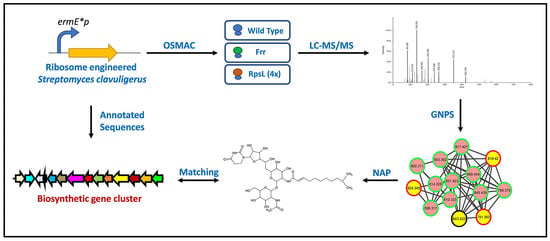
Specialized Metabolites from Ribosome Engineered Strains of Streptomyces clavuligerus
Abstract
1. Introduction
2. Results and Discussion
2.1. Overview of Metabolomics Analysis
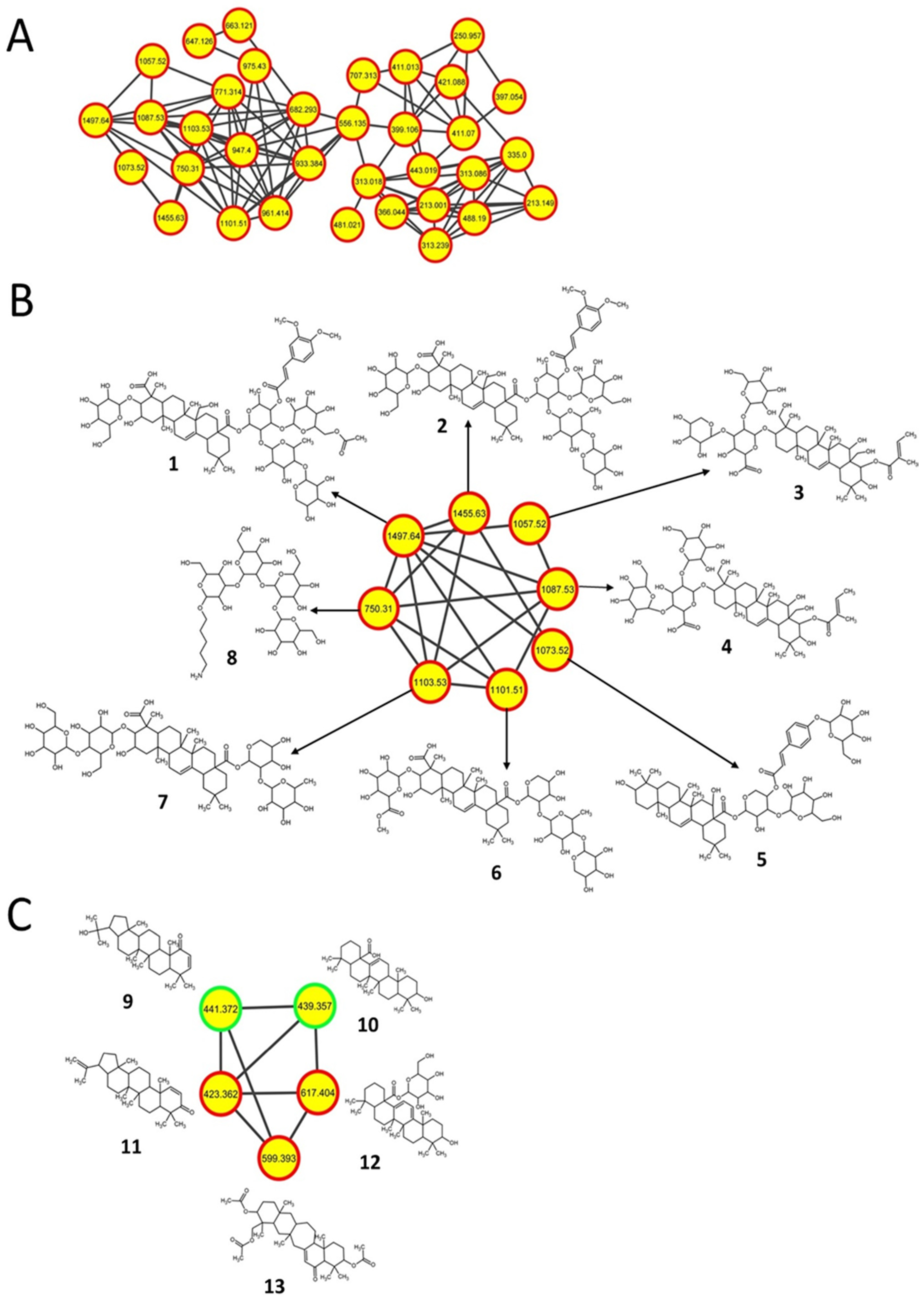
2.2. Triterpenoids and Derivatives
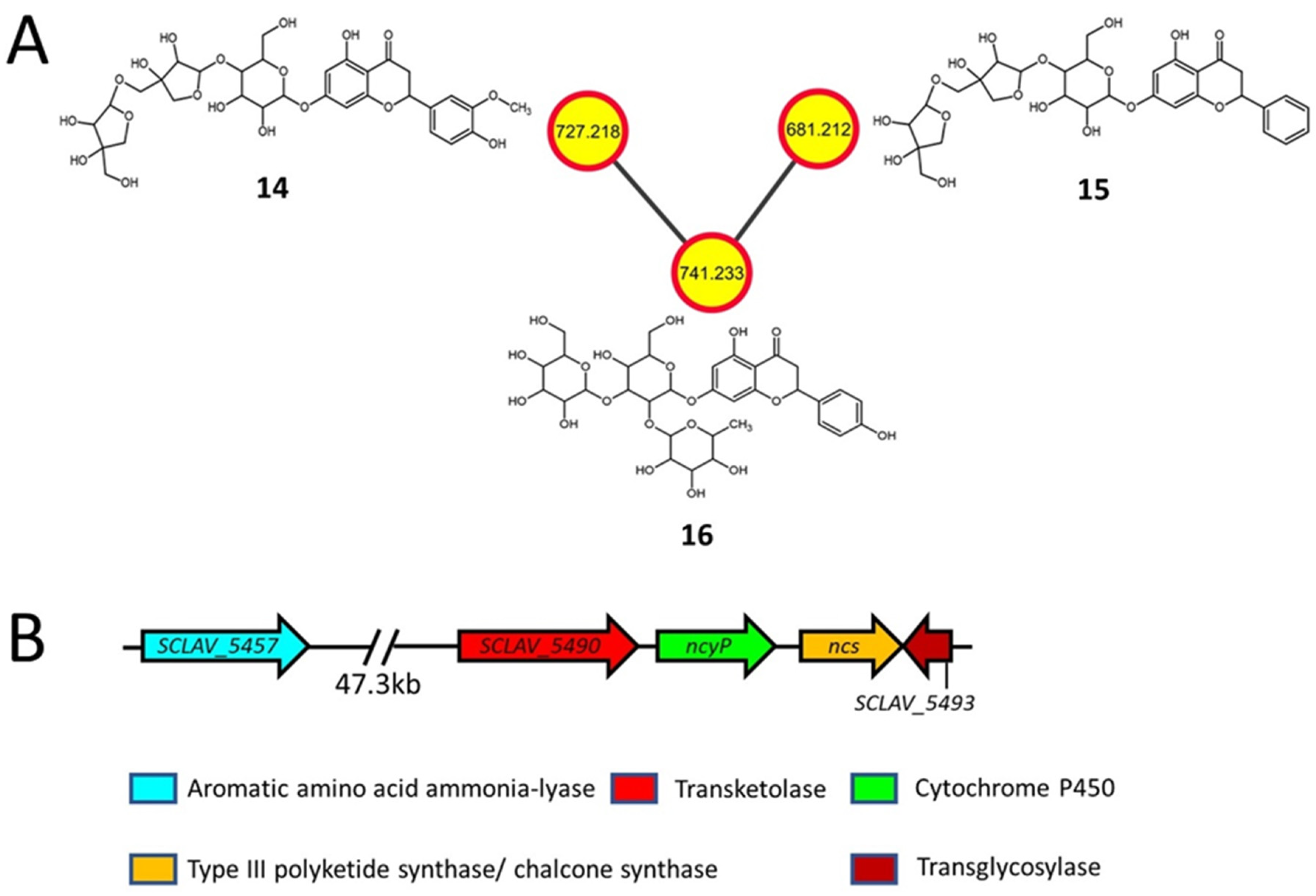
2.3. Flavonoids and Derivatives
2.4. Nucleoside Antibiotics
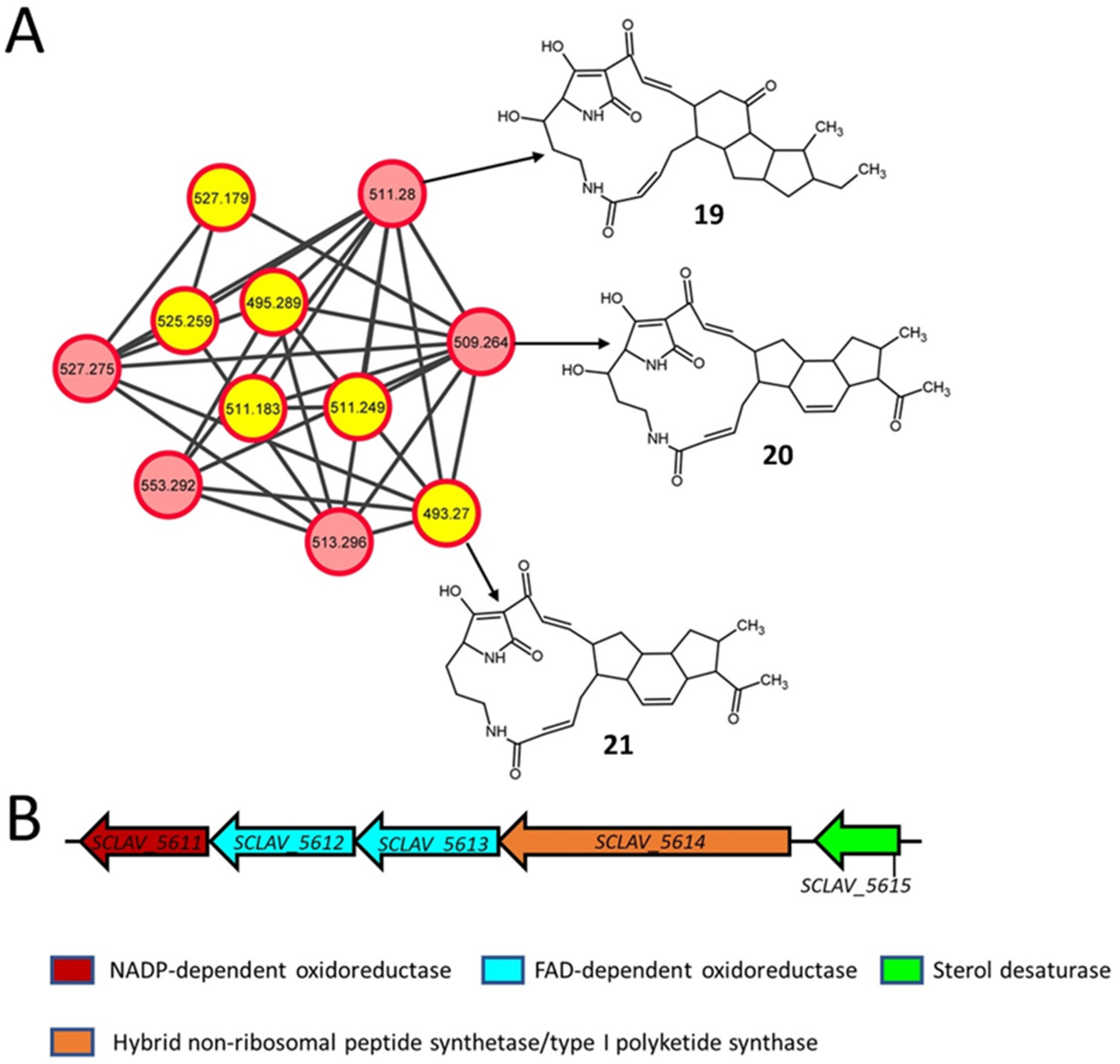
2.5. Polycyclic Tetramate Macrolactams
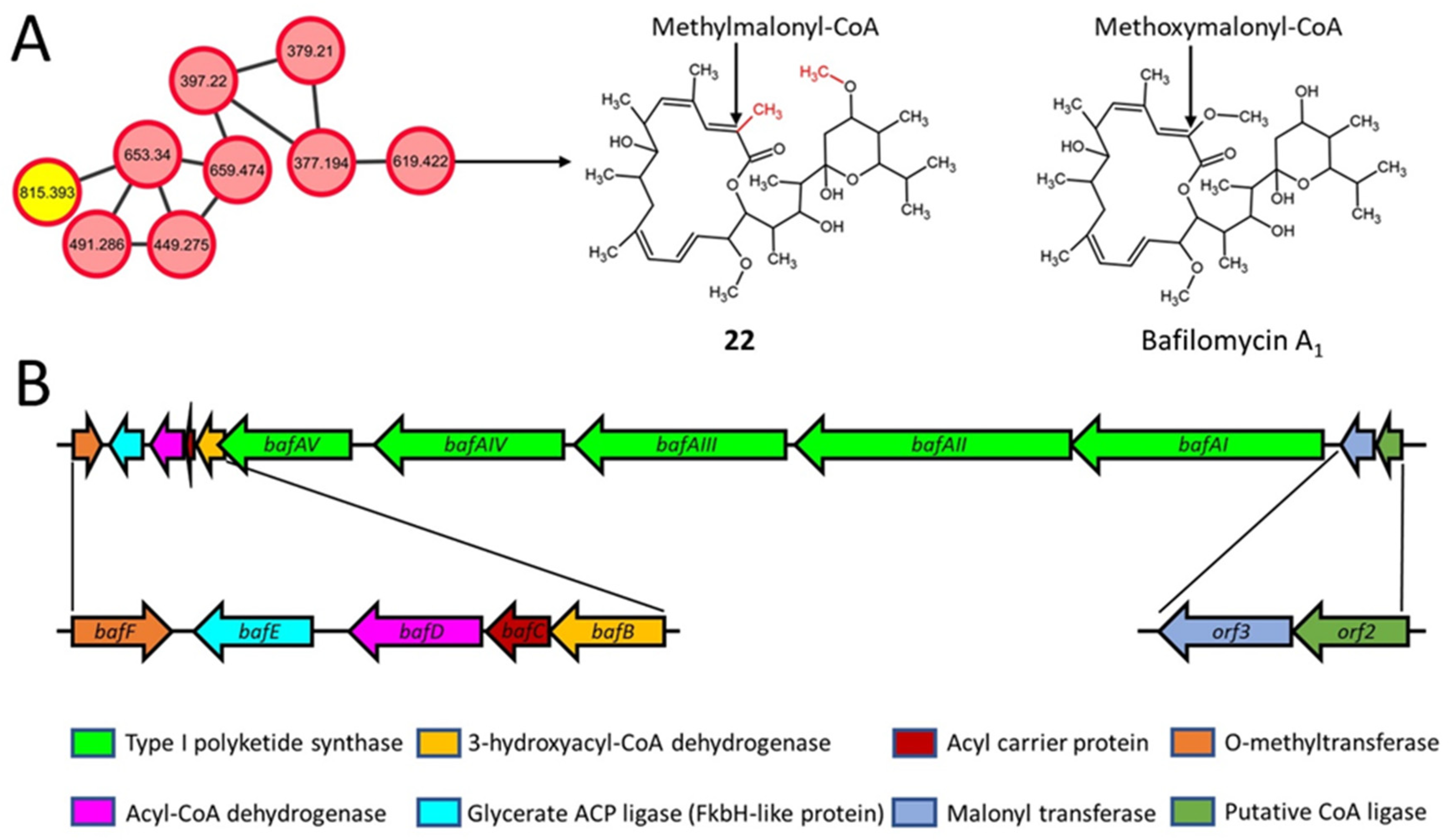
2.6. Macrolactone Plecomacrolide Antibiotics
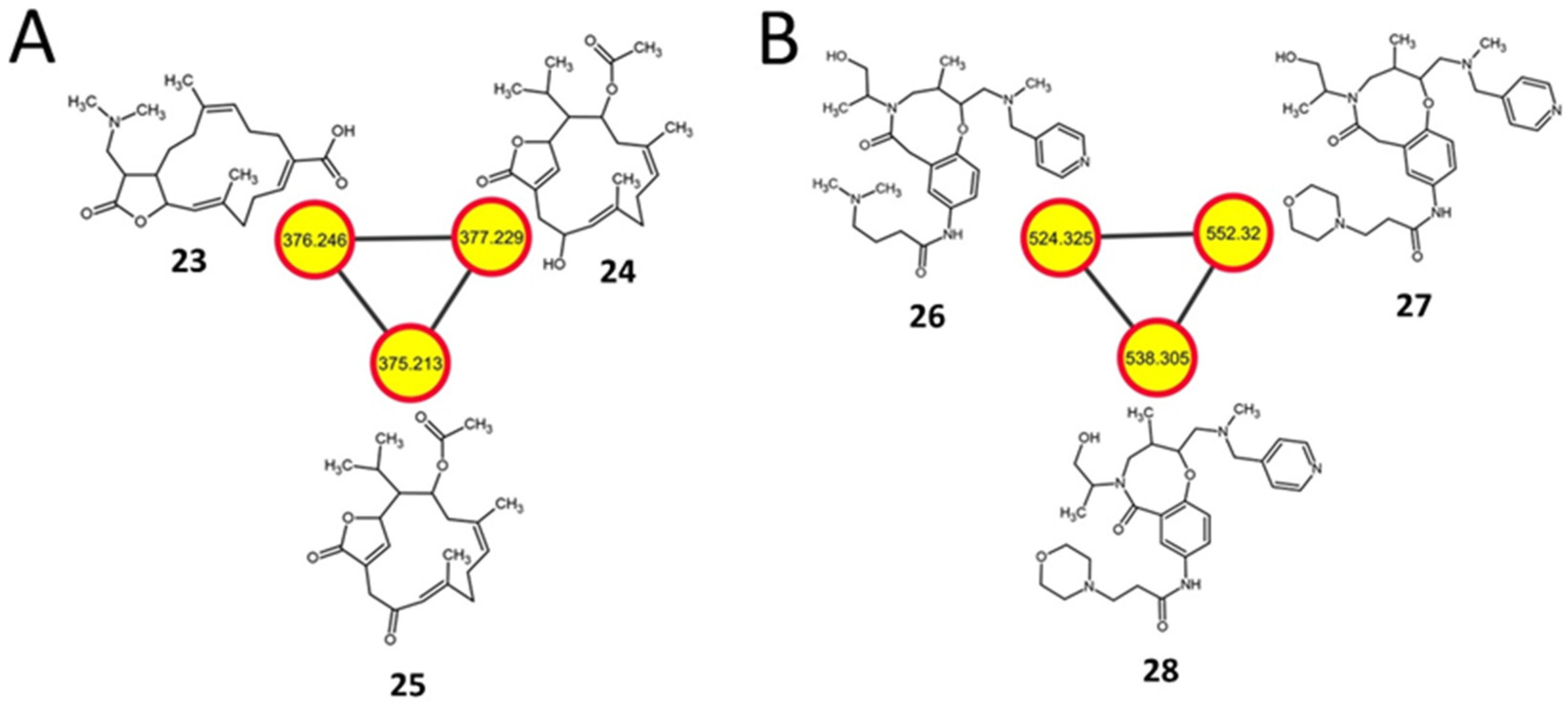
2.7. Diterpenoids and Organonitrogen Compounds with no Identifiable BGCs
3. Materials and Methods
3.1. Bacterial Strains, Plasmids, Culture Conditions and Molecular Methods
3.2. Metabolite Extraction, LC-MS/MS Analysis, and Molecular Networking
3.3. In Silico Annotation of the Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Meier-Kolthoff, J.P.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Belknap, K.C.; Park, C.J.; Barth, B.M.; Andam, C.P. Genome Mining of Biosynthetic and Chemotherapeutic Gene Clusters in Streptomyces Bacteria. Sci. Rep. 2020, 10, 2003. [Google Scholar] [CrossRef]
- Drawz, S.M.; Bonomo, R.A. Three Decades of β-Lactamase Inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Liras, P. Biosynthesis and Molecular Genetics of Cephamycins. Cephamycins Produced by Actinomycetes. Antonie Leeuwenhoek 1999, 75, 109–124. [Google Scholar] [CrossRef]
- Jensen, S.E. Biosynthesis of Clavam Metabolites. J. Ind. Microbiol. Biotechnol. 2012, 39, 1407–1419. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, R.; Botas, A.; Albillos, S.M.; Rumbero, A.; Martín, J.F.; Liras, P. Molecular Genetics of Naringenin Biosynthesis, a Typical Plant Secondary Metabolite Produced by Streptomyces Clavuligerus. Microb. Cell Fact. 2015, 14, 178. [Google Scholar] [CrossRef] [PubMed]
- AbuSara, N.F.; Piercey, B.M.; Moore, M.A.; Shaikh, A.A.; Nothias, L.F.; Srivastava, S.K.; Cruz-Morales, P.; Dorrestein, P.C.; Barona-Gómez, F.; Tahlan, K. Comparative Genomics and Metabolomics Analyses of Clavulanic Acid-Producing Streptomyces Species Provides Insight Into Specialized Metabolism. Front. Microbiol. 2019, 10, 2550. [Google Scholar] [CrossRef]
- Kenig, M.; Reading, C. Holomycin and an Antibiotic (MM 19290) Related to Tunicamycin, Metabolites of Streptomyces Clavuligerus. J. Antibiot. 1979, 32, 549–554. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, N.; Jeong, Y.; Lee, Y.; Kim, W.; Cho, S.; Palsson, B.O.; Cho, B.K. Primary Transcriptome and Translatome Analysis Determines Transcriptional and Translational Regulatory Elements Encoded in the Streptomyces Clavuligerus Genome. Nucleic Acids Res. 2019, 47, 6114–6129. [Google Scholar] [CrossRef]
- Medema, M.H.; Trefzer, A.; Kovalchuk, A.; Van Den Berg, M.; Müller, U.; Heijne, W.; Wu, L.; Alam, M.T.; Ronning, C.M.; Nierman, W.C.; et al. The Sequence of a 1.8-Mb Bacterial Linear Plasmid Reveals a Rich Evolutionary Reservoir of Secondary Metabolic Pathways. Genome Biol. Evol. 2010, 2, 212–224. [Google Scholar] [CrossRef]
- Hwang, K.S.; Kim, H.U.; Charusanti, P.; Palsson, B.T.; Lee, S.Y. Systems Biology and Biotechnology of Streptomyces Species for the Production of Secondary Metabolites. Biotechnol. Adv. 2014, 32, 255–268. [Google Scholar] [CrossRef]
- Baral, B.; Akhgari, A.; Metsä-Ketelä, M. Activation of Microbial Secondary Metabolic Pathways: Avenues and Challenges. Synth. Syst. Biotechnol. 2018, 3, 163–178. [Google Scholar] [CrossRef]
- Zhu, S.; Duan, Y.; Huang, Y. The Application of Ribosome Engineering to Natural Product Discovery and Yield Improvement in Streptomyces. Antibiotics 2019, 8, 133. [Google Scholar] [CrossRef]
- Sharma, D.; Cukras, A.R.; Rogers, E.J.; Southworth, D.R.; Green, R. Mutational Analysis of S12 Protein and Implications for the Accuracy of Decoding by the Ribosome. J. Mol. Biol. 2007, 374, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Okamoto-Hosoya, Y.; Hosaka, T.; Ochi, K. An Aberrant Protein Synthesis Activity Is Linked with Antibiotic Overproduction in RpsL Mutants of Streptomyces Coelicolor A3(2). Microbiology 2003, 149 Pt 11, 3299–3309. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Ohnishi-Kameyama, M.; Muramatsu, H.; Murakami, K.; Tsurumi, Y.; Kodani, S.; Yoshida, M.; Fujie, A.; Ochi, K. Antibacterial Discovery in Actinomycetes Strains with Mutations in RNA Polymerase or Ribosomal Protein S12. Nat. Biotechnol. 2009, 27, 462–464. [Google Scholar] [CrossRef]
- Hosaka, T.; Xu, J.; Ochi, K. Increased Expression of Ribosome Recycling Factor Is Responsible for the Enhanced Protein Synthesis during the Late Growth Phase in an Antibiotic-Overproducing Streptomyces Coelicolor Ribosomal RpsL Mutant. Mol. Microbiol. 2006, 61, 883–897. [Google Scholar] [CrossRef]
- Wang, G.; Hosaka, T.; Ochi, K. Dramatic Activation of Antibiotic Production in Streptomyces Coelicolor by Cumulative Drug Resistance Mutations. Appl. Environ. Microbiol. 2008, 74, 2834–2840. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Komatsu, M.; Okamoto, S.; Tokuyama, S.; Kaji, A.; Ikeda, H.; Ochi, K. Antibiotic Overproduction by RpsL and RsmG Mutants of Various Actinomycetes. Appl. Environ. Microbiol. 2009, 75, 4919–4922. [Google Scholar] [CrossRef]
- Shima, J.; Hesketh, A.; Okamoto, S.; Kawamoto, S.; Ochi, K. Induction of Actinorhodin Production by RpsL (Encoding Ribosomal Protein S12) Mutations That Confer Streptomycin Resistance in Streptomyces Lividans and Streptomyces Coelicolor A3(2). J. Bacteriol. 1996, 178, 7276–7284. [Google Scholar] [CrossRef]
- Okamoto-Hosoya, Y.; Okamoto, S.; Ochi, K. Development of Antibiotic-Overproducing Strains by Site-Directed Mutagenesis of the RpsL Gene in Streptomyces Lividans. Appl. Environ. Microbiol. 2003, 69, 4256–4259. [Google Scholar] [CrossRef]
- Koshla, O.; Lopatniuk, M.; Borys, O.; Misaki, Y.; Kravets, V.; Ostash, I.; Shemediuk, A.; Ochi, K.; Luzhetskyy, A.; Fedorenko, V.; et al. Genetically Engineered RpsL Merodiploidy Impacts Secondary Metabolism and Antibiotic Resistance in Streptomyces. World J. Microbiol. Biotechnol. 2021, 37, 62. [Google Scholar] [CrossRef]
- Hirokawa, G.; Nijman, R.M.; Raj, V.S.; Kaji, H.; Igarashi, K.; Kaji, A. The Role of Ribosome Recycling Factor in Dissociation of 70S Ribosomes into Subunits. RNA 2005, 11, 1317–1328. [Google Scholar] [CrossRef][Green Version]
- Ma, Z.; Tao, L.; Bechthold, A.; Shentu, X.; Bian, Y.; Yu, X. Overexpression of Ribosome Recycling Factor Is Responsible for Improvement of Nucleotide Antibiotic-Toyocamycin in Streptomyces Diastatochromogenes 1628. Appl. Microbiol. Biotechnol. 2014, 98, 5051–5058. [Google Scholar] [CrossRef]
- Li, L.; Guo, J.; Wen, Y.; Chen, Z.; Song, Y.; Li, J. Overexpression of Ribosome Recycling Factor Causes Increased Production of Avermectin in Streptomyces Avermitilis Strains. J. Ind. Microbiol. Biotechnol. 2010, 37, 673–679. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with GNPS. Nat. Biotechnol. 2017, 34, 828–837. [Google Scholar] [CrossRef]
- da Silva, R.R.; Wang, M.; Nothias, L.-F.; van der Hooft, J.J.J.; Caraballo-Rodríguez, A.M.; Fox, E.; Balunas, M.J.; Klassen, J.L.; Lopes, N.P.; Dorrestein, P.C. Propagating Annotations of Molecular Networks Using in Silico Fragmentation. PLoS Comput. Biol. 2018, 14, e1006089. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; John Innes Foundation: Norwich, UK, 2000; Volume 291. [Google Scholar]
- Motamedi, H.; Shafiee, A.; Cai, S.J. Integrative Vectors for Heterologous Gene Expression in Streptomyces Spp. Gene 1995, 160, 25–31. [Google Scholar] [CrossRef]
- Bierman, M.; Logan, R.; O’Brien, K.; Seno, E.T.; Rao, R.N.; Schoner, B.E. Plasmid Cloning Vectors for the Conjugal Transfer of DNA from Escherichia Coli to Streptomyces Spp. Gene 1992, 116, 43–49. [Google Scholar] [CrossRef]
- Alexander, D.C.; Jensen, S.E. Investigation of the Streptomyces Clavuligerus Cephamycin C Gene Cluster and Its Regulation by the CcaR Protein. J. Bacteriol. 1998, 180, 4068–4079. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit Cuticular Waxes as a Source of Biologically Active Triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial Activity of Oleanolic and Ursolic Acids and Their Derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Akihisa, T.; Ogihara, J.; Kato, J.; Yasukawa, K.; Ukiya, M.; Yamanouchi, S.; Oishi, K. Inhibitory Effects of Triterpenoids and Sterols on Human Immunodeficiency Virus-1 Reverse Transcriptase. Lipids 2001, 36, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical Anti-Inflammatory Activity of Salvia Officinalis L. Leaves: The Relevance of Ursolic Acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Kuo, R.-Y.; Qian, K.; Morris-Natschke, S.L.; Lee, K.-H. Plant-Derived Triterpenoids and Analogues as Antitumor and Anti-HIV Agents. Nat. Prod. Rep. 2009, 26, 1321–1344. [Google Scholar] [CrossRef]
- Kaneda, M.; Ishimaru, K.; Nakamura, S. Production of Oleanene Triterpenes by Streptomyces. Chem. Pharm. Bull. 1984, 32, 1287–1293. [Google Scholar] [CrossRef][Green Version]
- Ghimire, G.P.; Koirala, N.; Sohng, J.K. Activation of Cryptic Hop Genes from Streptomyces Peucetius ATCC 27952 Involved in Hopanoid Biosynthesis. J. Microbiol. Biotechnol. 2015, 25, 658–661. [Google Scholar] [CrossRef]
- Poralla, K.; Muth, G.; Härtner, T. Hopanoids Are Formed during Transition from Substrate to Aerial Hyphae in Streptomyces Coelicolor A3(2). FEMS Microbiol. Lett. 2000, 189, 93–95. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene Biosynthesis in Plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Tagousop, C.N.; Tamokou, J.-D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial Activities of Flavonoid Glycosides from Graptophyllum Grandulosum and Their Mechanism of Antibacterial Action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef] [PubMed]
- Ilboudo, O.; Bonzi, S.; Tapsoba, I.; Somda, I.; Bonzi-Coulibaly, Y.L. In Vitro Antifungal Activity of Flavonoid Diglycosides of Mentha Piperita and Their Oxime Derivatives against Two Cereals Fungi. Comptes Rendus Chim. 2016, 19, 857–862. [Google Scholar] [CrossRef]
- Lin, J.A.; Wu, C.H.; Fang, S.C.; Yen, G.C. Combining the Observation of Cell Morphology with the Evaluation of Key Inflammatory Mediators to Assess the Anti-Inflammatory Effects of Geranyl Flavonoid Derivatives in Breadfruit. Food Chem. 2012, 132, 2118–2125. [Google Scholar] [CrossRef]
- Ijaz, F.; Ahmad, N.; Ahmad, I.; Ul Haq, A.; Wang, F. Two New Anti-Plasmodial Flavonoid Glycosides from Duranta Repens. J. Enzyme Inhib. Med. Chem. 2010, 25, 773–778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanno, S.-I.; Tomizawa, A.; Hiura, T.; Osanai, Y.; Shouji, A.; Ujibe, M.; Ohtake, T.; Kimura, K.; Ishikawa, M. Inhibitory Effects of Naringenin on Tumor Growth in Human Cancer Cell Lines and Sarcoma S-180-Implanted Mice. Biol. Pharm. Bull. 2005, 28, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Chiou, Y.-N.; Lin, Y.-L. Phenolic Glycosides from Viscum Angulatum. J. Nat. Prod. 2002, 65, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Lee, H.; Ahn, J.H. Biosynthesis of Pinocembrin from Glucose Using Engineered Escherichia Coli. J. Microbiol. Biotechnol. 2014, 24, 1536–1541. [Google Scholar] [CrossRef]
- Cui, H.; Song, M.C.; Lee, J.Y.; Yoon, Y.J. Microbial Production of O-Methylated Flavanones from Methylated Phenylpropanoic Acids in Engineered Escherichia Coli. J. Ind. Microbiol. Biotechnol. 2019, 46, 1707–1713. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Choi, O.; Lee, J.K.; Hwang, B.Y.; Uhm, T.-B.; Hong, Y.-S. Artificial Biosynthesis of Phenylpropanoic Acids in a Tyrosine Overproducing Escherichia Coli Strain. Microb. Cell Fact. 2012, 11, 153. [Google Scholar] [CrossRef]
- Thrum, H.; Eckardt, K.; Bradler, G.; Fügner, R.; Tonew, E.; Tonew, M. Streptovirudins, New Antibiotics with Antibacterial and Antiviral Activity. I. Culture Taxonomy, Fermentation and Production of Streptovirudin Complex. J. Antibiot. 1975, 28, 514–521. [Google Scholar] [CrossRef]
- Eckardt, K. Tunicamycins, Streptovirudins, and Corynetoxins, a Special Subclass of Nucleoside Antibiotics. J. Nat. Prod. 1983, 46, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qu, D.; Zhai, L.; Tao, M.; Wang, Y.; Lin, S.; Price, N.P.J.; Deng, Z. Characterization of the Tunicamycin Gene Cluster Unveiling Unique Steps Involved in Its Biosynthesis. Protein Cell 2010, 1, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Burgo, Y.; Santos-Aberturas, J.; Rodríguez-García, A.; Barreales, E.G.; Tormo, J.R.; Truman, A.W.; Reyes, F.; Aparicio, J.F.; Liras, P. Activation of Secondary Metabolite Gene Clusters in Streptomyces Clavuligerus by the PimM Regulator of Streptomyces Natalensis. Front. Microbiol. 2019, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, T.; Suzuki, H.; Kariya, R.; Seo, T.; Minami, A.; Oikawa, H. Biosynthesis of the Structurally Unique Polycyclopropanated Polyketide-Nucleoside Hybrid Jawsamycin (FR-900848). Angew. Chem. Int. Ed. Engl. 2014, 53, 5423–5426. [Google Scholar] [CrossRef]
- Kaysser, L.; Tang, X.; Wemakor, E.; Sedding, K.; Hennig, S.; Siebenberg, S.; Gust, B. Identification of a Napsamycin Biosynthesis Gene Cluster by Genome Mining. ChemBioChem 2011, 12, 477–487. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, H.; Liang, J.; Wang, M.; Lu, L.; Shao, Z.; Cobb, R.E.; Zhao, H. Activation and Characterization of a Cryptic Polycyclic Tetramate Macrolactam Biosynthetic Gene Cluster. Nat. Commun. 2013, 4, 2894. [Google Scholar] [CrossRef]
- Xu, L.; Wu, P.; Wright, S.J.; Du, L.; Wei, X. Bioactive Polycyclic Tetramate Macrolactams from Lysobacter Enzymogenes and Their Absolute Configurations by Theoretical ECD Calculations. J. Nat. Prod. 2015, 78, 1841–1847. [Google Scholar] [CrossRef]
- Blodgett, J.A.V.; Oh, D.-C.; Cao, S.; Currie, C.R.; Kolter, R.; Clardy, J. Common Biosynthetic Origins for Polycyclic Tetramate Macrolactams from Phylogenetically Diverse Bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, W.; Saha, S.; Zhang, C. Recent Advances in Discovery, Biosynthesis and Genome Mining of Medicinally Relevant Polycyclic Tetramate Macrolactams. Curr. Top. Med. Chem. 2016, 16, 1727–1739. [Google Scholar] [CrossRef]
- Bertasso, M.; Holzenkämpfer, M.; Zeeck, A.; Stackebrandt, E.; Beil, W.; Fiedler, H.-P. Ripromycin and Other Polycyclic Macrolactams from Streptomyces Sp. Tü 6239: Taxonomy, Fermentation, Isolation and Biological Properties. J. Antibiot. 2003, 56, 364–371. [Google Scholar] [CrossRef]
- Jakobi, M.; Winkelmann, G.; Kaiser, D.; Kempler, C.; Jung, G.; Berg, G.; Bahl, H. Maltophilin: A New Antifungal Compound Produced by Stenotrophomonas Maltophilia R3089. J. Antibiot. 1996, 49, 1101–1104. [Google Scholar] [CrossRef]
- Jomon, K.; Kuroda, Y.; Ajisaka, M.; Sakai, H. A New Antibiotic, Ikarugamycin. J. Antibiot. 1972, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.; Heiss, E.H.; Ferk, F.; Peschel, A.; Knasmueller, S.; Dirsch, V.M.; Krupitza, G.; Kopp, B. Ikarugamycin Induces DNA Damage, Intracellular Calcium Increase, P38 MAP Kinase Activation and Apoptosis in HL-60 Human Promyelocytic Leukemia Cells. Mutat. Res. 2011, 709–710, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Blodgett, J.A.V.; Clardy, J. Targeted Discovery of Polycyclic Tetramate Macrolactams from an Environmental Streptomyces Strain. Org. Lett. 2010, 12, 4652–4654. [Google Scholar] [CrossRef]
- Lou, L.; Qian, G.; Xie, Y.; Hang, J.; Chen, H.; Zaleta-Rivera, K.; Li, Y.; Shen, Y.; Dussault, P.H.; Liu, F.; et al. Biosynthesis of HSAF, a Tetramic Acid-Containing Macrolactam from Lysobacter Enzymogenes. J. Am. Chem. Soc. 2011, 133, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ding, E.; Blodgett, J.A.V. Native and Engineered Clifednamide Biosynthesis in Multiple Streptomyces Spp. ACS Synth. Biol. 2018, 7, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, Q.; Ikeda, H.; Zhang, C.; Xu, L.-H. Hydroxylation of Steroids by a Microbial Substrate-Promiscuous P450 Cytochrome (CYP105D7): Key Arginine Residues for Rational Design. Appl. Environ. Microbiol. 2019, 85, e01530-19. [Google Scholar] [CrossRef]
- Greunke, C.; Antosch, J.; Gulder, T.A.M. Promiscuous Hydroxylases for the Functionalization of Polycyclic Tetramate Macrolactams--Conversion of Ikarugamycin to Butremycin. Chem. Commun. 2015, 51, 5334–5336. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Kim, H.S.; Kim, S.H.; Oh, H.R.; Nam, D.H. Organization and Characterization of a Biosynthetic Gene Cluster for Bafilomycin from Streptomyces Griseus DSM 2608. AMB Express 2013, 3, 24. [Google Scholar] [CrossRef]
- Carr, G.; Williams, D.E.; Díaz-Marrero, A.R.; Patrick, B.O.; Bottriell, H.; Balgi, A.D.; Donohue, E.; Roberge, M.; Andersen, R.J. Bafilomycins Produced in Culture by Streptomyces Spp. Isolated from Marine Habitats Are Potent Inhibitors of Autophagy. J. Nat. Prod. 2010, 73, 422–427. [Google Scholar] [CrossRef]
- Zhang, W.; Fortman, J.L.; Carlson, J.C.; Yan, J.; Liu, Y.; Bai, F.; Guan, W.; Jia, J.; Matainaho, T.; Sherman, D.H.; et al. Characterization of the Bafilomycin Biosynthetic Gene Cluster from Streptomyces Lohii. Chembiochem 2013, 14, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef] [PubMed]
- Meguro, A.; Tomita, T.; Nishiyama, M.; Kuzuyama, T. Identification and Characterization of Bacterial Diterpene Cyclases That Synthesize the Cembrane Skeleton. Chembiochem 2013, 14, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Gustafson, K.R.; Boyd, M.R. HIV-Inhibitory Cembrane Derivatives from a Philippines Collection of the Soft Coral Lobophytum Species. J. Nat. Prod. 2000, 63, 531–533. [Google Scholar] [CrossRef]
- Russell, D.W.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001; Volume 1. [Google Scholar]
- Tahlan, K.; Park, H.U.; Wong, A.; Beatty, P.H.; Jensen, S.E. Two Sets of Paralogous Genes Encode the Enzymes Involved in the Early Stages of Clavulanic Acid and Clavam Metabolite Biosynthesis in Streptomyces Clavuligerus. Antimicrob. Agents Chemother. 2004, 48, 930–939. [Google Scholar] [CrossRef][Green Version]
- Romero, J.; Liras, P.; Martín, J.F. Dissociation of Cephamycin and Clavulanic Acid Biosynthesis in Streptomyces Clavuligerus. Appl. Microbiol. Biotechnol. 1984, 20, 318–325. [Google Scholar] [CrossRef]
- Adusumilli, R.; Mallick, P. Data Conversion with ProteoWizard MsConvert. Methods Mol. Biol. 2017, 1550, 339–368. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Spicer, R.A.; Salek, R.; Steinbeck, C. Compliance with Minimum Information Guidelines in Public Metabolomics Repositories. Sci. Data 2017, 4, 170137. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag Relaunched: Incorporating Strategies beyond in Silico Fragmentation. J. Cheminform. 2016, 8, 1–16. [Google Scholar] [CrossRef]
- Böcker, S.; Letzel, M.C.; Lipták, Z.; Pervukhin, A. SIRIUS: Decomposing Isotope Patterns for Metabolite Identification. Bioinformatics 2009, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]



| Strain/Plasmid | Description a | Source/Reference b |
|---|---|---|
| Bacterial Strains | ||
| Escherichia coli DH5α | General laboratory cloning host | Promega |
| E. coli ET12567/pUZ8002 | DNA methylation deficient conjugation host containing the plasmid pUZ8002 (Cam R, Kan R) | [28] |
| Streptomyces clavuligerus ATCC 27064 | Wild type clavulanic acid producer | ATCC |
| S. clavuligerus ermE*p | S. clavuligerus harboring pSET-ermE*p, control strain | This study |
| S. clavuligerus frr | S. clavuligerus harboring pSET-frr, overexpression of frr | This study |
| S. clavuligerus rpsL | S. clavuligerus harboring pSET-rpsL, overexpression of rpsL | This study |
| S. clavuligerus rpsL-K88E | S. clavuligerus harboring pSET-rpsL-K88E, overexpression of rpsL-K88E | This study |
| S. clavuligerus rpsL-L90K | S. clavuligerus harboring pSET-rpsL-L90K, overexpression of rpsL-L90K | This study |
| S. clavuligerus rpsL-R94G | S. clavuligerus harboring pSET-rpsL-R94G, overexpression of rpsL-R94G | This study |
| Plasmids | ||
| pHM11a | Integrative Streptomyces expression vector containing the constitutive ermE*p (Hyg R) | [29] |
| pSET152-tsr | Integrative Streptomyces cloning vector (Apr R, Tsr R) | [30,31] |
| pSET-ermE*p | pSET152-tsr containing constitutive promoter ermE*p from pHM11a | This study |
| pSET-frr | pSET152 containing S. clavuligerus frr gene along with ermE*p from pHM11a | This study |
| pSET-rpsL | pSET152 containing S. clavuligerus rpsL gene along with ermE*p from pHM11a | This study |
| pSET-rpsL-K88E | A site-directed mutant of rpsL (Lys88Glu, K88E) in pSET-rpsL plasmid | This study |
| pSET-rpsL-L90K | A site-directed mutant of rpsL (Leu90Lys, L90K) in pSET-rpsL plasmid | This study |
| pSET-rpsL-R94G | A site-directed mutant of rpsL (Arg94Gly, R94G) in pSET-rpsL plasmid | This study |
| Label | Observed m/z [Adduct] | Name/ Database ID a | Strain Detected in | Molecular Formula (Weight, g/mol) | Metabolite Family |
|---|---|---|---|---|---|
| 1 | 1497.64 [M−H]− | Securioside A | K88E | C72H106O33 (1499.6) | Triterpenoid glycoside |
| 2 | 1455.63 [M−H]− | CID: 56924794 | K88E | C70H104O32 (1457.6) | Triterpenoid glycoside |
| 3 | 1057.52 [M−H]− | Eryngioside E | K88E | C52H82O22 (1059.2) | Triterpenoid glycoside |
| 4 | 1087.53 [M−H]− | CID: 10582011 | K88E, L90K | C53H84O23 (1089.2) | Triterpenoid glycoside |
| 5 | 1073.52 [M−H]− | Tragopogonsaponin Q | K88E, L90K | C56H82O20 (1075.2) | Triterpenoid glycoside |
| 6 | 1101.51 [M−H]− | SN00394245 | K88E | C53H82O24 (1103.2) | Triterpenoid glycoside |
| 7 | 1103.53 [M−H]− | CID: 101205416 | K88E, L90K | C53H84O24 (1105.2) | Triterpenoid glycoside |
| 8 | 750.31 [M−H]− | Mannooligosaccharide derivative | K88E, L90K | C29H53NO21 (751.3) | Saccharide |
| 9 | 441.372 [M+H]+ | 22-Hydroxy-2-hopen-1-oneb | K88E, L90K, R94G | C30H48O2 (440.7) | Hopanoid |
| 10 | 439.357 [M−H2O+H]+ | Oleanolic acidb | K88E | C30H48O3 (456.7) | Triterpenoid |
| 11 | 423.362 [M+H]+ | Glochidone | K88E, L90K, R94G | C30H46O (422.3) | Hopanoid |
| 12 | 617.404 [M+H]+ | CID: 10603865 | K88E | C36H56O8 (616.8) | Triterpenoid glycoside |
| 13 | 599.393 [M+H]+ | SN00379882 | K88E, L90K, R94G | C36H54O7 (598.4) | Triterpenoid |
| 14 | 727.218 [M−H]− | Viscumneoside V | K88E | C32H40O19 (728.2) | Flavonoid glycoside |
| 15 | 681.212 [M−H]− | CID: 42607862 | K88E | C31H38O17 (682.6) | Flavonoid glycoside |
| 16 | 741.233 [M−H]− | Monoglucosyl naringin | K88E | C33H42O19 (742.7) | Flavonoid glycoside |
| 17 | 819.42 [M+H]+ | Streptovirudin C1 | frr | C37H62N4O16 (818.9) | Nucleoside antibiotic |
| 18 | 791.393 [M+H]+ | Streptovirudin A1 | frr | C35H58N4O16 (790.9) | Nucleoside antibiotic |
| 19 | 511.28 [M+H]+ | Maltophilin | wt, frr, rpsL, K88E, L90K, R94G | C29H38N2O6 (510.6) | Polycyclic tetramate macrolactam |
| 20 | 509.264 [M+H]+ | Clifednamide B | wt, frr, rpsL, K88E, L90K, R94G | C29H36N2O6 (508.6) | Polycyclic tetramate macrolactam |
| 21 | 493.27 [M+H]+ | Clifednamide A | K88E, L90K, R94G | C29H36N2O5 (492.6) | Polycyclic tetramate macrolactam |
| 22 | 619.422 [M-H]− | Bafilomycin J | wt, frr, rpsL | C36H60O8 (620.9) | Macrolide |
| 23 | 376.246 [M+H]+ | 17-dimethylamino lobohedleolide | K88E | C22H33NO4 (375.2) | Cembrane diterpenoid |
| 24 | 377.229 [M+H]+ | CID: 11559852 | K88E, L90K, R94G | C22H32O5 (376.5) | Cembrane diterpenoid |
| 25 | 375.213 [M+H]+ | SN00398992 | K88E | C22H30O5 (374.2) | Cembrane diterpenoid |
| 26 | 524.325 [M−H]− | ChEBI:124407 | rpsL | C29H43N5O4 (525.3) | Organonitrogen |
| 27 | 552.32 [M−H]− | ChEBI:126491 | rpsL | C30H43N5O5 (553.3) | Organonitrogen |
| 28 | 538.305 [M−H]− | ChEBI:128695 | rpsL | C29H41N5O5 (539.3) | Organonitrogen |
| Primer Name | Sequence (5’→3’) | Description |
|---|---|---|
| Sc-frr-F | ATAGCCATATGATGGAGAAGGCCGTCGTGGTC | Primers for the amplification of frr from S. clavuligerus to prepare pSET-frr |
| Sc-frr-R | CTTACGGATCCTCAGACTTCGAGCAGCTCGG | |
| Sc-rpsL-F | ATAGCCATATGGTGCCTACGATCCAGCAGC | Primers for the amplification of rpsL from S. clavuligerus to prepare pSET-rpsL |
| Sc-rpsL-R | CTTACGGATCCTTACTTCTCCTTCTTGGCG | |
| Sc-rpsL-K88E-F | CCGGCAGGTCCTCCACACGGCCACC | Primers for introducing a single amino acid mutation (Lys88Glu) in rpsL from S. clavuligerus to prepare pSET-rpsL-K88E |
| Sc-rpsL-K88E-R | GGTGGCCGTGTGGAGGACCTGCCGG | |
| Sc-rpsL-L90K-F | TAACGAACACCCGGCTTGTCCTTCACACGGCC | Primers for introducing a single amino acid mutation (Leu90Lys) in rpsL from S. clavuligerus to prepare pSET-rpsL-L90K |
| Sc-rpsL-L90K-R | GGCCGTGTGAAGGACAAGCCGGGTGTTCGTTA | |
| Sc-rpsL-R94G-F | CGGATGATCTTGTAACCAACACCCGGCAGGTC | Primers for introducing a single amino acid mutation (Arg94Gly) in rpsL from S. clavuligerus to prepare pSET-rpsL-R94G |
| Sc-rpsL-R94G-R | GACCTGCCGGGTGTTGGTTACAAGATCATCCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, A.A.; Nothias, L.-F.; Srivastava, S.K.; Dorrestein, P.C.; Tahlan, K. Specialized Metabolites from Ribosome Engineered Strains of Streptomyces clavuligerus. Metabolites 2021, 11, 239. https://doi.org/10.3390/metabo11040239
Shaikh AA, Nothias L-F, Srivastava SK, Dorrestein PC, Tahlan K. Specialized Metabolites from Ribosome Engineered Strains of Streptomyces clavuligerus. Metabolites. 2021; 11(4):239. https://doi.org/10.3390/metabo11040239
Chicago/Turabian StyleShaikh, Arshad Ali, Louis-Felix Nothias, Santosh K. Srivastava, Pieter C. Dorrestein, and Kapil Tahlan. 2021. "Specialized Metabolites from Ribosome Engineered Strains of Streptomyces clavuligerus" Metabolites 11, no. 4: 239. https://doi.org/10.3390/metabo11040239
APA StyleShaikh, A. A., Nothias, L.-F., Srivastava, S. K., Dorrestein, P. C., & Tahlan, K. (2021). Specialized Metabolites from Ribosome Engineered Strains of Streptomyces clavuligerus. Metabolites, 11(4), 239. https://doi.org/10.3390/metabo11040239