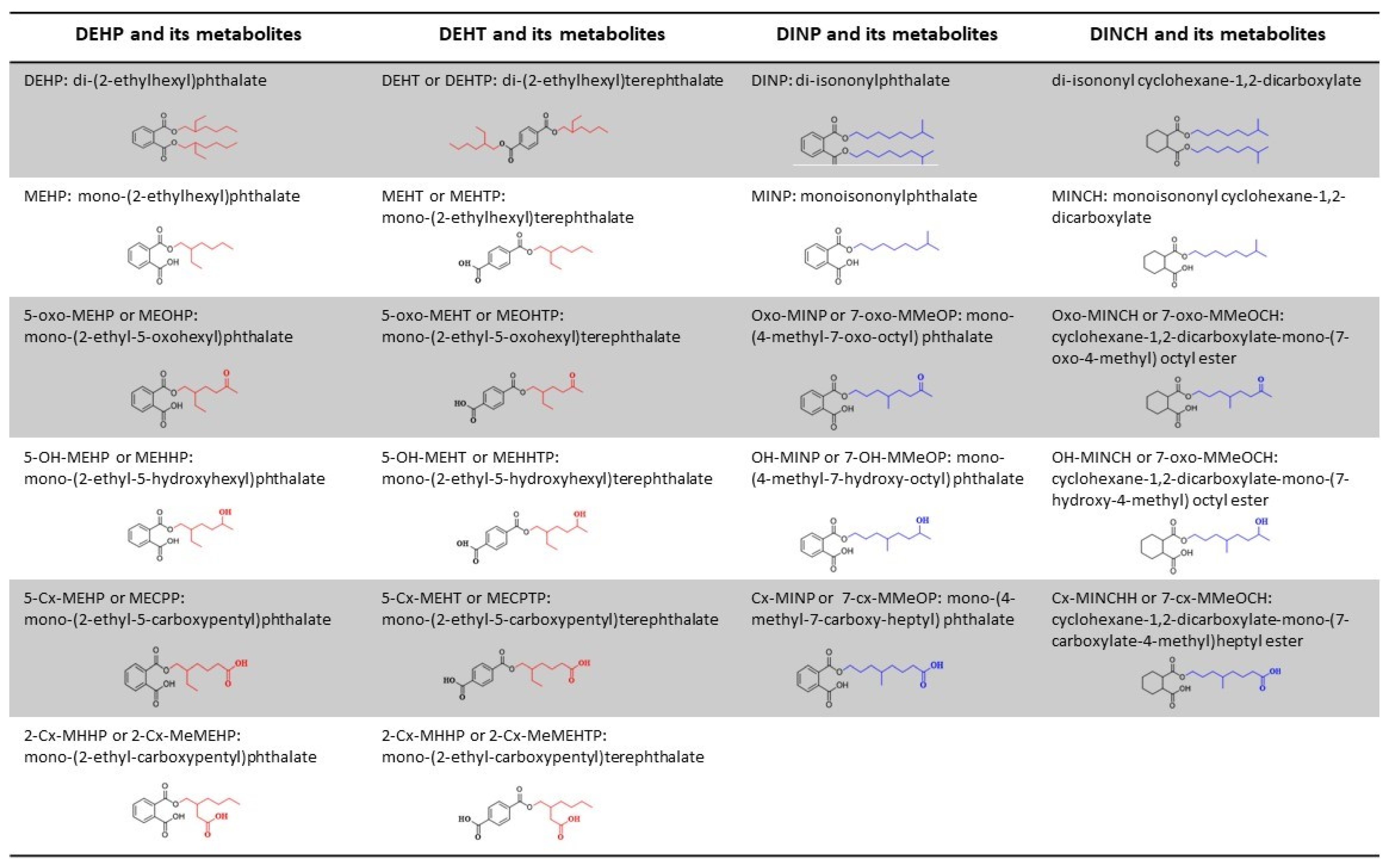
4.4. Assessment of Biomarkers in Urine
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANSM | Agence Nationale de Sécurité du Médicament et des Produits de Santé |
| ARMED | Assessment and Risk management of Medical Devices in plasticized polyvinyl chloride |
| ARs | Androgen receptors |
| CLP | Classification Labeling and Packaging |
| CMR | Cancerigen Mutagen or Reprotoxic |
| CRF | Case Report Form |
| DEHP | Di-ethylhexylphthalate |
| DEHT | Di-(2-ethylhexyl) terephthalate |
| DNEL | Derived no effect level |
| DINCH | Di-isononylcyclohexane-1,2-dicarboxylate |
| DINP | Di-isononylphthalate |
| ECHA | European CHemicals Agency |
| ECMO | Extra-Corporeal Membrane Oxygenation |
| EN | Enteral Nutrition |
| GC-MS | Gas Chromatography – Mass Spectrometry |
| HD | Hemodialysis |
| hERα | human estrogen receptor α |
| IPN | Infusion and Parenteral Nutrition |
| MD | Medical device |
| NICNAS | National Industrial Chemicals Notification and Assessment Scheme |
| NICU | Neonatal Intensive Care Unit |
| PN | Parenteral Nutrition |
| PVC | polyvinyl chloride |
| SHBG | Sex Hormone Binging Globulin |
| SPE-LC-MS/MS | (Solid Phase Extraction-Liquid Chromatography- Mass Spectrometry/MassSpectrometry) |
References
- Testai, E.; Hartemann, P.; Rastogi, S.C.; Bernauer, U.; Piersma, A.; De Jong, W.; Gulliksson, H.; Sharpe, R.; Schubert, D.; Rodríguez-Farre, E.; et al. The safety of medical devices containing DEHP plasticized PVC or other plasticizers on neonates and other groups possibly at risk (2015 update). Regul. Toxicol. Pharmacol. 2016, 76, 209–210. [Google Scholar] [CrossRef]
- Bernard, L.; Cueff, R.; Breysse, C.; Décaudin, B.; Sautou, V. Migrability of PVC plasticizers from medical devices into a simulant of infused solutions. Int. J. Pharm. 2015, 485, 341–347. [Google Scholar] [CrossRef]
- Bastiaensen, M.; Malarvannan, G.; Been, F.; Yin, S.; Yao, Y.; Huygh, J.; Clotman, K.; Schepens, T.; Jorens, P.G.; Covaci, A. Metabolites of phosphate flame retardants and alternative plasticizers in urine from intensive care patients. Chemosphere 2019, 233, 590–596. [Google Scholar] [CrossRef]
- Regulation (EC). No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 (Text. with EEA Relevance); Official Journal of the European Union: Luxembourg, 2008. [Google Scholar]
- Regulation (EU). 2017/745 of the European Parliament and of the Council of on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text. with EEA Relevance); Official Journal of the European Union: Luxembourg, 5 April 2017. [Google Scholar]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, K.R.; Mitchell, R.T. Effect of environmental and pharmaceutical exposures on fetal testis development and function: A systematic review of human experimental data. Hum. Reprod. Update 2019, 25, 397–421. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, J.; Xu, S.; Wan, Y.; Li, Y.; Jiang, Y.; Zhao, H.; Zhou, Y.; Liao, J.; Liu, H.; et al. Prenatal exposure to phthalates and neurocognitive development in children at two years of age. Environ. Int. 2019, 131, 105023. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Information on Chemicals. Available online: http://Echa.Europa.Eu/Information-on-Chemicals (accessed on 1 March 2020).
- Johns, E.L.; Ferguson, K.K.; Soldin, O.P.; E Cantonwine, D.; O Rivera-González, L.; Del Toro, L.V.A.; Calafat, A.M.; Ye, X.; Alshawabkeh, A.N.; Cordero, J.F.; et al. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: A longitudinal analysis. Reprod. Biol. Endocrinol. 2015, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghisari, M.; Bonefeld-Jorgensen, E.C. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol. Lett. 2009, 189, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-B.; Chuang, C.-J.; Su, P.-H.; Sun, C.-W.; Wang, C.-J.; Wu, M.-T.; Wang, S.-L. Prenatal and Childhood Exposure to Phthalate Diesters and Thyroid Function in a 9-Year Follow-up Birth Cohort Study. Epidemiology 2017, 28, S10–S18. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Vassilopoulou, L.; Mamoulakis, C.; Psycharakis, C.; Anifantaki, A.; Sifakis, S.; Docea, A.O.; Tsiaoussis, J.; Makrigiannakis, A.; Tsatsakis, A.M. Endocrine Disruptors Leading to Obesity and Related Diseases. Int. J. Environ. Res. Public Health 2017, 14, 1282. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Park, C.G.; Kim, S.H.; Kim, Y.J. The Effects of Mono-(2-Ethylhexyl) Phthalate (MEHP) on Human Estrogen Receptor (hER) and Androgen Receptor (hAR) by YES/YAS In Vitro Assay. Molecules 2019, 24, 1558. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; Sánchez, B.N.; Calafat, A.M.; Schettler, T.; Green, R.A.; Hu, H.; Hauser, R. Exposure to Phthalates in Neonatal Intensive Care Unit Infants: Urinary Concentrations of Monoesters and Oxidative Metabolites. Environ. Health Perspect. 2006, 114, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Hauser, R.; Calafat, A.M.; Weuve, J.; Schettler, T.; Ringer, S.; Huttner, K.; Hu, H. Use of Di(2-ethylhexyl) Phthalate–Containing Medical Products and Urinary Levels of Mono(2-ethylhexyl) Phthalate in Neonatal Intensive Care Unit Infants. Environ. Health Perspect. 2005, 113, 1222–1225. [Google Scholar] [CrossRef]
- Calafat, A.M.; Needham, L.L.; Silva, M.J.; Lambert, G. Exposure to Di-(2-Ethylhexyl) Phthalate among Premature Neonates in a Neonatal Intensive Care Unit. Pediatrics 2004, 113, e429–e434. [Google Scholar] [CrossRef]
- Demirel, A.; Çoban, A.; Yıldırım, Ş.; Doğan, C.; Sancı, R.; Ince, Z. Hidden Toxicity in Neonatal Intensive Care Units: Phthalate Exposure in Very Low Birth Weight Infants. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 298–304. [Google Scholar] [CrossRef]
- Gaynor, J.W.; Ittenbach, R.F.; Calafat, A.M.; Burnham, N.B.; Bradman, A.; Bellinger, D.C.; Henretig, F.M.; Wehrung, E.E.; Ward, J.L.; Russell, W.W.; et al. Perioperative Exposure to Suspect Neurotoxicants From Medical Devices in Newborns With Congenital Heart Defects. Ann. Thorac. Surg. 2019, 107, 567–572. [Google Scholar] [CrossRef]
- Rossi, M. Neonatal Exposure to DEHP (Di-2-Ethylhexyl Phthalate) and Opportunities for Prevention. Health Care Harm 2002, 24. [Google Scholar]
- Stroustrup, A.; Bragg, J.B.; Busgang, S.A.; Andra, S.S.; Curtin, P.; Spear, E.A.; Just, A.C.; Arora, M.; Gennings, C. Sources of clinically significant neonatal intensive care unit phthalate exposure. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 137–148. [Google Scholar] [CrossRef]
- Bernard, L.; Eljezi, T.; Clauson, H.; Lambert, C.; Bouattour, Y.; Chennell, P.; Pereira, B.; Sautou, V.; on behalf of the ARMED Study Group. Effects of flow rate on the migration of different plasticizers from PVC infusion medical devices. PLoS ONE 2018, 13, e0192369. [Google Scholar] [CrossRef]
- Bernard, L.; Décaudin, B.; Lecoeur, M.; Richard, D.; Bourdeaux, D.; Cueff, R.; Sautou, V. Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: A review. Talanta 2014, 129, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Consumer Product Safety Commission. Prohibition of Children’s Toys and Child Care Articles Containing Specified Phthalates. 2017. Available online: https://www.federalregister.gov/documents/2017/10/27/2017-23267/prohibition-of-childrens-toys-and-child-care-articles-containing-specified-phthalates (accessed on 29 September 2019).
- National Industrial Chemicals Notification and Assessment Scheme, L. 7 1,2-Cyclohexanedicarboxylic Acid, 1,2-Diisononyl Ester (‘Hexamoll DINCH’)—Full Public Report. Available online: https://www.nicnas.gov.au/search?query=dinch&collection=nicnas-meta (accessed on 1 March 2020).
- Sheikh, I.A.; Yasir, M.; Abu-Elmagd, M.; Dar, T.A.; Abuzenadah, A.M.; Damanhouri, G.A.; Al-Qahtani, M.; Beg, M.A.; Yasir, M. Human sex hormone-binding globulin as a potential target of alternate plasticizers: An in silico study. BMC Struct. Biol. 2016, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K. CPSC Staff Statement on University of Cincinnati Report: Toxicity Review for Di-2 -Ethylhexyl Terephthalate (DEHT); University of Cincinnati: Cincinnati, OH, USA, 2018. [Google Scholar]
- Kambia, N.K.; Séverin, I.; Farce, A.; Moreau, E.; Dahbi, L.; Duval, C.; Dine, T.; Sautou, V.; Chagnon, M.C. In vitro and in silico hormonal activity studies of di-(2-ethylhexyl)terephthalate, a di-(2-ethylhexyl)phthalate substitute used in medical devices, and its metabolites. J. Appl. Toxicol. 2019, 39, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, P.; Thomas, S.; Bousquet, C.; Maggio, A.F.; Civade, C.; Brenier, C.; Bonnet, P.A. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC–MS. J. Chromatogr. B 2014, 949-950, 99–108. [Google Scholar] [CrossRef]
- Schulz, C.; Wilhelm, M.; Heudorf, U.; Kolossa-Gehring, M. Update of the reference and HBM values derived by the German Human Biomonitoring Commission. Int. J. Hyg. Environ. Health 2011, 215, 26–35. [Google Scholar] [CrossRef]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef]
- Koch, H.M.; Angerer, J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int. J. Hyg. Environ. Health 2007, 210, 9–19. [Google Scholar] [CrossRef]
- Lin, S.; Ku, H.Y.; Su, P.H.; Chen, J.W.; Huang, P.C.; Angerer, J.; Wang, S.L. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere 2011, 82, 947–955. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.; Choi, K.; Kim, K.T. Comparative analysis of endocrine disrupting effects of major phthalates in employed two cell lines (MVLN and H295R) and embryonic zebrafish assay. Environ. Res. 2019, 172, 319–325. [Google Scholar] [CrossRef]
- Pelletier, M.; Glorennec, P.; Mandin, C.; Le Bot, B.; Ramalho, O.; Mercier, F.; Bonvallot, N. Chemical-by-chemical and cumulative risk assessment of residential indoor exposure to semivolatile organic compounds in France. Environ. Int. 2018, 117, 22–32. [Google Scholar] [CrossRef]
- Weiss, J.M.; Gustafsson, Å.; Gerde, P.; Bergman, Å.; Lindh, C.H.; Krais, A.M. Daily intake of phthalates, MEHP, and DINCH by ingestion and inhalation. Chemosphere 2018, 208, 40–49. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, B. Modeled Exposure Assessment via Inhalation and Dermal Pathways to Airborne Semivolatile Organic Compounds (SVOCs) in Residences. Environ. Sci. Technol. 2014, 48, 5691–5699. [Google Scholar] [CrossRef]
- Li, H.L.; Ma, W.L.; Liu, L.Y.; Zhang, Z.; Sverko, E.; Zhang, Z.F.; Song, W.W.; Sun, Y.; Li, Y.F. Phthalates in infant cotton clothing: Occurrence and implications for human exposure. Sci. Total Environ. 2019, 683, 109–115. [Google Scholar] [CrossRef]
- Morrison, G.C.; Weschler, C.J.; Bekö, G.; Koch, H.M.; Salthammer, T.; Schripp, T.; Toftum, J.; Clausen, G. Role of clothing in both accelerating and impeding dermal absorption of airborne SVOCs. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Domingo, J.L. Human health risks due to exposure to inorganic and organic chemicals from textiles: A review. Environ. Res. 2019, 168, 62–69. [Google Scholar] [CrossRef]
- Schütze, A.; Otter, R.; Modick, H.; Langsch, A.; Brüning, T.; Koch, H.M. Additional oxidized and alkyl chain breakdown metabolites of the plasticizer DINCH in urine after oral dosage to human volunteers. Arch. Toxicol. 2016, 91, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Schütze, A.; Pälmke, C.; Angerer, J.; Brüning, T. Metabolism of the plasticizer and phthalate substitute diisononyl-cyclohexane-1,2-dicarboxylate (DINCH®) in humans after single oral doses. Arch. Toxicol. 2012, 87, 799–806. [Google Scholar] [CrossRef]
- Zhou, C.; Flaws, J.A. Effects of an Environmentally Relevant Phthalate Mixture on Cultured Mouse Antral Follicles. Toxicol. Sci. 2016, 156, 217–229. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef] [PubMed]
- Gaudriault, P.; Mazaud-Guittot, S.; Lavoué, V.; Coiffec, I.; Lesné, L.; Dejucq-Rainsford, N.; Scholze, M.; Kortenkamp, A.; Jégou, B. Endocrine Disruption in Human Fetal Testis Explants by Individual and Combined Exposures to Selected Pharmaceuticals, Pesticides, and Environmental Pollutants. Environ. Health Perspect. 2017, 125, 087004. [Google Scholar] [CrossRef]
- Bernard, L.; Cueff, R.; Chagnon, M.; Abdoulouhab, F.; Décaudin, B.; Breysse, C.; Kauffmann, S.; Cosserant, B.; Souweine, B.; Sautou, V. Migration of plasticizers from PVC medical devices: Development of an infusion model. Int. J. Pharm. 2015, 494, 136–145. [Google Scholar] [CrossRef]
- Fick, A.V. On liquid diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Bourdeaux, D.; Yessaad, M.; Chennell, P.; Larbre, V.; Eljezi, T.; Bernard, L.; Sautou, V. Analysis of PVC plasticizers in medical devices and infused solutions by GC–MS. J. Pharm. Biomed. Anal. 2016, 118, 206–213. [Google Scholar] [CrossRef]
- Bouattour, Y.; Wasiak, M.; Bernard, L.; Pinguet, J.; Richard, D.; Le Rouzo-Grèves, M.; Dhifallah, I.; Lambert, C.; Pereira, B.; Chennell, P.; et al. Quantification of bis(2-ethylhexyl) phthalate released by medical devices during respiratory assistance and estimation of patient exposure. Chemosphere 2020, 255, 126978. [Google Scholar] [CrossRef]
- Demir, A.P.T.; Ulutan, S. Migration of phthalate and non-phthalate plasticizers out of plasticized PVC films into air. J. Appl. Polym. Sci. 2012, 128, 1948–1961. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.W.; Lin, Q.B.; Hu, C.Y. Study of the Migration of Stabilizer and Plasticizer from Polyethylene Terephthalate into Food Simulants. J. Chromatogr. Sci. 2016, 54, 939–951. [Google Scholar] [CrossRef]
- Takehisa, H.; Naoko, E.; Masahiko, S.; Katsuhide, T.; Moriyuki, O.; Keizoh, S.; Mutsuko, T.; Kenji, K.; Shin’Ichiro, N.; Toshio, O. Release behavior of diethylhexyl phthalate from the polyvinyl-chloride tubing used for intravenous administration and the plasticized PVC membrane. Int. J. Pharm. 2005, 297, 30–37. [Google Scholar] [CrossRef]
- Yuan, H.; Hao, Q.; Su, R.; Qi, W.; He, Z. Migration of phthalates from polyvinyl chloride film to fatty food simulants: Experimental studies and model application. J. Consum. Prot. Food Saf. 2019, 15, 135–143. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Lee, C.; Nah, J.; Hahn, A. DEHP Migration Behavior from Excessively Plasticized PVC Sheets. Bull. Korean Chem. Soc. 2003, 24, 345–349. [Google Scholar] [CrossRef]
- Pinguet, J.; Kerckhove, N.; Eljezi, T.; Lambert, C.; Moreau, E.; Bernard, L.; Boeuf, B.; Decaudin, B.; Genay, S.; Masse, M.; et al. New SPE-LC-MS/MS method for the simultaneous determination in urine of 22 metabolites of DEHP and alternative plasticizers from PVC medical devices. Talanta 2019, 198, 377–389. [Google Scholar] [CrossRef]
| Center 1 (n = 51) | Center 2 (n = 51) | Total (n = 102) | ||||
|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | |
| DEHP | 0.18 | 0.22 | 0.04 | 0.77 | 0.15 | 0.13 |
| DEHT | 0.20 | 0.16 | 0.15 | 0.31 | 0.08 | 0.42 |
| DINP | −0.06 | 0.65 | 0.09 | 0.54 | 0.03 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernard, L.; Bouattour, Y.; Masse, M.; Boeuf, B.; Decaudin, B.; Genay, S.; Lambert, C.; Moreau, E.; Pereira, B.; Pinguet, J.; et al. Association between Urinary Metabolites and the Exposure of Intensive Care Newborns to Plasticizers of Medical Devices Used for Their Care Management. Metabolites 2021, 11, 252. https://doi.org/10.3390/metabo11040252
Bernard L, Bouattour Y, Masse M, Boeuf B, Decaudin B, Genay S, Lambert C, Moreau E, Pereira B, Pinguet J, et al. Association between Urinary Metabolites and the Exposure of Intensive Care Newborns to Plasticizers of Medical Devices Used for Their Care Management. Metabolites. 2021; 11(4):252. https://doi.org/10.3390/metabo11040252
Chicago/Turabian StyleBernard, Lise, Yassine Bouattour, Morgane Masse, Benoît Boeuf, Bertrand Decaudin, Stéphanie Genay, Céline Lambert, Emmanuel Moreau, Bruno Pereira, Jérémy Pinguet, and et al. 2021. "Association between Urinary Metabolites and the Exposure of Intensive Care Newborns to Plasticizers of Medical Devices Used for Their Care Management" Metabolites 11, no. 4: 252. https://doi.org/10.3390/metabo11040252
APA StyleBernard, L., Bouattour, Y., Masse, M., Boeuf, B., Decaudin, B., Genay, S., Lambert, C., Moreau, E., Pereira, B., Pinguet, J., Richard, D., Sautou, V., & for the ARMED Study Group. (2021). Association between Urinary Metabolites and the Exposure of Intensive Care Newborns to Plasticizers of Medical Devices Used for Their Care Management. Metabolites, 11(4), 252. https://doi.org/10.3390/metabo11040252









