Long-Chain Acylcarnitines and Monounsaturated Fatty Acids Discriminate Heart Failure Patients According to Pulmonary Hypertension Status
Abstract
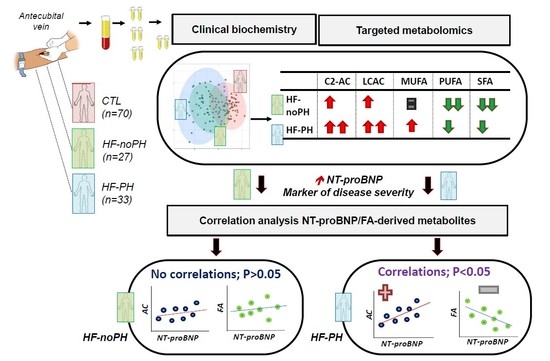
1. Introduction
2. Results
2.1. Population Characteristics
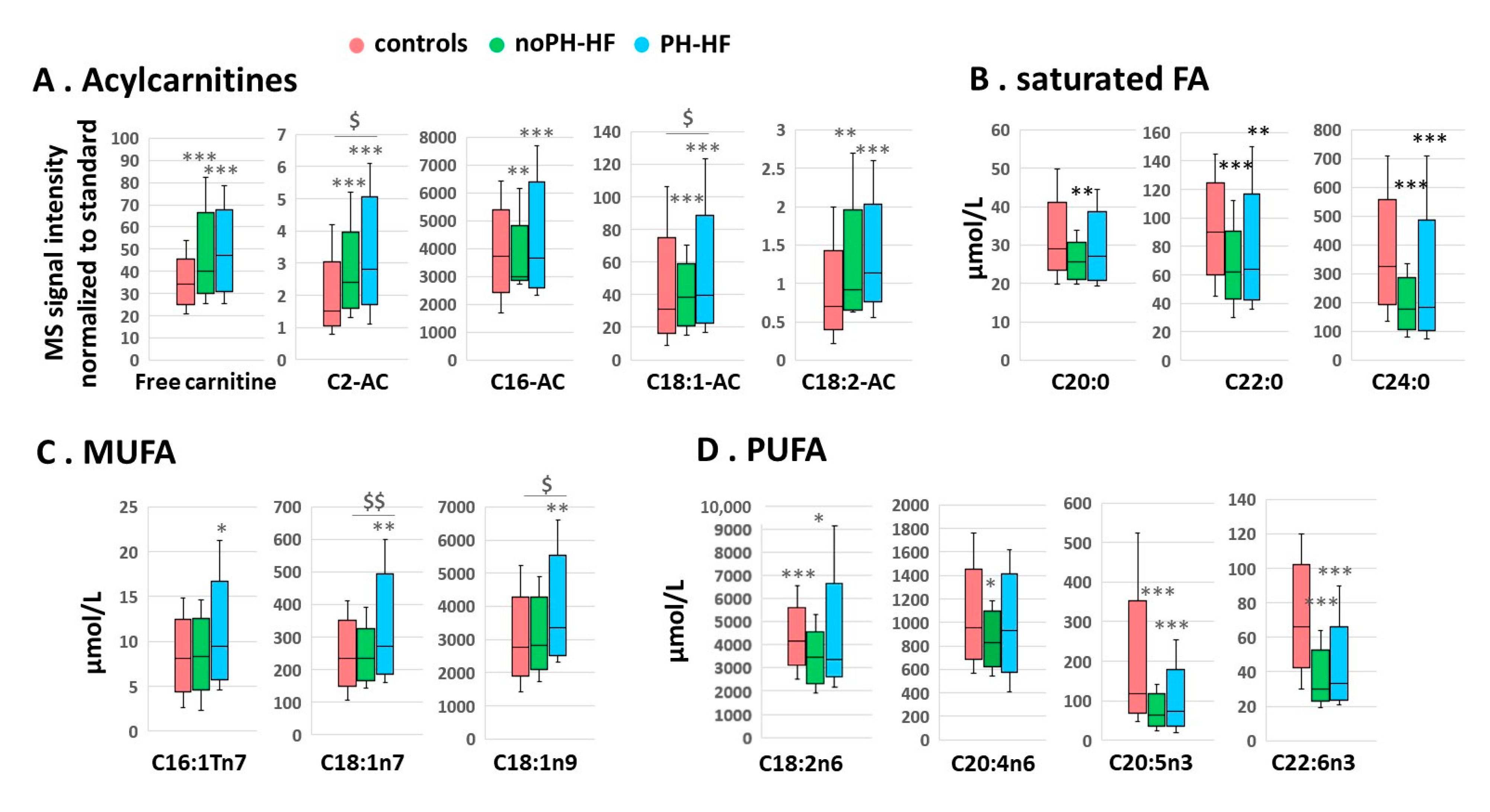
2.2. Differential Acylcarnitine and FA Profiles in noPH-HF and PH-HF Compared to Healthy Individuals
2.3. Acylcarnitine and MUFA Perturbations Are Greater in PH-HF Compared to noPH-HF
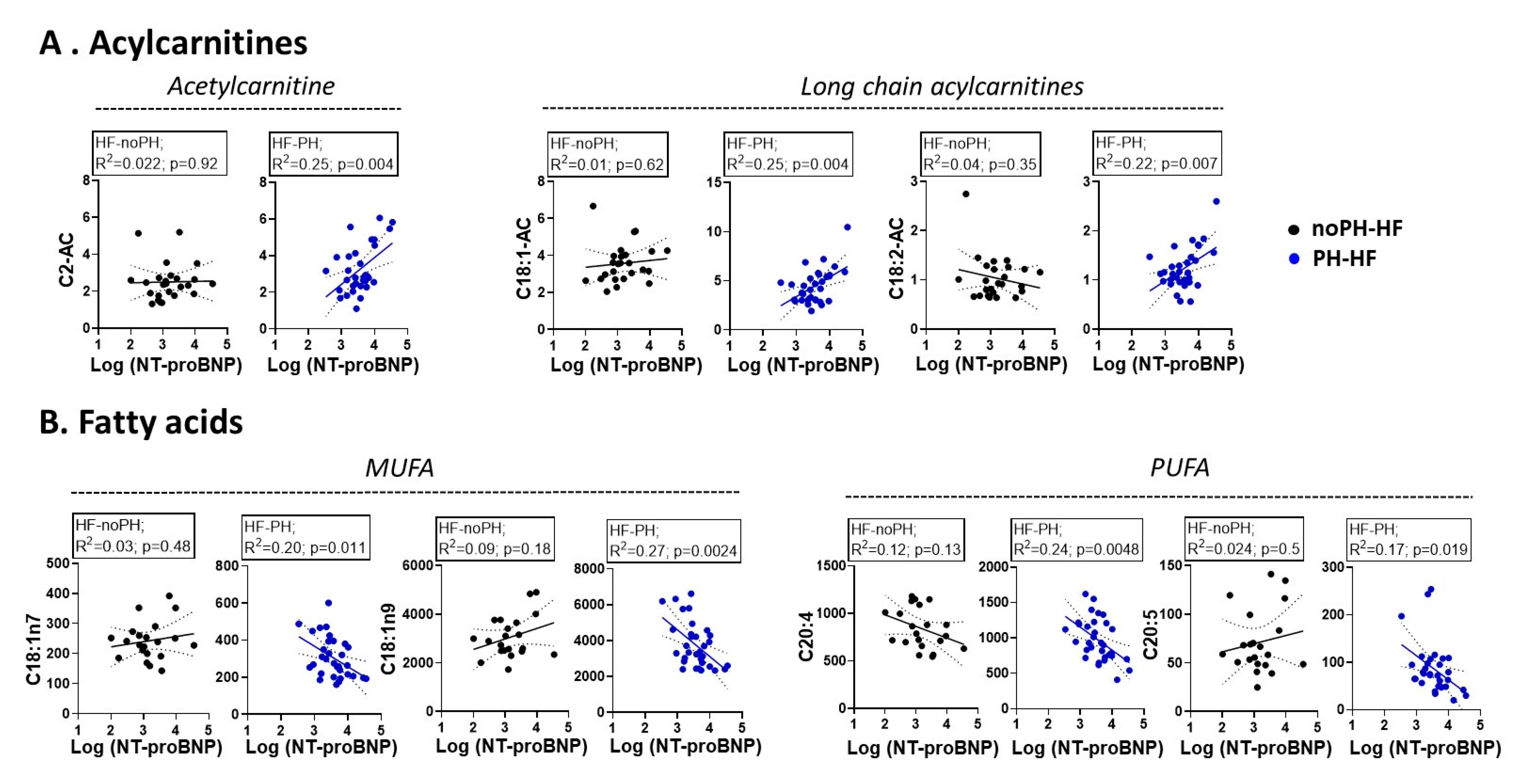
2.4. Different Associations between Metabolites and NT-proBNP According to PH Status
2.5. Metabolite Correlation with NT-proBNP Only in PH-HF
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Participants and Sample Collection
4.2. Data Collection and Patient Classification
4.3. Metabolite Profiling
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Guazzi, M. Pulmonary Hypertension and Heart Failure: A Dangerous Liaison. Heart Fail. Clin. 2018, 14, 297–309. [Google Scholar] [CrossRef]
- Neubauer, S. The failing heart—An engine out of fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Djousse, L.; Benkeser, D.; Arnold, A.; Kizer, J.R.; Zieman, S.J.; Lemaitre, R.N.; Tracy, R.P.; Gottdiener, J.S.; Mozaffarian, D.; Siscovick, D.S.; et al. Plasma free fatty acids and risk of heart failure: The Cardiovascular Health Study. Circ. Heart Fail. 2013, 6, 964–969. [Google Scholar] [CrossRef]
- Oie, E.; Ueland, T.; Dahl, C.P.; Bohov, P.; Berge, C.; Yndestad, A.; Gullestad, L.; Aukrust, P.; Berge, R.K. Fatty acid composition in chronic heart failure: Low circulating levels of eicosatetraenoic acid and high levels of vaccenic acid are associated with disease severity and mortality. J. Intern. Med. 2011, 270, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kelly, J.P.; McGarrah, R.W.; Hellkamp, A.S.; Fiuzat, M.; Testani, J.M.; Wang, T.S.; Verma, A.; Samsky, M.D.; Donahue, M.P.; et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility with Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2016, 67, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Kalim, S.; Clish, C.B.; Wenger, J.; Elmariah, S.; Yeh, R.W.; Deferio, J.J.; Pierce, K.; Deik, A.; Gerszten, R.E.; Thadhani, R.; et al. A plasma long-chain acylcarnitine predicts cardiovascular mortality in incident dialysis patients. J. Am. Heart Assoc. 2013, 2, e000542. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Labarthe, F.; Fortier, A.; Bouchard, B.; Thompson Legault, J.; Bolduc, V.; Rigal, O.; Chen, J.; Ducharme, A.; Crawford, P.A.; et al. Circulating acylcarnitine profile in human heart failure: A surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H768–H781. [Google Scholar] [CrossRef]
- Ryan, J.J.; Archer, S.L. Emerging concepts in the molecular basis of pulmonary arterial hypertension: Part I: Metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 2015, 131, 1691–1702. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Luther, J.M.; Rhodes, C.J.; Burgess, J.P.; Carlson, J.; Fan, R.; Fessel, J.P.; Fortune, N.; Gerszten, R.E.; Halliday, S.J.; et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Rhodes, C.J.; Ghataorhe, P.; Wharton, J.; Rue-Albrecht, K.C.; Hadinnapola, C.; Watson, G.; Bleda, M.; Haimel, M.; Coghlan, G.; Corris, P.A.; et al. Plasma Metabolomics Implicates Modified Transfer RNAs and Altered Bioenergetics in the Outcomes of Pulmonary Arterial Hypertension. Circulation 2017, 135, 460–475. [Google Scholar] [CrossRef]
- Luo, N.; Craig, D.; Ilkayeva, O.; Muehlbauer, M.; Kraus, W.E.; Newgard, C.B.; Shah, S.H.; Rajagopal, S. Plasma acylcarnitines are associated with pulmonary hypertension. Pulm. Circ. 2017, 7, 211–218. [Google Scholar] [CrossRef][Green Version]
- Asselin, C.; Ducharme, A.; Ntimbane, T.; Ruiz, M.; Fortier, A.; Guertin, M.C.; Lavoie, J.; Diaz, A.; Levy, E.; Tardif, J.C.; et al. Circulating levels of linoleic acid and HDL-cholesterol are major determinants of 4-hydroxynonenal protein adducts in patients with heart failure. Redox Biol. 2014, 2, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W., 3rd; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Brittain, E.L.; Talati, M.; Fessel, J.P.; Zhu, H.; Penner, N.; Calcutt, M.W.; West, J.D.; Funke, M.; Lewis, G.D.; Gerszten, R.E.; et al. Fatty Acid Metabolic Defects and Right Ventricular Lipotoxicity in Human Pulmonary Arterial Hypertension. Circulation 2016, 133, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.; Mizuno, S.; Szczepanek, K.; Van Tassell, B.; Natarajan, R.; dos Remedios, C.G.; Drake, J.I.; Farkas, L.; Kraskauskas, D.; Wijesinghe, D.S.; et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ. Heart Fail. 2013, 6, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Miller, D.P.; Frost, A.; Barst, R.J.; Krichman, A.M.; McGoon, M.D. Analysis of the lung allocation score estimation of risk of death in patients with pulmonary arterial hypertension using data from the REVEAL Registry. Transplantation 2010, 90, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Yamada, K.A.; Kanter, E.M.; Newatia, A. Long-chain acylcarnitine induces Ca2+ efflux from the sarcoplasmic reticulum. J. Cardiovasc. Pharmacol. 2000, 36, 14–21. [Google Scholar] [CrossRef]
- Bonnet, D.; Martin, D.; De Pascale, L.; Villain, E.; Jouvet, P.; Rabier, D.; Brivet, M.; Saudubray, J.M. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation 1999, 100, 2248–2253. [Google Scholar] [CrossRef]
- Criddle, D.N.; Dewar, G.H.; Radniknam, M.; Wathey, W.B.; Woodward, B. The synthesis, and structure-activity relationships of some long chain acyl carnitine esters on the coronary circulation of the rat isolated heart. J. Pharm. Pharmacol. 1991, 43, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Criddle, D.N.; Dewar, G.H.; Wathey, W.B.; Woodward, B. The effects of novel vasodilator long chain acyl carnitine esters in the isolated perfused heart of the rat. Br. J. Pharmacol. 1990, 99, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, L.; Zong, G.; Gao, X.; Zhang, H.; Xiong, Q.; Huo, S.; Niu, Z.; Sun, Q.; Zeng, R.; et al. Associations of Amino Acid and Acylcarnitine Profiles With Incident Hyperuricemia in Middle-Aged and Older Chinese Individuals. Arthritis Care Res. (Hoboken) 2020, 72, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Tuunanen, H.; Engblom, E.; Naum, A.; Nagren, K.; Hesse, B.; Airaksinen, K.E.; Nuutila, P.; Iozzo, P.; Ukkonen, H.; Opie, L.H.; et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation 2006, 114, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Abeywardena, M.Y.; Head, R.J. Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc. Res. 2001, 52, 361–371. [Google Scholar] [CrossRef]
- Sudheendran, S.; Chang, C.C.; Deckelbaum, R.J. N-3 vs. saturated fatty acids: Effects on the arterial wall. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 205–209. [Google Scholar] [CrossRef][Green Version]
- Vorn, R.; Yoo, H.Y. Differential effects of saturated and unsaturated fatty acids on vascular reactivity in isolated mesenteric and femoral arteries of rats. Korean J. Physiol. Pharmacol. 2019, 23, 403–409. [Google Scholar] [CrossRef]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Amsallem, M.; Sternbach, J.M.; Adigopula, S.; Kobayashi, Y.; Vu, T.A.; Zamanian, R.; Liang, D.; Dhillon, G.; Schnittger, I.; McConnell, M.V.; et al. Addressing the Controversy of Estimating Pulmonary Arterial Pressure by Echocardiography. J. Am. Soc. Echocardiogr. 2016, 29, 93–102. [Google Scholar] [CrossRef]
- Steckelberg, R.C.; Tseng, A.S.; Nishimura, R.; Ommen, S.; Sorajja, P. Derivation of mean pulmonary artery pressure from noninvasive parameters. J. Am. Soc. Echocardiogr. 2013, 26, 464–468. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Romeo, E.; Argiento, P.; D’Andrea, A.; Vanderpool, R.; Correra, A.; Bossone, E.; Sarubbi, B.; Calabro, R.; Russo, M.G.; et al. Accuracy and precision of echocardiography versus right heart catheterization for the assessment of pulmonary hypertension. Int. J. Cardiol. 2013, 168, 4058–4062. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Goek, O.N.; Doring, A.; Gieger, C.; Heier, M.; Koenig, W.; Prehn, C.; Romisch-Margl, W.; Wang-Sattler, R.; Illig, T.; Suhre, K.; et al. Serum metabolite concentrations and decreased GFR in the general population. Am. J. Kidney Dis. 2012, 60, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Control | noPH-HF | PH-HF | p-Value a | p-Value b | |
|---|---|---|---|---|---|
| Characteristics | (n = 72) | (n = 27) | (n = 33) | ||
| Age, years | 59 ± 9 | 64 ± 11 | 68 ± 10 | NS | <0.01 |
| Female sex, n (%) | 37 (51) | 18 (67) | 26 (79) | NS | 0.02 |
| Body mass index, kg/m2 | 26 ± 3 | 27 ± 4 | 27 ± 4 | NS | 0.50 |
| Ischemic cardiomyopathy, n (%) | 0 (0) | 14 (52) | 23 (70) | NS | <0.01 |
| Type 2 diabetes, n (%) | 0 (0) | 11 (41) | 17 (52) | NS | 0.01 |
| Smoking, n (%) | 0 (0) | 3 (11) | 5 (15) | NS | <0.01 |
| NYHA class 1, n (%) | NA | 1 (4) | 1 (4) | NS | NA |
| NYHA class 2, n (%) | NA | 12 (44) | 19 (57) | NS | NA |
| NYHA class 3, n (%) | NA | 14 (52) | 13 (39) | NS | NA |
| Systolic blood pressure, mmHg | 120 ± 13 | 107 ± 17 | 106 ± 18 | NS | <0.01 |
| Diastolic blood pressure, mmHg | 73 ± 8 | 60 ± 6 | 56 ± 9 | 0.051 | <0.01 |
| Echocardiographic parameters | |||||
| LVEF, % | NA | 27 ± 7 | 26 ± 7 | NS | NA |
| RV dysfunction, n (%) | NA | 2 (7) | 7 (21) | NS | NA |
| sPAP, mmHg | NA | 31 ± 3 | 54 ± 10 | <0.01 | NA |
| mPAP, mmHg | NA | 21 ± 2 | 35 ± 6 | <0.01 | NA |
| Medications | |||||
| Beta-blockers, n (%) | 3 (4) | 22 (81) | 27 (82) | NS | <0.01 |
| ACE inhibitor, n (%) | 7 (10) | 21 (78) | 29 (88) | NS | <0.01 |
| Mineralocorticoid receptor antagonist, n (%) | 0 (0) | 16 (59) | 23 (70) | NS | <0.01 |
| Diuretics, n (%) | 5 (7) | 24 (89) | 32 (97) | NS | <0.01 |
| Digoxin, n (%) | 0 (0) | 16 (59) | 22 (67) | NS | <0.01 |
| Nitrate, n (%) | 0 (0) | 16 (59) | 14 (42) | NS | <0.01 |
| Amiodarone, n (%) | 0 (0) | 9 (33) | 9 (27) | NS | <0.01 |
| Calcium channel blocker, n (%) | 3 (4) | 1 (4) | 2 (6) | NS | NS |
| Aspirin, n (%) | 10 (14) | 12 (44) | 20 (61) | NS | <0.01 |
| Warfarin, n (%) | 1 (1) | 11 (41) | 17 (52) | NS | <0.01 |
| Statin, n (%) | 7 (24) | 19 (70) | 25 (76) | NS | <0.01 |
| Oral hypoglycemic agent, n (%) | 0 (0) | 8 (30) | 13 (39) | NS | <0.01 |
| Levothyroxin, n (%) | 6 (8) | 7 (26) | 6 (18) | NS | 0.07 |
| Allopurinol, n (%) | 1 (1) | 6 (22) | 11 (33) | NS | <0.01 |
| Control (n = 72) | noPH-HF (n = 27) | PH-HF (n = 33) | p-Value a | p-Value b | |
|---|---|---|---|---|---|
| Biochemical Parameters | |||||
| Hemoglobin, g/L | 142 ± 10 | 132 ± 15 | 134 ± 15 | NS | <0.01 |
| Leucocytes, count × 109/L | 5.8 ± 1.3 | 7.6 ± 2.0 | 7.4 ± 1.8 | NS | <0.01 |
| Urea, nM | 6.1 ± 1.4 | 10.1 ± 3.7 | 12.8 ± 4.9 | <0.01 | <0.01 |
| Estimated glomerular filtration rate, mL/min | 83 ± 21 | 55 ± 25 | 46 ± 20 | NS | <0.01 |
| NT-proBNP, ng/mL | 57 ± (30–90) | 1273 (733–3377) | 3704 (1877–5954) | <0.01 | <0.01 |
| Elevated troponin, n (%) | 0 (0) | 4 (15) | 6 (18) | NS | <0.01 |
| Asparagine aminotransferase, U/L | 21 ± 6 | 24 ± 12 | 24 ± 8 | NS | NS |
| Alanine aminotransferase, U/L | 40 ± 9 | 42 ± 16 | 39 ± 11 | NS | NS |
| Alkaline phosphatase, U/L | 71 ± 18 | 112 ± 53 | 99 ± 42 | NS | <0.01 |
| Total bilirubin, μM | 11 ± 5 | 12 ± 6 | 13 ± 9 | NS | NS |
| Uric acid, μM | 281 ± 77 | 394 ± 127 | 468 ± 136 | 0.04 | <0.01 |
| Total cholesterol, mM | 5.1 ± 0.8 | 4.4 ± 0.9 | 3.9 ± 1.2 | NS | <0.01 |
| HDL-cholesterol, mM | 1.5 ± 0.4 | 1.0 ± 0.3 | 0.9 ± 0.2 | NS | <0.01 |
| LDL-cholesterol, mM | 3.2 ± 0.7 | 2.6 ± 0.7 | 2.4 ± 1.1 | NS | <0.01 |
| Triglycerides, mM | 1.1 ± 0.5 | 2.0 ± 1.3 | 1.4 ± 0.7 | NS | <0.01 |
| Glucose, mM | 4.6 ± 0.5 | 7.4 ± 3.1 | 7.0 ± 1.9 | NS | <0.01 |
| HOMA-IR | 23 ± 12 | 65 ± 40 | 45 ± 26 | 0.03 | <0.01 |
| C-reactive protein, μg/mL | 1.0 (0.5–2.1) | 2.9 (1.2–11.3) | 3.4 (2.0–9.3) | NS | <0.01 |
| TNF-α, pg/mL | 1.1 ± 0.4 | 2.1 ± 1.0 | 2.2 ± 0.6 | NS | <0.01 |
| Myeloperoxydase, ng/mL | 16.9 ± 6.7 | 23.1 ± 8.5 | 23.2 ± 15.0 | NS | <0.01 |
| A. ANCOVA 1 (Sex, Age, HOMA-IR, eGFR) | |||
| noPH-HF vs. Controls | PH-HF vs. Controls | PH-HF vs. noPH-HF | |
| Acylcarnitines | |||
| Free carnitine | <0.01 | <0.001 | NS |
| C2-AC | <0.01 | <0.001 | <0.05 |
| C16-AC | <0.05 | <0.01 | NS |
| C18:1-AC | <0.01 | <0.001 | 0.07 |
| C18:2-AC | <0.01 | <0.001 | NS |
| Saturated fatty acids | |||
| C20:0 | NS | NS | <0.05 |
| C22:0 | <0.01 | <0.05 | NS |
| C24:0 | <0.001 | <0.001 | NS |
| Monounsaturated fatty acids | |||
| C16:1Tn7 | NS | NS | NS |
| C18:1n7 | NS | <0.05 | <0.01 |
| C18:1n9 | NS | <0.05 | <0.01 |
| Polyunsaturated fatty acids | |||
| C18:2n6 | <0.05 | NS | NS |
| C20:4n6 | NS | NS | <0.05 |
| C20:5n3 | <0.001 | <0.01 | NS |
| C22:5n3 | <0.05 | NS | <0.05 |
| C22:4n6 | NS | <0.05 | <0.05 |
| C22:6n3 | <0.001 | <0.05 | NS |
| B. ANCOVA 2 (Sex, Age, HOMA-IR, eGFR, NT-proBNP) | |||
| noPH-HF vs. Controls | PH-HF vs. Controls | PH-HF vs. noPH-HF | |
| Acylcarnitines | |||
| Free carnitine | <0.05 | <0.01 | NS |
| C2-AC | NS | <0.01 | 0.06 |
| C16-AC | NS | <0.05 | NS |
| C18:1-AC | <0.01 | <0.001 | 0.07 |
| C18:2-AC | <0.05 | <0.001 | NS |
| Saturated fatty acids | |||
| C20:0 | NS | NS | <0.05 |
| C22:0 | NS | NS | NS |
| C24:0 | <0.001 | <0.001 | NS |
| Monounsaturated fatty acids | |||
| C16:1Tn7 | NS | <0.05 | NS |
| C18:1n7 | NS | <0.05 | <0.01 |
| C18:1n9 | NS | <0.01 | <0.01 |
| Polyunsaturated fatty acids | |||
| C18:2n6 | NS | NS | NS |
| C20:4n6 | NS | NS | <0.05 |
| C20:5n3 | <0.01 | NS | NS |
| C22:5n3 | NS | NS | NS |
| C22:4n6 | NS | NS | NS |
| C22:6n3 | <0.05 | NS | <0.05 |
| Interaction with NT-proBNP | ||
|---|---|---|
| noPH-HF | PH-HF | |
| Acylcarnitines | ||
| free carnitine | NS | NS |
| C2-AC | NS | <0.01 |
| C16-AC | NS | NS |
| C18:1-AC | NS | <0.01 |
| C18:2-AC | NS | <0.05 |
| Saturated fatty acids | ||
| C20:0 | NS | NS |
| C22:0 | NS | NS |
| C24:0 | NS | NS |
| Monounsaturated fatty acids | ||
| C16:1Tn7 | NS | NS |
| C18:1n7 | NS | <0.01 |
| C18:1n9 | NS | <0.01 |
| Polyunsaturated fatty acids | ||
| C18:2n6 | NS | NS |
| C20:4n6 | NS | <0.01 |
| C20:5n3 | NS | <0.05 |
| C22:4n6 | NS | NS |
| C22:5n3 | NS | NS |
| C22:6n3 | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremblay-Gravel, M.; Fortier, A.; Baron, C.; David, C.; Mehanna, P.; Ducharme, A.; Hussin, J.; Hu, Q.; Tardif, J.-C.; Des Rosiers, C.; et al. Long-Chain Acylcarnitines and Monounsaturated Fatty Acids Discriminate Heart Failure Patients According to Pulmonary Hypertension Status. Metabolites 2021, 11, 196. https://doi.org/10.3390/metabo11040196
Tremblay-Gravel M, Fortier A, Baron C, David C, Mehanna P, Ducharme A, Hussin J, Hu Q, Tardif J-C, Des Rosiers C, et al. Long-Chain Acylcarnitines and Monounsaturated Fatty Acids Discriminate Heart Failure Patients According to Pulmonary Hypertension Status. Metabolites. 2021; 11(4):196. https://doi.org/10.3390/metabo11040196
Chicago/Turabian StyleTremblay-Gravel, Maxime, Annik Fortier, Cantin Baron, Chloé David, Pamela Mehanna, Anique Ducharme, Julie Hussin, Qinghua Hu, Jean-Claude Tardif, Christine Des Rosiers, and et al. 2021. "Long-Chain Acylcarnitines and Monounsaturated Fatty Acids Discriminate Heart Failure Patients According to Pulmonary Hypertension Status" Metabolites 11, no. 4: 196. https://doi.org/10.3390/metabo11040196
APA StyleTremblay-Gravel, M., Fortier, A., Baron, C., David, C., Mehanna, P., Ducharme, A., Hussin, J., Hu, Q., Tardif, J.-C., Des Rosiers, C., Dupuis, J., & Ruiz, M. (2021). Long-Chain Acylcarnitines and Monounsaturated Fatty Acids Discriminate Heart Failure Patients According to Pulmonary Hypertension Status. Metabolites, 11(4), 196. https://doi.org/10.3390/metabo11040196