Application of Metabolomics in Pediatric Asthma: Prediction, Diagnosis and Personalized Treatment
Abstract
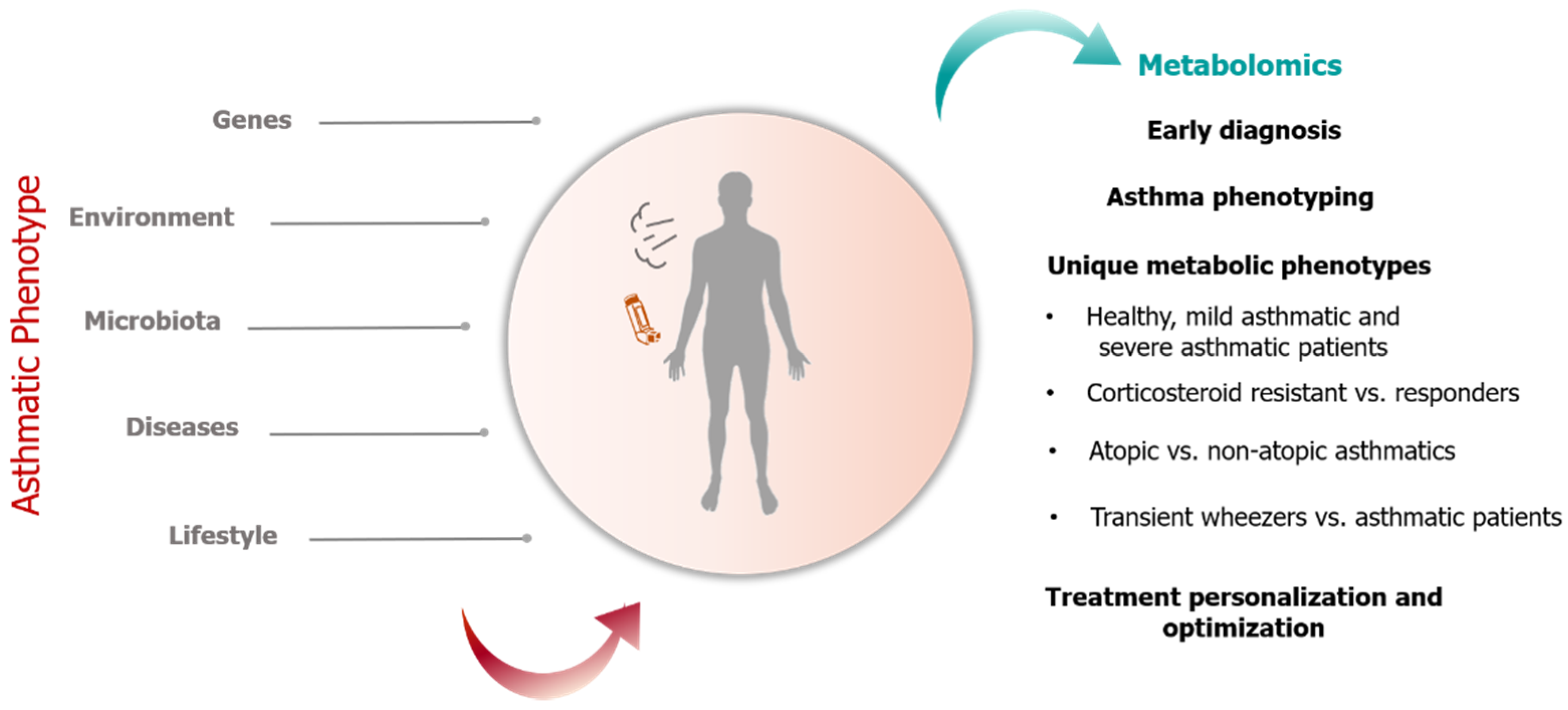
1. Introduction
1.1. Strategies in Metabolomics Research
1.2. Application of Metabolomics in Pediatric Asthma Research
2. Results
3. Discussion
3.1. Hypoxia and Energy Deficits
3.1.1. Citric Acid Cycle
3.1.2. Nicotinamide
3.2. Protein Synthesis/Degradation
3.3. One-Carbon Folate Cycle
3.4. Purine Metabolism
3.5. Lipid Metabolism and Inflammation
3.6. Oxidative Stress
3.7. Bile Acids
3.8. Gut Microbiota
3.9. Steroid Hormone Biosynthesis
3.10. Xenobiotics
4. Methods
5. Limitations/Strengths
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GINA. Pocket guide for Asthma Management and Prevention (For Adults and Children older than 5 years). In Global Initiative for Asthma; Global Initiative for Asthma: Fontana-on-Geneva Lake, WI, USA, 2019; Available online: www.ginasthma.org (accessed on 15 March 2021).
- Beasley, R.; Semprini, A.; Mitchell, E.A. Risk factors for asthma: Is prevention possible? Lancet 2015, 386, 1075–1085. [Google Scholar] [CrossRef]
- Asher, I.; Pearce, N. Global burden of asthma among children. Int. J. Tuberc. Lung Dis. 2014, 18, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Katsardis, C.V.; Alexandraki, S.; Paraskakis, E. Chapter 2: Spirometry in children 6-16 years old. In Paediatric Pulmonary Function Testing Indications and Interpretation; Katsardis, C., Koumbourlis, A., Anthracopoulos, M., Paraskakis, E., Eds.; NOVA Biomedical: New York, NY, USA, 2015; pp. 15–42. [Google Scholar]
- Frima, E.-S.; Theodorakopoulos, I.; Gidaris, D.; Karantaglis, N.; Chatziparasidis, G.; Plotas, P.; Anthracopoulos, M.; Fouzas, S. Lung Function Variability in Children and Adolescents With and Without Asthma (LUV Study): Protocol for a Prospective, Nonrandomized, Clinical Trial. JMIR Res. Protoc. 2020, 9, e20350. [Google Scholar] [CrossRef]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Wang, T.J.; Gona, P.; Larson, M.G.; Tofler, G.H.; Levy, D.; Newton-Cheh, C.; Jacques, P.F.; Rifai, N.; Selhub, J.; Robins, S.J.; et al. Multiple Biomarkers for the Prediction of First Major Cardiovascular Events and Death. N. Engl. J. Med. 2006, 355, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Fragoulakis, V.; Sarandi, E.; Docea, A.O.; Papakonstaninou, E.; Tsilimidos, G.; Anamaterou, C.; Fragkiadaki, P.; Aschner, M.; Tsatsakis, A.; et al. Targeted Metabolomic Analysis of Serum Fatty Acids for the Prediction of Autoimmune Diseases. Front. Mol. Biosci. 2019, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, D.; Fragoulakis, V.; Papakonstantinou, E.; Antonaki, M.; Vozikis, A.; Tsatsakis, A.; Buga, A.M.; Mitroi, M.; Calina, D. Prediction of Autoimmune Diseases by Targeted Metabolomic Assay of Urinary Organic Acids. Metabolites 2020, 10, 502. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Sarandi, E.; Thanasoula, M.; Anamaterou, C.; Papakonstantinou, E.; Geraci, F.; Papamichael, M.M.; Itsiopoulos, C.; Tsoukalas, D. Metabolic profiling of organic and fatty acids in chronic and autoimmune diseases. Adv. Appl. Microbiol. 2021, 101, 169–229. [Google Scholar] [CrossRef]
- Kelly, R.S.; Dahlin, A.; McGeachie, M.J.; Qiu, W.; Sordillo, J.; Wan, E.S.; Wu, A.C.; Lasky-Su, J. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest 2017, 151, 262–277. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.I.; Atherton, H.J.; Goodacre, R.; Griffin, J.L. Systems level studies of mammalian metabolomes: The roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 2011, 40, 387–426. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30–32. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Park, Y.; Brown, L.A.S.; Jones, D.P. Children with severe asthma have unique oxidative stress-associated metabolomic profiles. J. Allergy Clin. Immunol. 2014, 133, 258.e8–261.e8. [Google Scholar] [CrossRef] [PubMed]
- Mattarucchi, E.; Baraldi, E.; Guillou, C. Metabolomics applied to urine samples in childhood asthma; Differentiation between asthma phenotypes and identification of relevant metabolites. Biomed. Chromatogr. 2011, 26, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Van De Kant, K.D.G.; Van Berkel, J.J.B.N.; Jöbsis, Q.; Passos, V.L.; Klaassen, E.M.M.; Van Der Sande, L.; Van Schayck, O.C.P.; De Jongste, J.C.; Van Schooten, F.J.; Derks, E.; et al. Exhaled breath profiling in diagnosing wheezy preschool children. Eur. Respir. J. 2012, 41, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Davies, D.E. In vitro and ex vivo models of human asthma. Eur. J. Pharm. Biopharm. 2013, 84, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Carraro, S.; Baraldi, E.; Giordano, G.; Pirillo, P.; Stocchero, M.; Houben, M.; Bont, L. Metabolomic Profile of Amniotic Fluid and Wheezing in the First Year of Life—A Healthy Birth Cohort Study. J. Pediatr. 2018, 196, 264.e4–269.e4. [Google Scholar] [CrossRef]
- Chawes, B.L.; Giordano, G.; Pirillo, P.; Rago, D.; Rasmussen, M.A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Baraldi, E. Neonatal Urine Metabolic Profiling and Development of Childhood Asthma. Metabolites 2019, 9, 185. [Google Scholar] [CrossRef]
- Carraro, S.; Bozzetto, S.; Giordano, G.; El Mazloum, D.; Stocchero, M.; Pirillo, P.; Zanconato, S.; Baraldi, E. Wheezing preschool children with early-onset asthma reveal a specific metabolomic profile. Pediatr. Allergy Immunol. 2018, 29, 375–382. [Google Scholar] [CrossRef]
- Smolinska, A.; Klaassen, E.M.M.; Dallinga, J.W.; Van De Kant, K.D.G.; Jobsis, Q.; Moonen, E.J.C.; Van Schayck, O.C.P.; Dompeling, E.; Van Schooten, F.J. Profiling of Volatile Organic Compounds in Exhaled Breath As a Strategy to Find Early Predictive Signatures of Asthma in Children. PLoS ONE 2014, 9, e95668. [Google Scholar] [CrossRef]
- Klaassen, E.M.M.; Van De Kant, K.D.G.; Jöbsis, Q.; Van Schayck, O.C.P.; Smolinska, A.; Dallinga, J.W.; Van Schooten, F.J.; Hartog, G.J.M.D.; De Jongste, J.C.; Rijkers, G.T.; et al. Exhaled Biomarkers and Gene Expression at Preschool Age Improve Asthma Prediction at 6 Years of Age. Am. J. Respir. Crit. Care Med. 2015, 191, 201–207. [Google Scholar] [CrossRef]
- Tao, J.-L.; Chen, Y.-Z.; Dai, Q.-G.; Tian, M.; Wang, S.-C.; Shan, J.-J.; Ji, J.-J.; Lin, L.-L.; Li, W.-W.; Yuan, B. Urine metabolic profiles in paediatric asthma. Respirology 2019, 24, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Turi, K.N.; Romick-Rosendale, L.; Gebretsadik, T.; Watanabe, M.; Brunwasser, S.; Anderson, L.J.; Moore, M.L.; Larkin, E.K.; Peebles, R.S.; Hartert, T.V. Using urine metabolomics to understand the pathogenesis of infant respiratory syncytial virus (RSV) infection and its role in childhood wheezing. Metabolomics 2018, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Cheng, M.-L.; Chiang, M.-H.; Wang, C.-J.; Tsai, M.-H.; Lin, G. Metabolomic Analysis Reveals Distinct Profiles in the Plasma and Urine Associated with IgE Reactions in Childhood Asthma. J. Clin. Med. 2020, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Saude, E.J.; Skappak, C.D.; Regush, S.; Cook, K.; Ben-Zvi, A.; Becker, A.; Moqbel, R.; Sykes, B.D.; Rowe, B.H.; Adamko, D.J. Metabolomic profiling of asthma: Diagnostic utility of urine nuclear magnetic resonance spectroscopy. J. Allergy Clin. Immunol. 2011, 127, 757.e6–764.e6. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, M.M.; Katsardis, C.; Erbas, B.; Itsiopoulos, C.; Tsoukalas, D.; Katsardis, C. Urinary organic acids as biomarkers in the assessment of pulmonary function in children with asthma. Nutr. Res. 2019, 61, 31–40. [Google Scholar] [CrossRef]
- Kelly, R.S.; Virkud, Y.; Giorgio, R.; Celedón, J.C.; Weiss, S.T.; Lasky-Su, J. Metabolomic profiling of lung function in Costa-Rican children with asthma. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1590–1595. [Google Scholar] [CrossRef]
- Carraro, S.; Giordano, G.; Reniero, F.; Carpi, D.; Stocchero, M.; Sterk, P.; Baraldi, E. Asthma severity in childhood and metabolomic profiling of breath condensate. Allergy 2012, 68, 110–117. [Google Scholar] [CrossRef]
- Park, Y.H.; Fitzpatrick, A.M.; Medriano, C.A.; Jones, D.P. High-resolution metabolomics to identify urine biomarkers in corticosteroid-resistant asthmatic children. J. Allergy Clin. Immunol. 2017, 139, 1518.e4–1524.e4. [Google Scholar] [CrossRef][Green Version]
- Barlotta, A.; Pirillo, P.; Stocchero, M.; Donato, F.; Giordano, G.; Bont, L.; Zanconato, S.; Carraro, S.; Baraldi, E. Metabolomic Profiling of Infants With Recurrent Wheezing After Bronchiolitis. J. Infect. Dis. 2018, 219, 1216–1223. [Google Scholar] [CrossRef]
- Dallinga, J.W.; Robroeks, C.M.H.H.T.; Van Berkel, J.J.B.N.; Moonen, E.J.C.; Godschalk, R.W.L.; Jöbsis, Q.; Dompeling, E.; Wouters, E.F.M.; Van Schooten, F.J. Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin. Exp. Allergy 2009, 40, 68–76. [Google Scholar] [CrossRef]
- Atzei, A.; Atzori, L.; Moretti, C.; Barberini, L.; Noto, A.; Ottonello, G.; Pusceddu, E.; Fanos, V. Metabolomics in paediatric respiratory diseases and bronchiolitis. J. Matern. Neonatal. Med. 2011, 24, 59–62. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Lin, G.; Cheng, M.-L.; Chiang, M.-H.; Tsai, M.-H.; Su, K.-W.; Hua, M.-C.; Liao, S.-L.; Lai, S.-H.; Yao, T.-C.; et al. Longitudinal urinary metabolomic profiling reveals metabolites for asthma development in early childhood. Pediatr. Allergy Immunol. 2018, 29, 496–503. [Google Scholar] [CrossRef]
- Gahleitner, F.; Guallar-Hoyas, C.; Beardsmore, C.S.; Pandya, H.C.; Thomas, C.P. Metabolomics pilot study to identify volatile organic compound markers of childhood asthma in exhaled breath. Bioanalysis 2013, 5, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Guo, Z.-G.; He, B.; Yao, W.-Z. Metabolic alterations in the sera of Chinese patients with mild persistent asthma: A GC-MS-based metabolomics analysis. Acta Pharmacol. Sin. 2015, 36, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Aragonés, J.; Fraisl, P.; Baes, M.; Carmeliet, P. Oxygen Sensors at the Crossroad of Metabolism. Cell Metab. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Ho, W.E.; Xu, Y.-J.; Xu, F.; Cheng, C.; Peh, H.Y.; Tannenbaum, S.R.; Wong, W.S.F.; Ong, C.N. Metabolomics Reveals Altered Metabolic Pathways in Experimental Asthma. Am. J. Respir. Cell Mol. Biol. 2013, 48, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.-H.; Lee, H.-S.; Choi, G.S.; Jung, Y.-S.; Ryu, D.H.; Park, H.-S.; Hwang, G.-S. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin. Exp. Allergy 2013, 43, 425–433. [Google Scholar] [CrossRef]
- Infantino, V.; Iacobazzi, V.; Menga, A.; Avantaggiati, M.L.; Palmieri, F. A key role of the mitochondrial citrate carrier (SLC25A1) in TNFα- and IFNγ-triggered inflammation. Biochim. Biophys. Acta Bioenerg. 2014, 1839, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef]
- Litwack, G. Pantothenic Acid in Chapter 20—Vitamins and Nutrition. In Human Biochemistry; Litwack, G., Ed.; Academic Press: Boston, MA, USA, 2018; pp. 645–680. [Google Scholar]
- Nelson, D.; Cox, M.L. Principles of Biochemistry; W.H Freeman & Co Ltd.: New York, NY, USA, 2017. [Google Scholar]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Hettling, H.; Van Beek, J.H.G.M. Analyzing the Functional Properties of the Creatine Kinase System with Multiscale “Sloppy” Modeling. PLoS Comput. Biol. 2011, 7, e1002130. [Google Scholar] [CrossRef]
- Upadhyay, D.; Dave, S.T.C. Rhabdomyolysis in Acute Severe Asthma: A Case Report and Literature Review. Int. J. Asthma Allergy Immunol. 2001, 2, 1–4. Available online: https://ispub.com/IJAAI/2/2/6514 (accessed on 15 March 2021).
- Demos, M.A.; Gitin, E.L.; Kagen, L.J. Exercise myoglobinemia and acute exertional rhabdomyolysis. Arch. Intern. Med. 1974, 134, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kumar, M.; Mabalirajan, U.; Pattnaik, B.; Aggarwal, S.; Singh, R.; Singh, S.; Mukerji, M.; Ghosh, B.; Agrawal, A. Hypoxia Response in Asthma. Am. J. Respir. Cell Mol. Biol. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Comhair, S.A.A.; McDunn, J.; Bennett, C.; Fettig, J.; Erzurum, S.C.; Kalhan, S.C.; Fetig, J. Metabolomic Endotype of Asthma. J. Immunol. 2015, 195, 643–650. [Google Scholar] [CrossRef]
- Gebicki, J.; Sysa-Jedrzejowska, A.; Adamus, J.; Woźniacka, A.; Rybak, M.; Zielonka, J. 1-Methylnicotinamide: A potent anti-inflammatory agent of vitamin origin. Pol. J. Pharmacol. 2003, 55, 109–112. [Google Scholar] [PubMed]
- Biedroń, R.; Ciszek, M.; Tokarczyk, M.; Bobek, M.; Kurnyta, M.; Słomińska, E.M.; Smolenski, R.T.; Marcinkiewicz, J. 1-Methylnicotinamide and nicotinamide: Two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch. Immunol. Ther. Exp. 2008, 56, 127–134. [Google Scholar] [CrossRef]
- Oka, T.; Itoi, T.; Terada, N.; Nakanishi, H.; Taguchi, R.; Hamaoka, K. Change in the Membranous Lipid Composition Accelerates Lipid Peroxidation in Young Rat Hearts Subjected to 2 Weeks of Hypoxia Followed by Hyperoxia. Circ. J. 2008, 72, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.F.; Pepine, C.J. The role of carnitine in myocardial dysfunction. Am. J. Kidney Dis. 2003, 41, S35–S43. [Google Scholar] [CrossRef]
- Baker, P.R.S.; Cramer, S.D.; Kennedy, M.; Assimos, D.G.; Holmes, R.P. Glycolate and glyoxylate metabolism in HepG2 cells. Am. J. Physiol. Physiol. 2004, 287, C1359–C1365. [Google Scholar] [CrossRef]
- Kalapos, M. Possible physiological roles of acetone metabolism in humans. Med. Hypotheses 1999, 53, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Horne, S.; Sharp, P.; Sharps, R.; Kippelen, P. Effect of Creatine Supplementation on the Airways of Youth Elite Soccer Players. Med. Sci. Sports Exerc. 2019, 51, 1582–1590. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Toledo, A.C.; Hage, M.; Santos, A.B.G.; Medeiros, M.C.R.; Martins, M.A.; Carvalho, C.; Dolhnikoff, M.; Vieira, R.P. Creatine Activates Airway Epithelium in Asthma. Endoscopy 2010, 31, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Kiyatake, I.; Koide, H.; Jung, K.Y.; Endou, H. Biosynthesis of Guanidinoacetic Acid in Isolated Renal Tubules. Clin. Chem. Lab. Med. 1992, 30, 325–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strapková, A.; Antošová, M. Glutamate receptors and the airways hyperreactivity. Gen. Physiol. Biophys. 2012, 31, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Hasegawa, K.; Wong, B.M.C.; Ajami, N.J.; Petrosino, J.F.; Piedra, P.A.; Espinola, M.J.A.; Tierney, M.C.N.; Camargo, J.C.A.; Mansbach, J.M. Respiratory Syncytial Virus and Rhinovirus Bronchiolitis Are Associated With Distinct Metabolic Pathways. J. Infect. Dis. 2017, 217, 1160–1169. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y.; Liu, S.; Mao, S.; Ling, Y.; Liu, D.; He, X.; Wang, X. Metabonomic Profiling of Serum and Urine by 1H NMR-Based Spectroscopy Discriminates Patients with Chronic Obstructive Pulmonary Disease and Healthy Individuals. PLoS ONE 2013, 8, e65675. [Google Scholar] [CrossRef]
- Kassel, D.B.; Martin, M.; Schall, W.; Sweeley, C.C. Urinary metabolites ofL-threonine in type 1 diabetes determined by combined gas chromatography/chemical ionization mass spectrometry. J. Mass Spectrom. 1986, 13, 535–540. [Google Scholar] [CrossRef]
- Sanchez Jimenez, J.; Herrero Espinet, F.J.; Mengibar Garrido, J.M.; Roca Antonio, J.; Penos Mayor, S.; Penas Boira, M.D.M.; Roca Comas, A.; Ballester Martinez, A. Asthma and insulin resistance in obese children and adolescents. Pediatr. Allergy Immunol. 2014, 25, 699–705. [Google Scholar] [CrossRef] [PubMed]
- NCBI. PubChem Compound Summary for Creatinine. USA: National Center for Biotechnology Information. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Creatinine (accessed on 7 January 2021).
- Atzori, L. 1H NMR-based metabolomic analysis of urine from preterm and term neonates. Front. Biosci. 2011, 3, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.M.; Gómez, A.H.; Abitbol, C.L.; Chandar, J.J.; Duara, S.; Zilleruelo, G.E. Histomorphometric Analysis of Postnatal Glomerulogenesis in Extremely Preterm Infants. Pediatr. Dev. Pathol. 2004, 7, 17–25. [Google Scholar] [CrossRef]
- Aguirre, M.; Oliveros, R.; Vallo, A. Long-term renal follow-up of extremely low birth weight infants. Pediatr. Nephrol. 2005, 20, 579–584. [Google Scholar] [CrossRef]
- Licari, A.; Fuchs, D.; Marseglia, G.; Ciprandi, G. Tryptophan metabolic pathway and neopterin in asthmatic children in clinical practice. Ital. J. Pediatr. 2019, 45, 1–4. [Google Scholar] [CrossRef]
- Wedes, S.H.; Wu, W.; Comhair, S.A.; McDowell, K.M.; DiDonato, J.A.; Erzurum, S.C.; Hazen, S.L. Urinary Bromotyrosine Measures Asthma Control and Predicts Asthma Exacerbations in Children. J. Pediatr. 2011, 159, 248.e1–255.e1. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, IJTR.S2129–IJTR.S2160. [Google Scholar] [CrossRef]
- Kepert, I.; Fonseca, J.; Müller, C.; Milger, K.; Hochwind, K.; Kostric, M.; Fedoseeva, M.; Ohnmacht, C.; Dehmel, S.; Nathan, P.; et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J. Allergy Clin. Immunol. 2017, 139, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Van Der Sluijs, K.F.; Van De Pol, M.A.; Kulik, W.; Dijkhuis, A.; Smids, B.S.; Van Eijk, H.W.; Karlas, J.A.; Molenkamp, R.; Wolthers, K.C.; Johnston, S.L.; et al. Systemic tryptophan and kynurenine catabolite levels relate to severity of rhinovirus-induced asthma exacerbation: A prospective study with a parallel-group design. Thorax 2013, 68, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Bharath, A. Manipulation of indoleamine 2,3 dioxygenase; a novel therapeutic target for treatment of diseases. Expert Opin. Ther. Targets 2009, 13, 987–1012. [Google Scholar] [CrossRef]
- Gostner, J.M.; Becker, K.; Kofler, H.; Strasser, B.; Fuchs, D. Tryptophan Metabolism in Allergic Disorders. Int. Arch. Allergy Immunol. 2016, 169, 203–215. [Google Scholar] [CrossRef]
- Ünüvar, S.; Erge, D.; Kılıçarslan, B.; Bağ, H.G.G.; Çatal, F.; Girgin, G.; Baydar, T. Neopterin Levels and Indoleamine 2,3-Dioxygenase Activity as Biomarkers of Immune System Activation and Childhood Allergic Diseases. Ann. Lab. Med. 2019, 39, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J. Allergy Clin. Immunol. 2008, 122, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Noto, A.; Fanos, V.; Barberini, L.; Grapov, D.; Fattuoni, C.; Zaffanello, M.; Casanova, A.; Fenu, G.; De Giacomo, A.; De Angelis, M.; et al. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J. Matern. Neonat. Med. 2014, 27, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.A.; Carson, M.J.; Nair, M.G. Non-traditional cytokines: How catecholamines and adipokines influence macrophages in immunity, metabolism and the central nervous system. Cytokine 2015, 72, 210–219. [Google Scholar] [CrossRef]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garcés-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavón, L. Immunomodulatory Effects Mediated by Serotonin. J. Immunol. Res. 2015, 2015, 1–21. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound Summary for CID 4740700, 3-Phenylpropionate; National Center for Biotechnology Information, National Library of Medicine: Bethesda, MD, USA, 2021. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3-Phenylpropionate (accessed on 18 March 2021).
- MacPherson, J.C.; Comhair, S.A.A.; Erzurum, S.C.; Klein, D.F.; Lipscomb, M.F.; Kavuru, M.S.; Samoszuk, M.K.; Hazen, S.L. Eosinophils Are a Major Source of Nitric Oxide-Derived Oxidants in Severe Asthma: Characterization of Pathways Available to Eosinophils for Generating Reactive Nitrogen Species. J. Immunol. 2001, 166, 5763–5772. [Google Scholar] [CrossRef]
- Wu, W.; Samoszuk, M.K.; Comhair, S.A.A.; Thomassen, M.J.; Farver, C.F.; Dweik, R.A.; Kavuru, M.S.; Erzurum, S.C.; Hazen, S.L. Eosinophils generate brominating oxidants in allergen-induced asthma. J. Clin. Investig. 2000, 105, 1455–1463. [Google Scholar] [CrossRef]
- HMDB. Methyl-Imidazole Acetic Acid; Human Metabolome Database: Edmonton, AB, Canada, 2021. Available online: https://hmdb.ca/metabolites/HMDB0002820 (accessed on 18 March 2021).
- Yamauchi, K.; Ogasawara, M. The Role of Histamine in the Pathophysiology of Asthma and the Clinical Efficacy of Antihistamines in Asthma Therapy. Int. J. Mol. Sci. 2019, 20, 1733. [Google Scholar] [CrossRef]
- Neumann, D. Role of the Histamine H4-Receptor in Bronchial Asthma. Organotypic Model. Drug Dev. 2016, 241, 347–359. [Google Scholar] [CrossRef]
- Çakmak, A.; Zeyrek, D.; Atas, A.; Çelik, H.; Aksoy, N.; Erel, O. Serum prolidase activity and oxidative status in patients with bronchial asthma. J. Clin. Lab. Anal. 2009, 23, 132–138. [Google Scholar] [CrossRef]
- Nishitani, S.; Matsumura, T.; Fujitani, S.; Sonaka, I.; Miura, Y.; Yagasaki, K. Leucine promotes glucose uptake in skeletal muscles of rats. Biochem. Biophys. Res. Commun. 2002, 299, 693–696. [Google Scholar] [CrossRef]
- Liu, H.; Liu, R.; Xiong, Y.; Li, X.; Wang, X.; Ma, Y.; Guo, H.; Hao, L.; Yao, P.; Liu, L.; et al. Leucine facilitates the insulin-stimulated glucose uptake and insulin signaling in skeletal muscle cells: Involving mTORC1 and mTORC2. Amino Acids 2014, 46, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Ochiai, K.; Imai, Y.; Moriyasu, F.; Imawari, M. Restoration of innate host defense responses by oral supplementation of branched-chain amino acids in decompensated cirrhotic patients. Hepatol. Res. 2007, 37, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Branched-Chain Amino Acids and Immunity. J. Nutr. 2006, 136, 288S–293S. [Google Scholar] [CrossRef]
- Lara, A.; Khatri, S.B.; Wang, Z.; Comhair, S.A.A.; Xu, W.; Dweik, R.A.; Bodine, M.; Levison, B.S.; Hammel, J.; Bleecker, E.; et al. Alterations of the Arginine Metabolome in Asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 673–681. [Google Scholar] [CrossRef]
- Morris, C.R.; Poljakovic, M.; Lavrisha, L.; Machado, L.; Kuypers, F.A.; Morris, S.M. Decreased Arginine Bioavailability and Increased Serum Arginase Activity in Asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 148–153. [Google Scholar] [CrossRef]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar]
- Mahmoud, A.M.; Ali, M.M. Methyl Donor Micronutrients that Modify DNA Methylation and Cancer Outcome. Nutrients 2019, 11, 608. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.G. SAM/SAH Analogs as Versatile Tools for SAM-Dependent Methyltransferases. ACS Chem. Biol. 2016, 11, 583–597. [Google Scholar] [CrossRef]
- Su, X.; Wellen, K.E.; Rabinowitz, J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef]
- Martino, D.; Prescott, S. Epigenetics and Prenatal Influences on Asthma and Allergic Airways Disease. Chest 2011, 139, 640–647. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Brosnan, M.E.; Brosnan, J.T. Formate: The Neglected Member of One-Carbon Metabolism. Annu. Rev. Nutr. 2016, 36, 369–388. [Google Scholar] [CrossRef]
- Sinha, A.; Desiraju, K.; Aggarwal, K.; Kutum, R.; Roy, S.; Lodha, R.; Kabra, S.K.; Ghosh, B.; Sethi, T.; Agrawal, A. Exhaled breath condensate metabolome clusters for endotype discovery in asthma. J. Transl. Med. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Zeisel, S.H. Diet, Methyl Donors and DNA Methylation: Interactions between Dietary Folate, Methionine and Choline. J. Nutr. 2002, 132, 2333S–2335S. [Google Scholar] [CrossRef]
- Boisvert, F.-M.; Richard, S. Arginine Methylation Regulates the Cytokine Response. Mol. Cell 2004, 15, 492–494. [Google Scholar] [CrossRef]
- Schwartz, D.A. Epigenetics and Environmental Lung Disease. Proc. Am. Thorac. Soc. 2010, 7, 123–125. [Google Scholar] [CrossRef]
- Yu, M.; Cui, F.-X.; Jia, H.-M.; Zhou, C.; Yang, Y.; Zhang, H.-W.; Ding, G.; Zou, Z.-M. Aberrant purine metabolism in allergic asthma revealed by plasma metabolomics. J. Pharm. Biomed. Anal. 2016, 120, 181–189. [Google Scholar] [CrossRef]
- Li, L.; Wan, C.; Wen, F. An unexpected role for serum uric acid as a biomarker for severity of asthma exacerbation. Asian Pac. J. Allergy Immunol. 2013, 32, 93–99. [Google Scholar] [CrossRef]
- Abdulnaby, N.K.; Sayed, A.O.; Shalaby, N.M. Predictive value of serum uric acid in hospitalized adolescents and adults with acute asthma. Ther. Clin. Risk Manag. 2016, 12, 1701–1708. [Google Scholar] [CrossRef]
- Kool, M.; Willart, M.A.; Van Nimwegen, M.; Bergen, I.; Pouliot, P.; Virchow, J.C.; Rogers, N.; Osorio, F.; Sousa, C.R.E.; Hammad, H.; et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma. Immunity 2011, 34, 527–540. [Google Scholar] [CrossRef]
- Spicuzza, L.; Di Maria, G.; Polosa, R. Adenosine in the airways: Implications and applications. Eur. J. Pharmacol. 2006, 533, 77–88. [Google Scholar] [CrossRef]
- Driver, A.G.; Kukoly, C.A.; Ali, S.; Mustafa, S.J. Adenosine in bronchoalveolar lavage fluid in asthma. Am. Rev. Respir. Dis. 1993, 148, 91–97. [Google Scholar] [CrossRef]
- Huszár, É.; Vass, G.; Vizi, É.; Csoma, Z.; Barát, E.; Világos, G.M.; Herjavecz, I.; Horváth, I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur. Respir. J. 2002, 20, 1393–1398. [Google Scholar] [CrossRef]
- Brown, R.A.; Spina, D.; Page, C.P. Adenosine receptors and asthma. Br. J. Pharmacol. 2008, 153, S446–S456. [Google Scholar] [CrossRef]
- HMDB. N-Methyladenosine; Human Metabolome Database: Edmonton, AB, Canada, 2021. Available online: https://hmdb.ca/metabolites/HMDB0004044 (accessed on 15 March 2021).
- HMDB. Hypoxanthine; Human Metabolome Database: Edmonton, AB, Canada, 2021. Available online: https://hmdb.ca/metabolites/HMDB0000157 (accessed on 15 March 2021).
- Lee, M.-Y.; Lee, N.-H.; Jung, D.; Lee, J.-A.; Seo, C.-S.; Lee, H.; Kim, J.-H.; Shin, H.-K. Protective effects of allantoin against ovalbumin (OVA)-induced lung inflammation in a murine model of asthma. Int. Immunopharmacol. 2010, 10, 474–480. [Google Scholar] [CrossRef]
- Pan, L.; Han, P.; Ma, S.; Peng, R.; Wang, C.; Kong, W.; Cong, L.; Fu, J.; Zhang, Z.; Yu, H.; et al. Abnormal metabolism of gut microbiota reveals the possible molecular mechanism of nephropathy induced by hyperuricemia. Acta Pharm. Sin. B 2020, 10, 249–261. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef]
- Ferraro, V.A.; Carraro, S.; Pirillo, P.; Gucciardi, A.; Poloniato, G.; Stocchero, M.; Giordano, G.; Zanconato, S.; Baraldi, E. Breathomics in Asthmatic Children Treated with Inhaled Corticosteroids. Metabolites 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Gallart-Ayala, H.; Gómez, C.; Checa, A.; Fauland, A.; Naz, S.; Kamleh, M.A.; Djukanović, R.; Hinks, T.S.; Wheelock, C.E. Metabolomics analysis identifies different metabotypes of asthma severity. Eur. Respir. J. 2017, 49, 1601740. [Google Scholar] [CrossRef] [PubMed]
- Comhair, S.A.A.; Bochenek, G.; Baicker-McKee, S.; Wang, Z.; Stachura, T.; Sanak, M.; Hammel, J.P.; Hazen, S.L.; Erzurum, S.C.; Nizankowska-Mogilnicka, E. The utility of biomarkers in diagnosis of aspirin exacerbated respiratory disease. Respir. Res. 2018, 19, 210. [Google Scholar] [CrossRef]
- Miyata, J.; Fukunaga, K.; Kawashima, Y.; Ohara, O.; Kawana, A.; Asano, K.; Arita, M. Dysregulated metabolism of polyunsaturated fatty acids in eosinophilic allergic diseases. Prostaglandins Other Lipid Mediat. 2020, 150, 106477. [Google Scholar] [CrossRef]
- Sanak, M. Eicosanoid Mediators in the Airway Inflammation of Asthmatic Patients: What is New? Allergy Asthma Immunol. Res. 2016, 8, 481–490. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.J. Exhaled leukotrienes and prostaglandins in asthma. J. Allergy Clin. Immunol. 2002, 109, 615–620. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Liang, C.-L.; Li, G.-M.; Yu, C.-Y.; Yin, M. Stearic acid protects primary cultured cortical neurons against oxidative stress. Acta Pharmacol. Sin. 2007, 28, 315–326. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; García-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G.; the ISAAC Phase III Study Group. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef]
- Nagel, G.; Weinmayr, G.; Kleiner, A.; Garcia-Marcos, L.; Strachan, D.P.; the ISAAC Phase Two Study Group. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax 2010, 65, 516–522. [Google Scholar] [CrossRef]
- Calder, P.C.; Kremmyda, L.-S.; Vlachava, M.; Noakes, P.S.; Miles, E.A. Is there a role for fatty acids in early life programming of the immune system? Proc. Nutr. Soc. 2010, 69, 373–380. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Shrestha, S.K.; Itsiopoulos, C.; Erbas, B. The role of fish intake on asthma in children: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 350–360. [Google Scholar] [CrossRef]
- Biong, A.S.; Berstad, P.; Pedersen, J.I. Biomarkers for intake of dairy fat and dairy products. Eur. J. Lipid Sci. Technol. 2006, 108, 827–834. [Google Scholar] [CrossRef]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef]
- La Marca, G.; Rizzo, C. Analysis of organic acids and acylglycines for the diagnosis of related inborn errors of metabolism by GC- and HPLC-MS. In Metabolic Profiling, Methods in Molecular Biology; Metz, T.O., Ed.; Springer: Berlin, Germany, 2011; pp. 73–98. [Google Scholar]
- HMDB. 3-Hydroxytetradecanedioic Acid; Human Metabolome Database: Edmonton, AB, Canada, 2021. Available online: https://hmdb.ca/metabolites/HMDB0000394 (accessed on 15 March 2021).
- Kumps, A.; Duez, P.; Mardens, Y. Metabolic, nutritional, iatrogenic, and artifactual sources of urinary organic acids: A comprehensive table. Clin. Chem. 2002, 48, 708–717. [Google Scholar] [PubMed]
- Quinn, K.D.; Schedel, M.; Nkrumah-Elie, Y.; Joetham, A.; Armstrong, M.; Cruickshank-Quinn, C.; Reisdorph, N.; Gelfand, E.W. Dysregulation of metabolic pathways in a mouse model of allergic asthma. Allergy 2017, 72, 1327–1337. [Google Scholar] [CrossRef]
- Yoder, M.; Zhuge, Y.; Yuan, Y.; Holian, O.; Kuo, S.; Van Breemen, R.; Thomas, L.L.; Lum, H. Bioactive Lysophosphatidylcholine 16:0 and 18:0 Are Elevated in Lungs of Asthmatic Subjects. Allergy Asthma Immunol. Res. 2014, 6, 61–65. [Google Scholar] [CrossRef]
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Ono, J.G.; Worgall, T.S.; Worgall, S. Airway reactivity and sphingolipids—Implications for childhood asthma. Mol. Cell. Pediatr. 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Mishra, V.; Banga, J.; Silveyra, P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol. Ther. 2018, 181, 169–182. [Google Scholar] [CrossRef]
- Niwa, T. Indoxyl Sulfate Is a Nephro-Vascular Toxin. J. Ren. Nutr. 2010, 20, S2–S6. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Pujos-Guillot, E.; Hubert, J.; Martin, J.-F.; Lyan, B.; Quintana, M.; Claude, S.; Chabanas, B.; Rothwell, J.A.; Bennetau-Pelissero, C.; Scalbert, A.; et al. Mass Spectrometry-based Metabolomics for the Discovery of Biomarkers of Fruit and Vegetable Intake: Citrus Fruit as a Case Study. J. Proteome Res. 2013, 12, 1645–1659. [Google Scholar] [CrossRef]
- Motta, A.; Paris, D.; D’Amato, M.; Melck, D.; Calabrese, C.; Vitale, C.; Stanziola, A.A.; Corso, G.; Sofia, M.; Maniscalco, M. NMR Metabolomic Analysis of Exhaled Breath Condensate of Asthmatic Patients at Two Different Temperatures. J. Proteome Res. 2014, 13, 6107–6120. [Google Scholar] [CrossRef]
- Albert, E.; Walker, J.; Thiesen, A.; Churchill, T.; Madsen, K. cis-Urocanic Acid Attenuates Acute Dextran Sodium Sulphate-Induced Intestinal Inflammation. PLoS ONE 2010, 5, e13676. [Google Scholar] [CrossRef]
- Dawson, H.D.; Collins, G.; Pyle, R.; Key, M.; Weeraratna, A.; Deep-Dixit, V.; Nadal, C.N.; Taub, D.D. Direct and indirect effects of retinoic acid on human Th2 cytokine and chemokine expression by human T lymphocytes. BMC Immunol. 2006, 7, 27. [Google Scholar] [CrossRef]
- Druilhe, A.; Zahm, J.-M.; Benayoun, L.; El Mehdi, D.; Grandsaigne, M.; Dombret, M.-C.; Mosnier, I.; Feger, B.; Depondt, J.; Aubier, M.; et al. Epithelium Expression and Function of Retinoid Receptors in Asthma. Am. J. Respir. Cell Mol. Biol. 2008, 38, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.U.; Kenyon, N.J.; Stephensen, C.B. Vitamin A Deficiency Decreases and High Dietary Vitamin A Increases Disease Severity in the Mouse Model of Asthma. J. Immunol. 2008, 180, 1834–1842. [Google Scholar] [CrossRef]
- Arora, P.; Kumar, V.; Batra, S. Vitamin A status in children with asthma. Pediatr. Allergy Immunol. 2002, 13, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Jat, K.R.; Khairwa, A. Vitamin D and asthma in children: A systematic review and meta-analysis of observational studies. Lung India 2017, 34, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Brehm, J.M.; Schuemann, B.; Fuhlbrigge, A.L.; Hollis, B.W.; Strunk, R.C.; Zeiger, R.S.; Weiss, S.T.; Litonjua, A.A. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. J. Allergy Clin. Immunol. 2010, 126, 52.e5–58.e5. [Google Scholar] [CrossRef]
- Chinellato, I.; Piazza, M.; Sandri, M.; Peroni, D.G.; Cardinale, F.; Piacentini, G.L.; Boner, A.L. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur. Respir. J. 2010, 37, 1366–1370. [Google Scholar] [CrossRef]
- Uysalol, M.; Mutlu, L.C.; Saracoglu, G.V.; Karasu, E.; Guzel, S.; Kayaoglu, S.; Uzel, N. Childhood asthma and vitamin D deficiency in Turkey: Is there cause and effect relationship between them? Ital. J. Pediatr. 2013, 39, 78. [Google Scholar] [CrossRef][Green Version]
- Chinellato, I.; Piazza, M.; Sandri, M.; Peroni, D.; Piacentini, G.; Boner, A.L. Vitamin D Serum Levels and Markers of Asthma Control in Italian Children. J. Pediatr. 2011, 158, 437–441. [Google Scholar] [CrossRef]
- Gupta, A.; Sjoukes, A.; Richards, D.; Banya, W.; Hawrylowicz, C.; Bush, A.; Saglani, S. Relationship between Serum Vitamin D, Disease Severity, and Airway Remodeling in Children with Asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 1342–1349. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Itsiopoulos, C.; Lambert, K.; Katsardis, C.; Tsoukalas, D.; Erbas, B. Sufficient vitamin D status positively modified ventilatory function in asthmatic children following a Mediterranean diet enriched with fatty fish intervention study. Nutr. Res. 2020, 82, 99–109. [Google Scholar] [CrossRef]
- Hall, S.C.; Agrawal, D.K. Vitamin D and Bronchial Asthma: An Overview of Data From the Past 5 Years. Clin. Ther. 2017, 39, 917–929. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Jones, D.P.; Brown, L.A.S. Glutathione Redox Control of Asthma: From Molecular Mechanisms to Therapeutic Opportunities. Antioxid. Redox Signal. 2012, 17, 375–408. [Google Scholar] [CrossRef]
- Holguin, F. Oxidative Stress in Airway Diseases. Ann. Am. Thorac. Soc. 2013, 10, S150–S157. [Google Scholar] [CrossRef]
- Husain, Q.; Ahmad, A.; Shameem, M. Relation of oxidant-antioxidant imbalance with disease progression in patients with asthma. Ann. Thorac. Med. 2012, 7, 226–232. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Teague, W.G.; Holguin, F.; Yeh, M.; Brown, L.A.S. Airway glutathione homeostasis is altered in children with severe asthma: Evidence for oxidant stress. J. Allergy Clin. Immunol. 2009, 123, 146.e8–152.e8. [Google Scholar] [CrossRef]
- Ueda, N.; Tsuboi, K.; Uyama, T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 1274–1285. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.V.; Schmid, P.C.; Krebsbach, R.J.; Hillard, C.J.; Huang, C.; Chen, N.; Dong, Z.; Schmid, H.H.O. Cannabinoid-receptor-independent cell signalling by N-acylethanolamines. Biochem. J. 2001, 360, 67–75. [Google Scholar] [CrossRef]
- Duncan, R.S.; Chapman, K.D.; Koulen, P. The neuroprotective properties of palmitoylethanolamine against oxidative stress in a neuronal cell line. Mol. Neurodegener. 2009, 4, 50. [Google Scholar] [CrossRef]
- Boots, A.W.; Van Berkel, J.J.B.N.; Dallinga, J.W.; Smolinska, A.; Wouters, E.F.; Van Schooten, F.J. The versatile use of exhaled volatile organic compounds in human health and disease. J. Breath Res. 2012, 6, 027108. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, M.; Perestrelo, R.; Barros, A.; Bilelo, M.; Morête, A.; Câmara, J.; Rocha, S. Allergic asthma exhaled breath metabolome: A challenge for comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2012, 1254, 87–97. [Google Scholar] [CrossRef]
- Loureiro, C.C.; Duarte, I.F.; Gomes, J.; Carrola, J.; Barros, A.; Gil, A.M.; Bousquet, J.; Bom, A.T.; Rocha, S.M. Urinary metabolomic changes as a predictive biomarker of asthma exacerbation. J. Allergy Clin. Immunol. 2014, 133, 261.e5–263.e5. [Google Scholar] [CrossRef]
- Paredi, P.; Kharitonov, S.A.; Barnes, P.J. Analysis of Expired Air for Oxidation Products. Am. J. Respir. Crit. Care Med. 2002, 166, S31–S37. [Google Scholar] [CrossRef]
- Chau-Etchepare, F.; Hoerger, J.L.; Kuhn, B.T.; Zeki, A.A.; Haczku, A.; Louie, S.; Kenyon, N.J.; Davis, C.E.; Schivo, M. Viruses and non-allergen environmental triggers in asthma. J. Investig. Med. 2019, 67, 1029–1041. [Google Scholar] [CrossRef]
- NCBI. PubChem Compound Summary for 4-Isopropyl-1-Methylcyclohexane-1-Hydroperoxide; National Center for Biotechnology: Bethesda, MD, USA, 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Isopropyl-1-methylcyclohexane-1-hydroperoxide (accessed on 15 March 2021).
- Elliott, L.; Longnecker, M.P.; Kissling, G.E.; London, S.J. Volatile Organic Compounds and Pulmonary Function in the Third NationalHealth and Nutrition Examination Survey, 1988–1994. Environ. Health Perspect. 2006, 114, 1210–1214. [Google Scholar] [CrossRef]
- Guarneri, F.; Barbuzza, O.; Vaccaro, M.; Galtieri, G. Allergic contact dermatitis and asthma caused by limonene in a labourer handling citrus fruits. Contact Dermat. 2008, 58, 315–316. [Google Scholar] [CrossRef]
- Rumchev, K.; Spickett, J.; Bulsara, M.; Phillips, M.; Stick, S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 2004, 59, 746–751. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef]
- Joyce, S.A.; Gahan, C.G. Disease-Associated Changes in Bile Acid Profiles and Links to Altered Gut Microbiota. Dig. Dis. 2017, 35, 169–177. [Google Scholar] [CrossRef]
- Menon, R.; Jones, J.; Gunst, P.R.; Kacerovsky, M.; Fortunato, S.J.; Saade, G.R.; Basraon, S. Amniotic Fluid Metabolomic Analysis in Spontaneous Preterm Birth. Reprod. Sci. 2014, 21, 791–803. [Google Scholar] [CrossRef]
- Viaene, L.; Thijs, L.; Jin, Y.; Liu, Y.; Gu, Y.; Meijers, B.; Claes, K.; Staessen, J.A.; Evenepoel, P. Heritability and Clinical Determinants of Serum Indoxyl Sulfate and p-Cresyl Sulfate, Candidate Biomarkers of the Human Microbiome Enterotype. PLoS ONE 2014, 9, e79682. [Google Scholar] [CrossRef]
- Nakada, E.M.; Bhakta, N.R.; Korwin-Mihavics, B.R.; Kumar, A.; Chamberlain, N.; Bruno, S.R.; Chapman, D.G.; Hoffman, S.M.; Daphtary, N.; Aliyeva, M.; et al. Conjugated bile acids attenuate allergen-induced airway inflammation and hyperresposiveness by inhibiting UPR transducers. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Shaik, F.B.; Panati, K.; Narasimha, V.R.; Narala, V.R. Chenodeoxycholic acid attenuates ovalbumin-induced airway inflammation in murine model of asthma by inhibiting the T H 2 cytokines. Biochem. Biophys. Res. Commun. 2015, 463, 600–605. [Google Scholar] [CrossRef]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The Role of the Microbiome in Asthma: The Gut–Lung Axis. Int. J. Mol. Sci. 2018, 20, 123. [Google Scholar] [CrossRef]
- Abrahamsson, T.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin. Exp. Allergy 2014, 44, 842–850. [Google Scholar] [CrossRef]
- Martinez, F.D.; Guerra, S. Early Origins of Asthma. Role of Microbial Dysbiosis and Metabolic Dysfunction. Am. J. Respir. Crit. Care Med. 2018, 197, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Davies, S.S. Microbial metabolism of dietary components to bioactive metabolites: Opportunities for new therapeutic interventions. Genome Med. 2016, 8, 1–18. [Google Scholar] [CrossRef]
- Juge, N.; Tailford, L.; Owen, C.D. Sialidases from gut bacteria: A mini-review. Biochem. Soc. Trans. 2016, 44, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.S.; Sordillo, J.E.; Lasky-Su, J.; Dahlin, A.; Perng, W.; Rifas-Shiman, S.L.; Weiss, S.T.; Gold, D.R.; Litonjua, A.A.; Hivert, M.-F.; et al. Plasma metabolite profiles in children with current asthma. Clin. Exp. Allergy 2018, 48, 1297–1304. [Google Scholar] [CrossRef]
- Kannisto, S.; Laatikainen, A.; Taivainen, A.; Savolainen, K.; Tukiainen, H.; Voutilainen, R. Serum dehydroepiandrosterone sulfate concentration as an indicator of adrenocortical suppression during inhaled steroid therapy in adult asthmatic patients. Eur. J. Endocrinol. 2004, 150, 687–690. [Google Scholar] [CrossRef]
- Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Brix, S.; Bisgaard, H.F. Neonates with reduced neonatal lung function have systemic low-grade inflammation. J. Allergy Clin. Immunol. 2015, 135, 1450.e1–1456.e1. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Chapter 3: Chemistry and toxicology of cigarette smoke and biomarkers of exposure and harm. In How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon; Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, US Department of Health and Human Services Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010; pp. 27–102. Available online: https://www.ncbi.nlm.nih.gov/books/NBK53017 (accessed on 7 January 2021).
- Turi, K.N.; Romick-Rosendale, L.; Ryckman, K.K.; Hartert, T.V. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. J. Allergy Clin. Immunol. 2018, 141, 1191–1201. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Docea, A.O.; Tsilimidos, G.; Calina, D.; Tsatsakis, A. Metabolic Fingerprint of Chronic Obstructive Lung Diseases: A New Diagnostic Perspective. Metabolites 2019, 9, 290. [Google Scholar] [CrossRef]
- Maniscalco, M.; Paris, D.; Melck, D.J.; D’Amato, M.; Zedda, A.; Sofia, M.; Stellato, C.; Motta, A. Coexistence of obesity and asthma determines a distinct respiratory metabolic phenotype. J. Allergy Clin. Immunol. 2017, 139, 1536.e5–1547.e5. [Google Scholar] [CrossRef]
- Papamichael, M.M.; Katsardis, C.; Tsoukalas, D.; Erbas, B.; Itsiopoulos, C. Weight Status and Respiratory Health in Asthmatic Children. Lung 2019, 197, 777–782. [Google Scholar] [CrossRef]
- Forno, E.; Weiner, D.J.; Mullen, J.; Sawicki, G.; Kurland, G.; Han, Y.Y.; Cloutier, M.M.; Canino, G.; Weiss, S.T.; Litonjua, A.A.; et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am. J. Respir. Crit. Care Med. 2016, 195, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
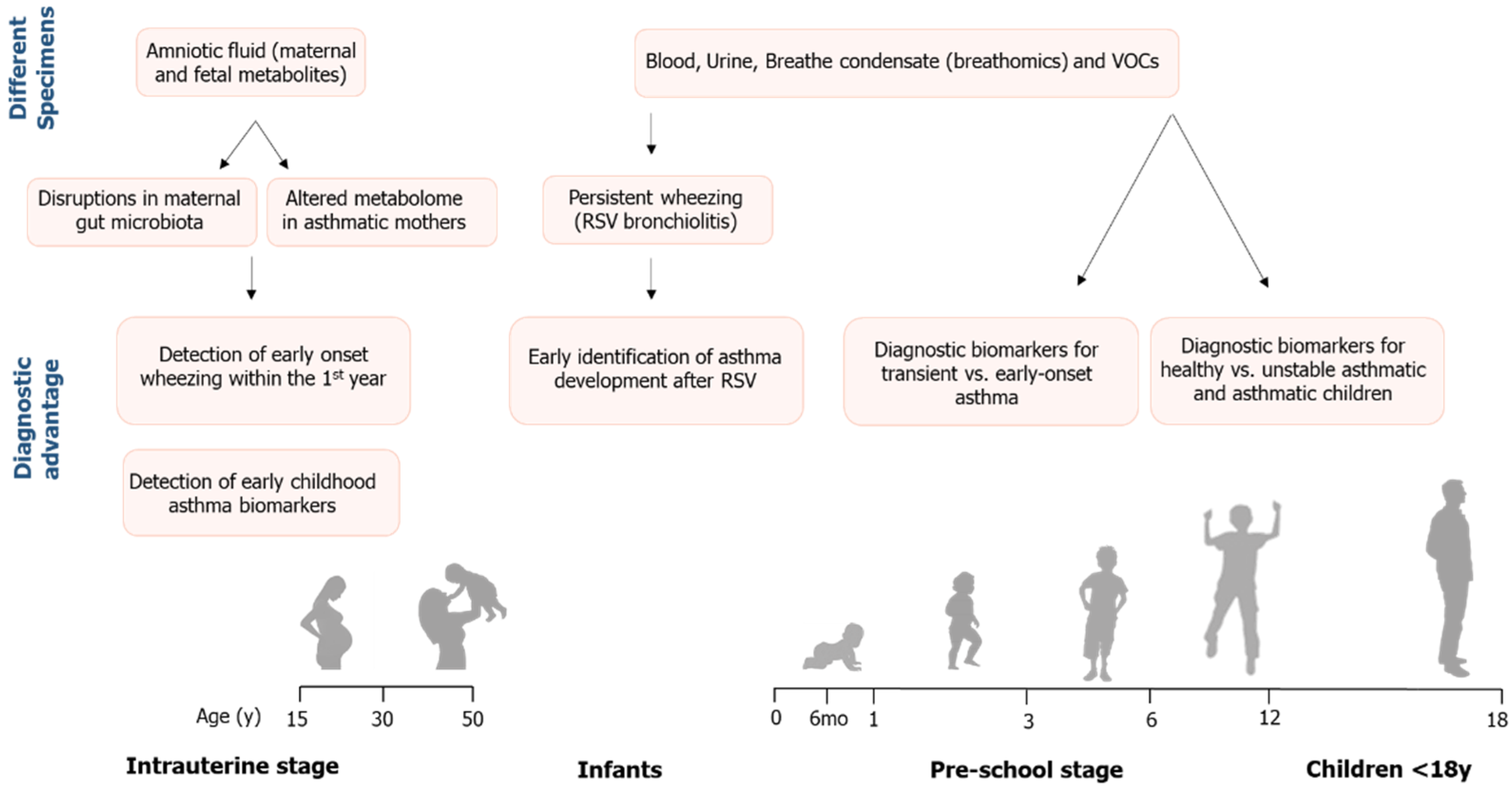
| Author Year/Study Design/ Country | Follow-Up | Population (n) | Group Allocated | Asthma or Bronchiolitis Diagnosis | Sample/ Metabolomic Technique | Metabolites Isolated | Annotated Pathways | Conclusions |
|---|---|---|---|---|---|---|---|---|
| NEONATES | ||||||||
| Carraro et al., 2018 [19] Birth Cohort Netherlands | 1 year | 292 mothers @ 38–42 weeks gestation 142 analyzed. | wheezers n = 86 non-wheezers n = 56 | Parent’s symptom report | Amniotic fluid (LC-MS) Untargeted | High levels in the wheezing group: Indoxyl sulfate, p-cresol glucuronide, 2-methoxyestrone-3-sulfate, S-adenosylhomocysteine, 1,3,7,12-tetrahydroxycholan- 24-oic acid, glycocholic acid, α- Ν-phenylacetyl-l-glutamine, corticosterone High levels in non-wheezers: 3-hydroxyphenylacetic acid, 3,4-dihydroxyphenyllactic acid methyl ester, ferulic acid 4-O-glucuronide, chenodeoxycholic acid 3- sulfate, 3a-hydroxy-7,12-dioxo-5β- cholan-24-oic acid, 4-hydroxystachydrine, 5-hydroxyindolepyruvate, dehydroepiandrosterone sulfate | Steroid hormone Biosynthesis, Phenylalanine metabolism, gluconeogenesis, bile acid synthesis, products of gut microbiota, oxidative stress and epigenetic dysregulation | Amniotic fluid collected at delivery differed in neonates that experienced wheezing at 1 year than in non-wheezers. |
| Chawes et al., 2019 [20] Birth Cohort Denmark | 6 years | Asthmatic mothers COPSA C2000 n = 171 neonates @ 4 weeks of age. COPSAC 2010 n = 161 | Persistent wheezers or asthmatics in the first 6 years of life | Physician | Urine (LC-MS) Untargeted | Higher in asthmatic children vs. healthy controls: Taurochenodeoxycholate-3-sulfate, 3-hydroxy-tetradecanedioic acid Lower in asthmatic children vs. control: Glucoronidated steroid | Steroid, fatty acid metabolism and bile acids | Metabolic profiles discriminated children developing asthma from healthy children. In both cohorts, urine metabolite levels measured at four weeks were related to asthma development before six years of age. |
| INFANTS | ||||||||
| Chiu et al., 2018 [35] Longitudinal Taiwan | 1, 2, 3, 4 years | PATCH Cohort n = 60 | Asthma n = 30, Healthy controls n = 30 | Physician | Urine (NMR) Untargeted | Lower levels in asthmatic children vs. healthy controls: Dimethylamine, allantoin, guanidoacetic acid, 1-methylnicotinamide | Purine and amino acid metabolism, nicotinamide/ nicotinate metabolism, methane metabolism and gut microbiota imbalance. | Metabolomic profiling provided a link of microbe-environment Interactions in the development of childhood. |
| Barlotta et al., 2019 [32] Prospective Italy | 6 months, 12 months, 2 years | n = 52 @ 1 year old patients with acute bronchiolitis | Wheezers Non-wheezers | BronchiolitisPhysician | Urine (LC-MS) Untargeted | Bronchiolitis-induced recurrent wheeze vs. non-wheezers: Isocitrate, citric acid, oxoglutaric acid, lysine, cysteine, methionine. Isobutyrylglycine, N-butyrylglycine. | Citric acid cycle, fatty acid and amino acid metabolism, gut microbial dysbiosis | Metabolomic profiling of urine specimens from infants with bronchiolitis identified children at increased risk of developing recurrent wheezing. |
| Atzei et al., 2011 [34] Case-study Italy | N/A | n = 2 @ 33-37 weeks gestation < 28 days Patients with RSV bronchiolitis | Physician | Urine (NMR) | Associated with RSV bronchiolitis: Betaine, creatinine, glycine | Creatine metabolism and epigenetic regulation | 1H-NMR can be potentially applied to identify metabolic alterations in urine samples related to the differences in the inflammation of bronchioles. | |
| Turi et al., 2018 [25] INSPIRE Cohort USA | 1, 2, 3 years | n = 140 120 days old | Healthy n = 60, HRV n = 10, RSV n = 70 | Physician | Urine (NMR) Untargeted | 11 metabolites were significantly different between RSV ARI, HRV ARI vs. healthy control infant groups: 1-methylnicotinamide, 4-deoxythreonic acid, citrate, creatine, hypoxanthine, alanine, succinate, 3-hydroxyisovalerate, acetone, valine, 2-aminobutyrate | Citric acid cycle, amino acid metabolism, nicotinamide/ nicotinate metabolism, catecholamine biosynthesis, glucose-alanine cycle, glutamate metabolism, arginine-proline metabolism | Metabolomics may aid in prophylaxis against bronchiolitis in infants. |
| PRE-SCHOOL | ||||||||
| Carraro et al., 2018 [21] Cohort Italy | 3 years | n = 47 2–5 years | Wheezing n = 34, Healthy n = 13 | Physician | Urine (LC-MS) Untargeted | Higher levels in transient wheezers vs. early-onset asthma: Oxoadipic acid, epinephrine, L-tyrosine, 3-hydroxyhippuric acid, benzoic acid, 3 hydroxy-sebacic acid, dihydroferulic acid 4-sulfate, p-cresol, indolelactic acid, N-acetyl-l-phenylalanine, N2-acetyl-ornithine Higher levels in early-onset asthma vs. transient wheezers: 4-(4-deoxy-α-d-gluc-4-enuronosyl)-d-galacturonate, glutaric acid, 4-hydroxy nonenal, phosphatidylglycerol, 3-methyluridine, steroid O-sulfate, 5-hydroxy-l-tryptophan,3-indoleacetic-acid, tiglylglycine, indole, cytosine, N-acetylputrescine, indole-3-acetamide, 6-methyladenine, 5-methylcytosine, N-acryloylglycine, hydroxyphenyllactic acid. | Tryptophan metabolism, fatty acid metabolism and microbial derivatives | Urine metabolites distinguished between transient wheezers and early-onset asthma. |
| Smolinska et al., 2014 [22] Prospective Cohort Netherlands | 6 years | ADEM Study n = 252 2–4 years | Recurrent Wheeze n = 202 Healthy controls n = 50 At age 6 years: Healthy n = 49, Transient wheezers n = 121, Early-onset Asthma n = 76 | Physician | VOC (GC-MS) Targeted | High levels in early-onset asthma vs. transient wheezers: 2,4-dimethylpentane, 2,4-dimethylheptane, 2-undecenal, octane, 2-methylpentane, 2,4-demethylheptane, 2-methylhexane Low levels in early-onset asthma vs. transient wheezers: Acetone, 2,2,4-trimethylheptane, 1-methyl-4-(1-methylethenyl) Cyclohexen, 2, 3, 6-trimethyloctane, biphenyl, 2-ethenylnaptalene, 2, 6, 10-trimethyldodecane | Hydrocarbons produced during lipid peroxidation | VOCs profile in exhaled breath discriminated healthy, transient wheezing and true asthmatic children. VOCs predictive of early-onset asthma. |
| Klaassen et al., 2015 [23] Prospective Cohort Netherlands | 6 years | ADEM study n = 202 2–4 years | Recurrent wheezers n= 202 At age 6 years: Healthy n = 4, Asthma n =76, Transient wheeze n =122 | Physician | VOC (GC-TOF-MS) Targeted | High levels in asthmatics: Octane, 2-methylhexane, 2, 3, 6 -trimethyloctane, 2, 4-dimethylheptane Low levels in asthmatics: Acetone, 2-undecenal, 2, 6, 10-trimethyldodecane, 2,4-dimethylpentane, 2-methylpentane | Hydrocarbons produced during airway inflammation | VOCs profile plus Asthma Predictive Index (API) status improved asthma diagnosis at preschool age. VOCs could be a valuable monitoring tool for airway inflammation and in predicting asthma onset. |
| Chiu et al., 2020 [26] Cross-sectional Taiwan | N/A | n = 54 3–5 years | Asthma n = 28, Control n = 26 | Physician | Plasma Urine (NMR) Untargeted | Higher in asthma vs. control: Histidine Lower in asthma vs. control: 1-methylnicotinamide, trimethylamine N-oxide (TMAO). Related to allergic sensitization (Ig E)/Food allergy: N-phenylacetylglycine, pyruvate, valine, leucine, isoleucine | Histadine metabolism, nicotinamide and pyruvate metabolism, phenylalanine metabolism, amino acid metabolism and products of microbial metabolism | Plasma pyruvate metabolism associated with Ig E production. Urinary branched-chain amino acids were associated with food allergic reactions. |
| SCHOOL CHILDREN | ||||||||
| Saude et al., 2011 [27] Cross-sectional Canada | N/A | n = 135 4–16 years SAGE Birth Cohort | Stable n = 73, Unstable asthma n = 20, Healthy controls n = 42 | Physician | Urine (NMR) Targeted | Protective against asthma exacerbation: 1-methylnicotinamide Asthma vs. healthy controls: 1–methylhistamine, 1-methyl-nicotinamide, 2- methylglutarate, 2-oxoglutarate, 3-OH-3-methyl-glutarate, 3-methyladipate, 4-aminohippurate, acetone, adenine, alanine, creatine, dimethylamine, formate, fumarate, glucose, glycolate, imidazole, lactate, methylamine, O-acetylcarnitine, oxaloacetate, phenylacetylglycine, phenylalanine, tryptophan, tyrosine, cis-aconitate, Myo-inositol, trans-aconitate. Separating stable vs. unstable asthma: 2-oxaloglutarate, succinate, fumarate, 3-hydroxy 3-methylglutarate, threonine, aconitate, acetylcarnitine, trimethylamine, threonine, taurine, 4-aminohippurate Stable vs. unstable vs. healthy controls: 4-aminohippurate, carnitine, homovanillate, kynurenine, O-acetylcarnitine, succinate, taurine, threonine, trimethylamine. | Citric acid cycle, nicotinamide metabolism, lipid metabolism, Protein metabolism, purine metabolism, glucose metabolism, tryptophan metabolism, histamine biosynthesis including catecholamine synthesis | 1H-NMR can be used to differentiate stable asthma from controls and unstable asthma. |
| Tao et al., 2019 [24] Cohort China | N/A | n = 109 6–11 years | Healthy n = 29, Uncontrolled asthma n = 37, Controlled asthma n = 43 | Physician | Urine (GC-MS) Untargeted | Asthma diagnosis and discrimination of controlled vs. uncontrolled asthma: Uric acid, stearic acid, threitol, acetylgalactosamine, heptadecanoic acid, aspartic acid, xanthosine, hypoxanthine Healthy vs. uncontrolled/ Controlled asthma: Glycine, serine, threonine, Pantothenate and CoA synthesis, BCAA synthesis, tyrosine, inosine, adenosine, arginine, proline, alanine, aspartate, glutamate, pyruvate, tryptophan | Citric acid cycle, purine metabolism, lipid and carbohydrate metabolism, amino acid and phenylalanine metabolism, pantothenate and Coenzyme A biosynthesis | Urine metabolomics discriminated asthma as well as controlled and uncontrolled sub-types and elucidated the biological mechanisms of pediatric asthma. |
| Dallinga et al., 2010 [33] Prospective Netherlands | N/A | n = 120 5–16 years | Asthma n = 63, Healthy controls n = 57 | Physician | VOC (GC-TOF-MS) Untargeted | Metabolites differentiated between asthma vs. healthy controls: Branched hydrocarbons (C13H28, C11H24), carbon disulfide, 1-penten-2-on, butanoic acid, 3-(1-methylethyl)-benzene, unsaturated hydrocarbon (C15H26), benzoic acid, p-xylene | Hydrocarbons produced during lipid peroxidation | EBC samples and comparing VOCs differentiated children with asthma from healthy controls. |
| Gahleitner et al., 2013 [36] Experimental UK | N/A | n = 23 8–16 years | Asthma n = 11, Healthy n = 12 | Physician | VOC (GC-MS) Targeted | Metabolites differentiated between asthma vs healthy: 1-(methylsulfanyl)propane, ethylbenzene, 1,4-dichlorobenzene, 4-isopropenyl-1-methylcyclohexene, 2-octenal, octadecyne, 1-isopropyl-3-methylbenzene, 1,7 dimethylnaphtalene | Organic compounds from external sources. Used in food manufacturing (flavorings) and disinfectants. | VOCs discriminated between asthmatic and healthy children. The application of breath markers could be a potential non-invasive and low-cost technique for the management of pediatric asthma. |
| Asthma Phenotype | Author Year/ Study Design /Country | Population (n) | Group Allocated | Asthma Diagnosis | Sample/ Metabolomic Technique | Metabolites Isolated | Annotated Pathways | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Mild | Papamichael et al., 2019 [28] Cross-sectional Greece | n = 65 5–12 years | N/A | Physician ACQ | Urine (GC-MS) Targeted | Metabolites correlated with PFTs (FEV1, FVC, FEV1/FVC, PEF, FeNO) and asthma control: Lactic, 4-hydroxyphenylacetic, 5-hydroxyindoleacetic, glycolic, malic acid. | Tryptophan and tyrosine metabolism, lactic acidosis, catecholamine synthesis and alterations in gut microbiota. | Metabolomics is a promising approach in the research for novel biomarkers for asthma monitoring |
| Mild-Moderate | Kelly et al., 2017 [29] Cohort Costa Rica | n = 380 6–14 years | N/A | Physician | Plasma (LC-MS) Untargeted | 574 Metabolites isolated in mild-moderate asthmatics. 91 associated with AHR, 102 with pre- FEV1/FVC and 155 with post-FEV1/FVC 24 metabolites common to all 3 parameters | Metabolites common to AHR, pre and post-FEV1/FVC related to: Glycerophospholipids, linoleic acid and pyrimidine metabolism. Metabolites pertaining to AHR: Disturbances in Glycerophospholipid and linoleic acid metabolism, d -glutamine/ glutamate, sphingolipid and pyrimidine metabolism, as well as Nitrogen metabolism. Pre and post bronchodilation FEV1/FVC: Citric acid cycle, lipid metabolism, alanine, aspartate and glutamate metabolism, arginine—proline metabolism, glycine, threonine and serine metabolism, pyrimidine metabolism, BCAA and nitrogen metabolism, pantothenate and CoA biosynthesis and aminoacyl tRNA biosynthesis. Post FEV1/FVC: Pantothenate and CoA biosynthesis | Metabolites and metabolomic profiles distinguished children with asthma by the degree of lung function as reflected by spirometric parameters, thus confirming the existence of an asthma severity metabolome. |
| Severe | Carraro et al., 2013 [30] Cross-sectional Italy | n = 57 8–17 years | Severe n = 11, Non-severe n = 31 (17 taking medication) Healthy controls n = 15 | Physician | EBC (LC-MS) Untargeted | Severe asthma: Retinoic acid, deoxyadenosine Non-severe: 20-hydroxy-PGF2a, Thromboxane B2, 6 keto-prostaglandin F1a Healthy controls: Ercalcitriol (active vitamin D2) | Compounds related to: Retinoic acid, adenosine and vitamin D | Breathomics discriminated between severe, non-severe and healthy child asthmatics. |
| Cortico- steroid Resistant | Fitzpatrick et al., 2014 [15] Cross-sectional US | n = 57 6–17 years | Mild asthma n = 22, Severe n = 35 | Physician | Plasma (LC-MS) Untargeted | Severe asthma: Glycine, serine, threonine, N-acylethanolamine, N-acyltransferase pathway | Biosynthesis of purine /pyrimidines, phospho-glycerides, sphingo-lipid, glycolipids. Folate cycle, glutathione synthesis. Oxidative stress | Metabolomics revealed that oxidative stress is a contributory factor to corticosteroid refractory severe asthma in children. |
| Cortico- steroid Resistant | Park et al., 2017 [31] Cross-sectional US | n = 30 6–17 years | Corticosteroid resistant n = 15, Corticosteroid responders n = 15 | Physician | Urine (LC-MS) Untargeted | Metabolites discriminating corticosteroid responders from non-responders: 3,6-dihydronicotinic, 3-methoxy-4-hydroxyphenyl(ethylene)glycol, 3,4-dihydroxy-phenylalanine, γ-glutamylcysteine, Cys-Gly, reduced Flavin mononucleotide High in the corticosteroid resistant (non-responders) group: Cyst-Gly 3,6-dihydronicotinic, 3,4-dihydroxy-phenylalanine, 1,2-dihydronaphthalene- 1,2-diol, 3-methoxy-4-hydroxyphenyl(ethylene)glycol Low in corticosteroid resistant group (non-responders): γ-glutamylcysteine | Tyrosine metabolism, catecholamine biosynthesis, and glutathione metabolism. 3,6-dihydronicotinic and 1,2-dihydronaphthalene- 1,2-diol are present in cigarette smoke | Putative biomarkers isolated using the metabolomics approach differentiated corticosteroid resistant non-responders) from responders in pediatric asthma. |
| Atopic | Mattarucchi et al., 2012 [16] Cross-sectional Italy | Atopic n = 41, Healthy n= 12 Median age 11 years | Well-controlled with β-agonists n = 14, Well-controlled with daily controller drugs n = 16, Poorly-controlled with daily controller drugs n = 11 | Physician | Urine (LC-MS) Untargeted | Low levels in asthmatics: Urocanic, methyl-imidazoleacetic, Ile-Pro fragment | Histamine metabolism. Urocanic acid related to inflammation/immunity and Ile-Pro to prolidase activity. | Metabolic profiling offers the potential of asthma characterization and identification of inflammation- related metabolites. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papamichael, M.M.; Katsardis, C.; Sarandi, E.; Georgaki, S.; Frima, E.-S.; Varvarigou, A.; Tsoukalas, D. Application of Metabolomics in Pediatric Asthma: Prediction, Diagnosis and Personalized Treatment. Metabolites 2021, 11, 251. https://doi.org/10.3390/metabo11040251
Papamichael MM, Katsardis C, Sarandi E, Georgaki S, Frima E-S, Varvarigou A, Tsoukalas D. Application of Metabolomics in Pediatric Asthma: Prediction, Diagnosis and Personalized Treatment. Metabolites. 2021; 11(4):251. https://doi.org/10.3390/metabo11040251
Chicago/Turabian StylePapamichael, Maria Michelle, Charis Katsardis, Evangelia Sarandi, Spyridoula Georgaki, Eirini-Sofia Frima, Anastasia Varvarigou, and Dimitris Tsoukalas. 2021. "Application of Metabolomics in Pediatric Asthma: Prediction, Diagnosis and Personalized Treatment" Metabolites 11, no. 4: 251. https://doi.org/10.3390/metabo11040251
APA StylePapamichael, M. M., Katsardis, C., Sarandi, E., Georgaki, S., Frima, E.-S., Varvarigou, A., & Tsoukalas, D. (2021). Application of Metabolomics in Pediatric Asthma: Prediction, Diagnosis and Personalized Treatment. Metabolites, 11(4), 251. https://doi.org/10.3390/metabo11040251








