Metabolic Signature of Arrhythmogenic Cardiomyopathy
Abstract
1. Introduction
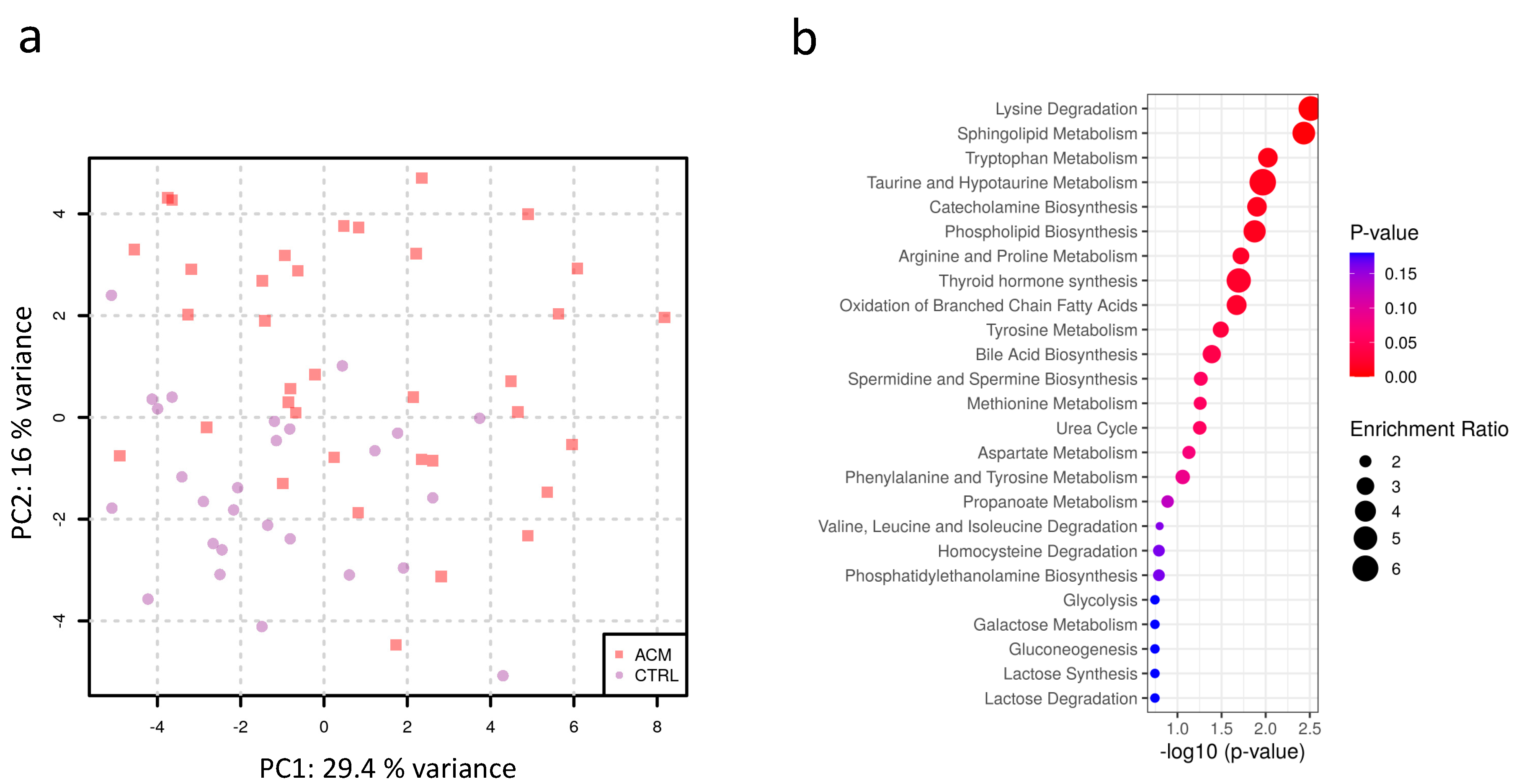
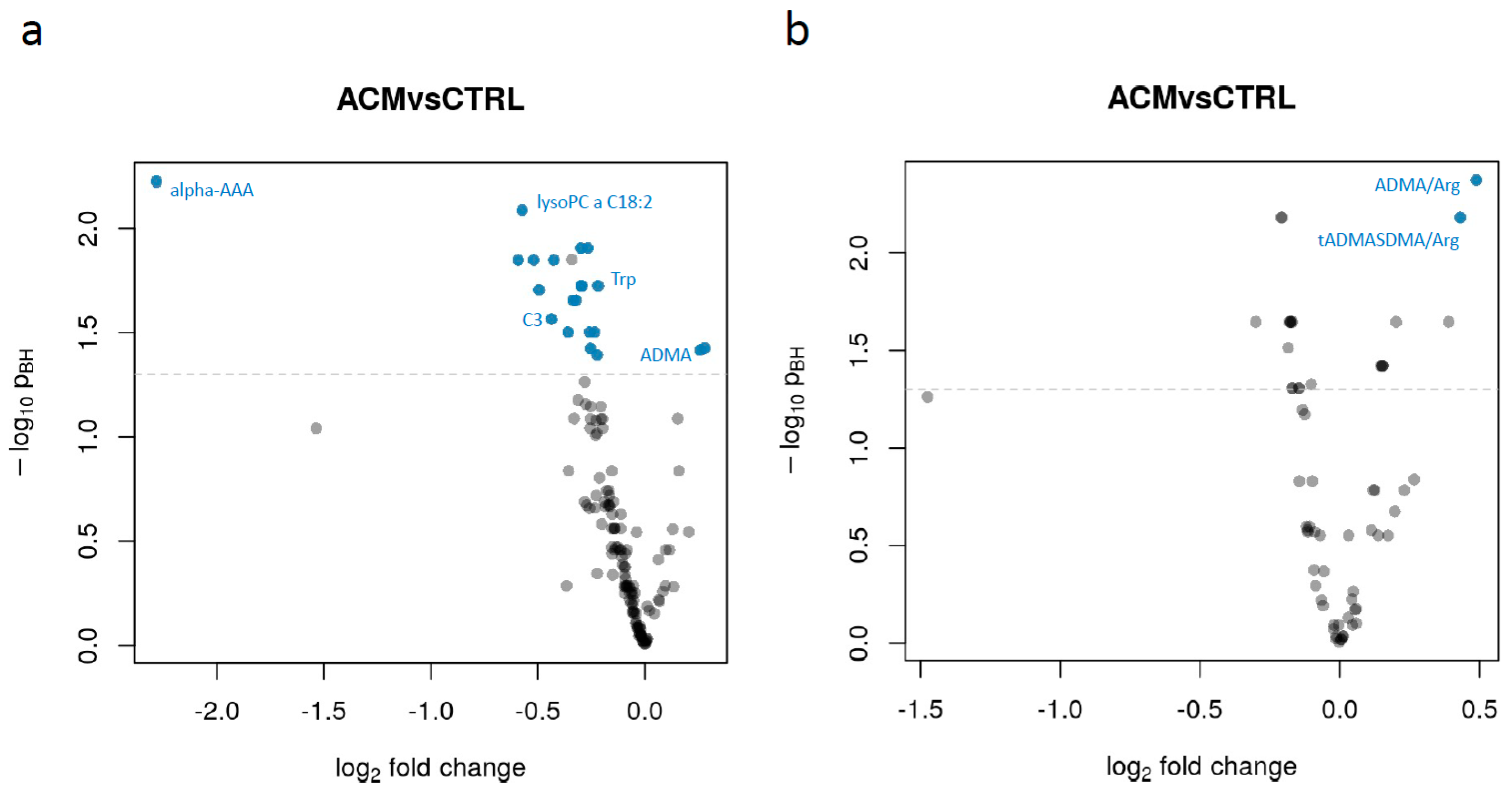
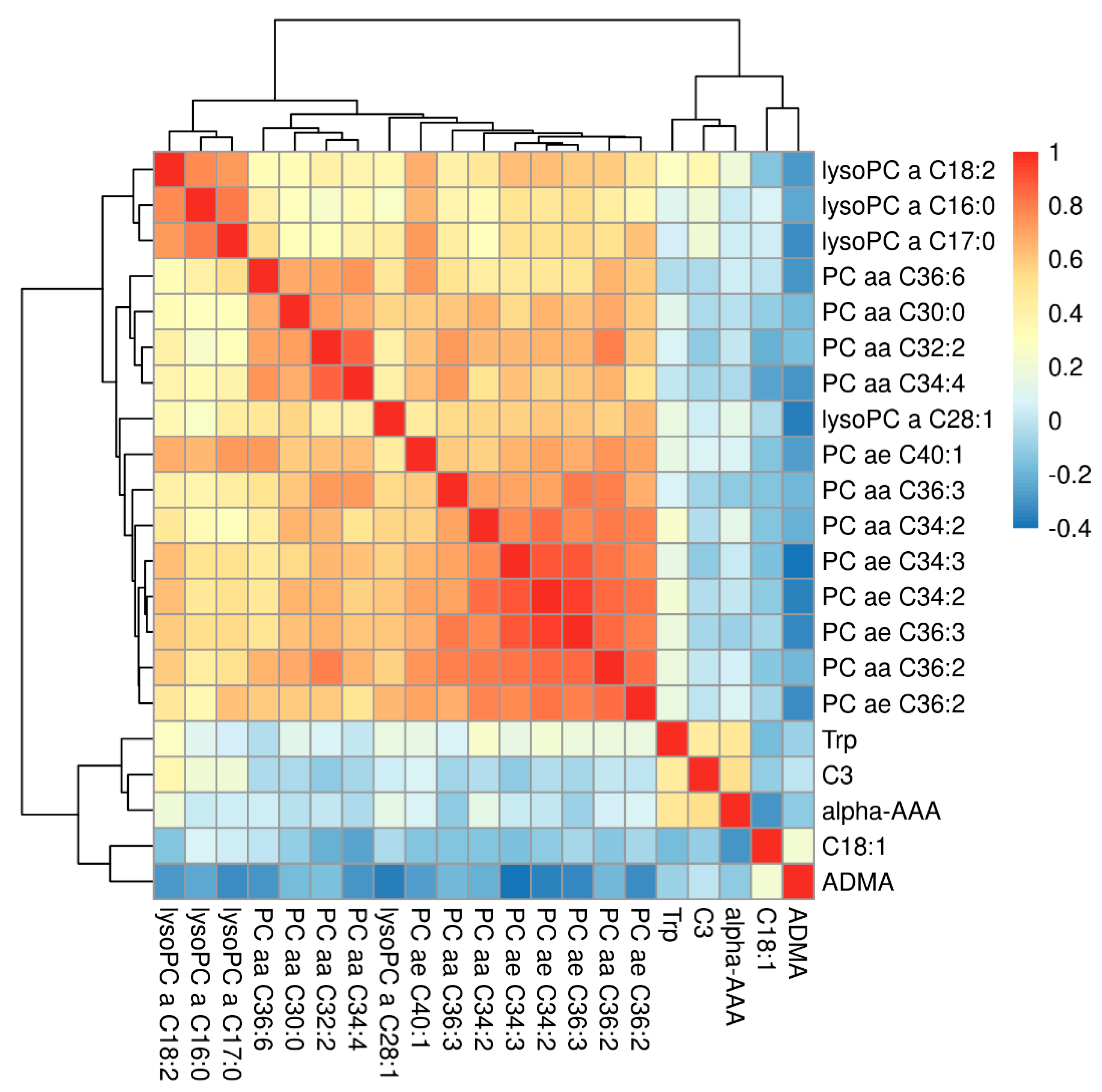
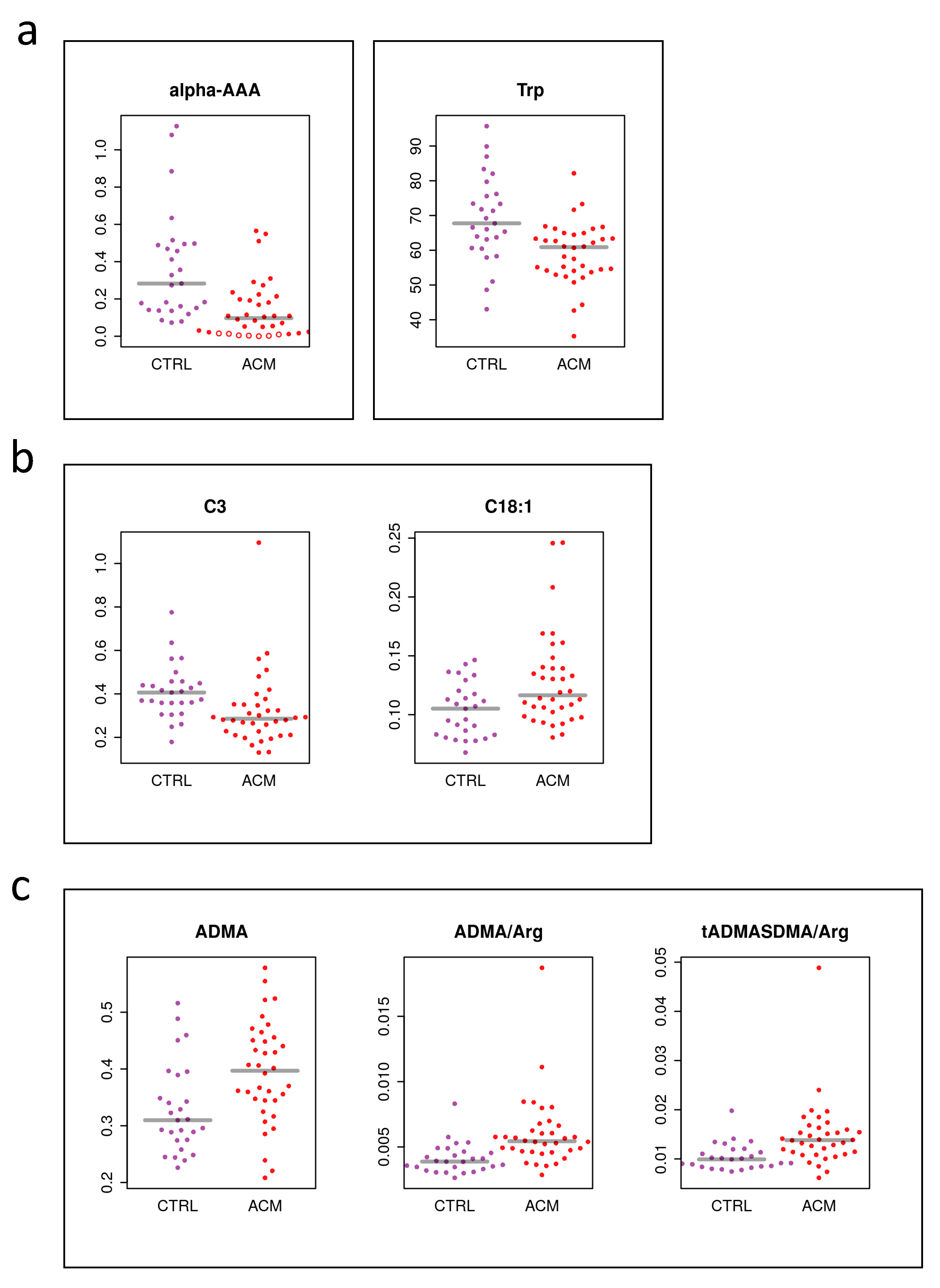
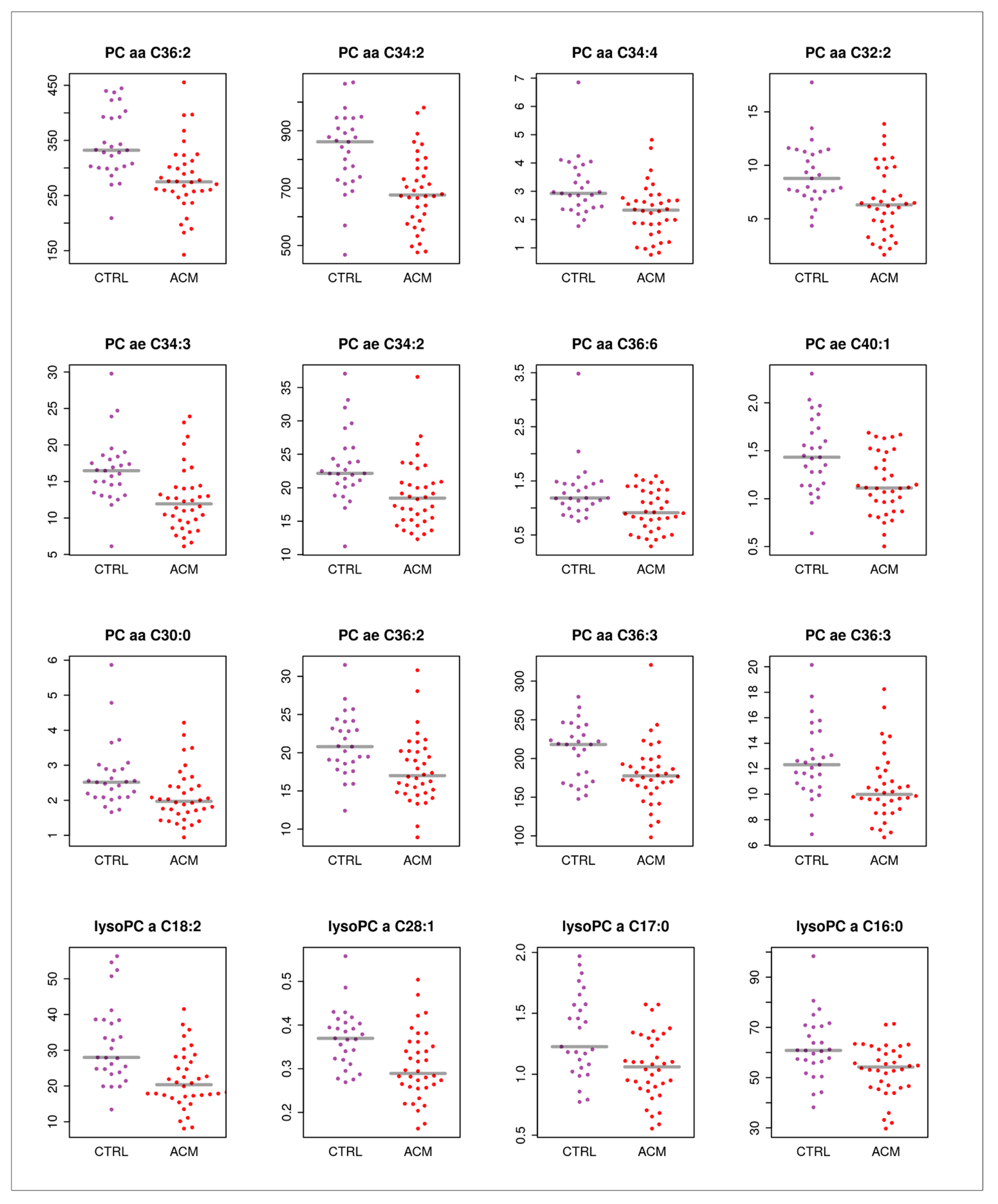
2. Results
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Participants
4.3. Plasma Preparation
4.4. Metabolite Quantification
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Nicholson, J.K. Global systems biology, personalized medicine and molecular epidemiology. Mol. Syst. Biol. 2006, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Donatti, A.; Canto, A.M.; Godoi, A.B.; Da Rosa, D.C.; Lopes-Cendes, I. Circulating Metabolites as Potential Biomarkers for Neurological Disorders—Metabolites in Neurological Disorders. Metabolites 2020, 10, 389. [Google Scholar] [CrossRef]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.-F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef]
- Dias, D.A.; Koal, T. Progress in Metabolomics Standardisation and its Significance in Future Clinical Laboratory Medicine. EJIFCC 2016, 27, 331–343. [Google Scholar]
- Monnerie, S.; Comte, B.; Ziegler, D.; Morais, J.A.; Pujos-Guillot, E.; Gaudreau, P. Metabolomic and Lipidomic Signatures of Metabolic Syndrome and its Physiological Components in Adults: A Systematic Review. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rankin, N.J.; Preiss, D.; Welsh, P.; Sattar, N. Applying metabolomics to cardiometabolic intervention studies and trials: Past experiences and a roadmap for the future: Table 1. Int. J. Epidemiol. 2016, 45, 1351–1371. [Google Scholar] [CrossRef]
- Zhu, M.; Han, Y.; Zhang, Y.; Zhang, S.; Wei, C.; Cong, Z.; Du, W. Metabolomics Study of the Biochemical Changes in the Plasma of Myocardial Infarction Patients. Front. Physiol. 2018, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Surendran, A.; Aliani, M.; Ravandi, A. Metabolomic characterization of myocardial ischemia-reperfusion injury in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz-Siemion, M.; Ciborowski, M.; Kretowski, A.; Musial, W.; Kaminski, K. Metabolomics—A wide-open door to personalized treatment in chronic heart failure? Int. J. Cardiol. 2016, 219, 156–163. [Google Scholar] [CrossRef]
- Shimada, Y.J.; Batra, J.; Kochav, S.M.; Patel, P.; Jung, J.; Maurer, M.S.; Hasegawa, K.; Reilly, M.P.; Fifer, M.A. Difference in Metabolomic Response to Exercise between Patients with and without Hypertrophic Cardiomyopathy. J. Cardiovasc. Transl. Res. 2020, 1–10. [Google Scholar] [CrossRef]
- Del Greco M, F.; Foco, L.; Teumer, A.; Verweij, N.; Paglia, G.; Meraviglia, V.; Melotti, R.; Vukovic, V.; Rauhe, W.; Joshi, P.K.; et al. Lipidomics, Atrial Conduction, and Body Mass Index. Circ. Genom. Precis. Med. 2019, 12, e002384. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Yu, B.; Sun, Y.V.; Chen, L.Y.; Loehr, L.R.; O’Neal, W.T.; Soliman, E.Z.; Boerwinkle, E. Serum Metabolomics and Incidence of Atrial Fibrillation (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2019, 123, 1955–1961. [Google Scholar] [CrossRef]
- Alexander, D.; Lombardi, R.; Rodriguez, G.; Mitchell, M.M.; Marian, A.J. Metabolomic distinction and insights into the pathogenesis of human primary dilated cardiomyopathy. Eur. J. Clin. Investig. 2010, 41, 527–538. [Google Scholar] [CrossRef]
- Jorgenrud, B.; Jalanko, M.; Helio, T.; Jaaskelainen, P.; Laine, M.; Hilvo, M.; Nieminen, M.S.; Laakso, M.; Hyotylainen, T.; Oresic, M.; et al. The Metabolome in Finnish Carriers of the MYBPC3-Q1061X Mutation for Hypertrophic Cardiomyopathy. PLoS ONE 2015, 10, e0134184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corrado, D.; Basso, C.; Schiavon, M.; Thiene, G. Screening for Hypertrophic Cardiomyopathy in Young Athletes. N. Engl. J. Med. 1998, 339, 364–369. [Google Scholar] [CrossRef]
- Riele, A.S.T.; Ajijola, O.A.; Shivkumar, K.; Tandri, H. Role of Bilateral Sympathectomy in the Treatment of Refractory Ventricular Arrhythmias in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2016, 9, e003713. [Google Scholar] [CrossRef]
- Corrado, D.; Link, M.S.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy. N. Engl. J. Med. 2017, 376, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Trümmel, M.; Meyners, W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int. J. Cardiol. 2004, 97, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Pavei, A.; Michieli, P.; Schiavon, M.; Thiene, G. Trends in Sudden Cardiovascular Death in Young Competitive Athletes After Implementation of a Preparticipation Screening Program. JAMA 2006, 296, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; Edson, S.; Towbin, J.A. Genetics of Arrhythmogenic Right Ventricular Cardiomyopathy. J. Am. Coll. Cardiol. 2013, 61, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Delmar, M.; McKenna, W.J. The Cardiac Desmosome and Arrhythmogenic Cardiomyopathies. Circ. Res. 2010, 107, 700–714. [Google Scholar] [CrossRef]
- De Bortoli, M.; Postma, A.V.; Poloni, G.; Calore, M.; Minervini, G.; Mazzotti, E.; Rigato, I.; Ebert, M.; Lorenzon, A.; Vazza, G.; et al. Whole-Exome Sequencing Identifies Pathogenic Variants in TJP1 Gene Associated With Arrhythmogenic Cardiomyopathy. Circ. Genom. Precis. Med. 2018, 11, e002123. [Google Scholar] [CrossRef] [PubMed]
- Mayosi, B.M.; Fish, M.; Shaboodien, G.; Mastantuono, E.; Kraus, S.; Wieland, T.; Kotta, M.-C.; Chin, A.; Laing, N.; Ntusi, N.B.; et al. Identification of Cadherin 2 (CDH2) Mutations in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Genet. 2017, 10. [Google Scholar] [CrossRef]
- Van Hengel, J.; Calore, M.; Bauce, B.; Dazzo, E.; Mazzotti, E.; De Bortoli, M.; Lorenzon, A.; Li Mura, I.E.; Beffagna, G.; Rigato, I.; et al. Mutations in the area composita protein alphaT-catenin are associated with arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2013, 34, 201–210. [Google Scholar] [CrossRef]
- Dalal, D.; James, C.; Devanagondi, R.; Tichnell, C.; Tucker, A.; Prakasa, K.; Spevak, P.J.; Bluemke, D.A.; Abraham, T.; Russell, S.D.; et al. Penetrance of mutations in plakophilin-2 among families with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1416–1424. [Google Scholar] [CrossRef]
- Corrado, D.; Van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.W.; Anastastakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brunckhorst, C.; Bucciarelli-Ducci, C.; et al. Arrhythmogenic right ventricular cardiomyopathy: Evaluation of the current diagnostic criteria and differential diagnosis. Eur. Heart J. 2019, 41, 1414–1429. [Google Scholar] [CrossRef]
- Hong, T.-T.; Cogswell, R.; James, C.A.; Kang, G.; Pullinger, C.R.; Malloy, M.J.; Kane, J.P.; Wojciak, J.; Calkins, H.; Scheinman, M.M.; et al. Plasma BIN1 correlates with heart failure and predicts arrhythmia in patients with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2012, 9, 961–967. [Google Scholar] [CrossRef]
- Broch, K.; Leren, I.S.; Saberniak, J.; Ueland, T.; Edvardsen, T.; Gullestad, L.; Haugaa, K.H. Soluble ST2 is associated with disease severity in arrhythmogenic right ventricular cardiomyopathy. Biomarkers 2017, 22, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Onur, I.; Elitok, A.; Ademoglu, E.; Altun, I.; Bilge, A.K.; Adalet, K. Galectin-3 correlates with arrhythmogenic right ventricular cardiomyopathy and predicts the risk of ventricular -arrhythmias in patients with implantable defibrillators. Acta Cardiol. 2017, 72, 453–459. [Google Scholar] [CrossRef]
- Stadiotti, I.; Pompilio, G.; Maione, A.S.; Pilato, C.A.; D’Alessandra, Y.; Sommariva, E. Arrhythmogenic cardiomyopathy: What blood can reveal? Heart Rhythm. 2019, 16, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, E.; D’Alessandra, Y.; Farina, F.M.; Casella, M.; Cattaneo, F.; Catto, V.; Chiesa, M.; Stadiotti, I.; Brambilla, S.; Russo, A.D.; et al. MiR-320a as a Potential Novel Circulating Biomarker of Arrhythmogenic CardioMyopathy. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Kim, C.; Wong, J.; Wen, J.; Wang, S.; Wang, C.; Spiering, S.; Kan, N.G.; Forcales, S.; Puri, P.L.; Leone, T.C.; et al. Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature 2013, 494, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-P.; Chen, L.; Chen, X.; Ren, J.; Zhang, N.-N.; Tirasawasdichai, T.; Hu, Z.-L.; Hua, W.; Hu, Y.-R.; Tang, H.-R.; et al. Elevated plasma β-hydroxybutyrate predicts adverse outcomes and disease progression in patients with arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2020, 12, eaay8329. [Google Scholar] [CrossRef]
- Barth, A.S.; Tomaselli, G.F. Cardiac Metabolism and Arrhythmias. Circ. Arrhythmia Electrophysiol. 2009, 2, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Paglia, G.; Del Greco, F.M.; Sigurdsson, B.B.; Rainer, J.; Volani, C.; Hicks, A.A.; Pramstaller, P.P.; Smarason, S.V.; Del Greco, M.F. Influence of collection tubes during quantitative targeted metabolomics studies in human blood samples. Clin. Chim. Acta 2018, 486, 320–328. [Google Scholar] [CrossRef]
- Floegel, A.; Kühn, T.; Sookthai, D.; Johnson, T.; Prehn, C.; Rolle-Kampczyk, U.; Otto, W.; Weikert, C.; Illig, T.; Von Bergen, M.; et al. Serum metabolites and risk of myocardial infarction and ischemic stroke: A targeted metabolomic approach in two German prospective cohorts. Eur. J. Epidemiol. 2018, 33, 55–66. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; De Angelis, M.H.; Peters, A.; et al. Identification of Serum Metabolites Associated With Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2012, 62, 639–648. [Google Scholar] [CrossRef]
- Kühn, T.; Floegel, A.; Sookthai, D.; Johnson, T.; Rolle-Kampczyk, U.; Otto, W.; Von Bergen, M.; Boeing, H.; Kaaks, R. Higher plasma levels of lysophosphatidylcholine 18:0 are related to a lower risk of common cancers in a prospective metabolomics study. BMC Med. 2016, 14, 1–9. [Google Scholar] [CrossRef]
- Suhre, K.; Meisinger, C.; Doring, A.; Altmaier, E.; Belcredi, P.; Gieger, C.; Chang, D.; Milburn, M.V.; Gall, W.E.; Weinberger, K.M.; et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS ONE 2010, 5, e13953. [Google Scholar] [CrossRef] [PubMed]
- Siskos, A.P.; Jain, P.; Romisch-Margl, W.; Bennett, M.; Achaintre, D.; Asad, Y.; Marney, L.; Richardson, L.; Koulman, A.; Griffin, J.L.; et al. Interlaboratory Reproducibility of a Targeted Metabolomics Platform for Analysis of Human Serum and Plasma. Anal. Chem. 2017, 89, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Marcus, F.I.; McKenna, W.J.; Sherrill, D.; Basso, C.; Bauce, B.; Bluemke, D.A.; Calkins, H.; Corrado, D.; Cox, M.G.; Daubert, J.P.; et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the Task Force Criteria. Eur. Heart J. 2010, 31, 806–814. [Google Scholar] [CrossRef] [PubMed]
- COMPOSIZIONE COMITATO COCIS. Protocolli Cardiologici per il Giudizio di Idoneità allo Sport Agonistico Casa; Casa Editrice Scientifica Internazionale: Rome, Italy, 2017. [Google Scholar]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.C.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy: Executive summary. Heart Rhythm. 2019, 16, e373–e407. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Wang, P.; Van Bilsen, M.; Hazebroek, M.R.; Merken, J.J.; Vanhoutte, E.K.; Henkens, M.; Van Den Wijngaard, A.; Glatz, J.F.C.; Krapels, I.P.C.; et al. Metabolic Profiling Associates with Disease Severity in Nonischemic Dilated Cardiomyopathy. J. Card. Fail 2020, 26, 212–222. [Google Scholar] [CrossRef]
- D’Apolito, O.; Paglia, G.; Tricarico, F.; Garofalo, D.; Pilotti, A.; Lamacchia, O.; Cignarelli, M.; Corso, G. Development and validation of a fast quantitative method for plasma dimethylarginines analysis using liquid chromatography-tandem mass spectrometry. Clin. Biochem. 2008, 41, 1391–1395. [Google Scholar] [CrossRef]
- Paglia, G.; D’Apolito, O.; Tricarico, F.; Garofalo, D.; Corso, G. Evaluation of mobile phase, ion pairing, and temperature influence on an HILIC-MS/MS method for L-arginine and its dimethylated derivatives detection. J. Sep. Sci. 2008, 31, 2424–2429. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Chu, R.; Yu, D.; Chu, J.; Lin, M.; Yu, H. Prognostic efficacy of circulating asymmetric dimethylarginine in patients with peripheral arterial disease: A meta-analysis of prospective cohort studies. Vascular 2018, 26, 322–330. [Google Scholar] [CrossRef]
- Schulze, F.; Lenzen, H.; Hanefeld, C.; Bartling, A.; Osterziel, K.J.; Goudeva, L.; Schmidt-Lucke, C.; Kusus, M.; Maas, R.; Schwedhelm, E.; et al. Asymmetric dimethylarginine is an independent risk factor for coronary heart disease: Results from the multicenter Coronary Artery Risk Determination investigating the Influence of ADMA Concentration (CARDIAC) study. Am. Heart J. 2006, 152, 493.e1–493.e8. [Google Scholar] [CrossRef]
- Cavusoglu, E.; Ruwende, C.; Chopra, V.; Yanamadala, S.; Eng, C.; Pinsky, D.J.; Marmur, J.D. Relationship of baseline plasma ADMA levels to cardiovascular outcomes at 2 years in men with acute coronary syndrome referred for coronary angiography. Coron. Artery. Dis. 2009, 20, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Krempl, T.K.; Maas, R.; Sydow, K.; Meinertz, T.; Böger, R.H.; Kähler, J. Elevation of asymmetric dimethylarginine in patients with unstable angina and recurrent cardiovascular events. Eur. Heart J. 2005, 26, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Ruwende, C.; Chopra, V.; Poludasu, S.; Yanamadala, S.; Frishman, W.H.; Eng, C.; Pinsky, D.J.; Marmur, J.D. Relation of baseline plasma ADMA levels to cardiovascular morbidity and mortality at two years in men with diabetes mellitus referred for coronary angiography. Atherosclerosis 2010, 210, 226–231. [Google Scholar] [CrossRef]
- Abedini, S.; Meinitzer, A.; Holme, I.; März, W.; Weihrauch, G.; Fellstrøm, B.; Jardine, A.; Holdaas, H. Asymmetrical dimethylarginine is associated with renal and cardiovascular outcomes and all-cause mortality in renal transplant recipients. Kidney Int. 2010, 77, 44–50. [Google Scholar] [CrossRef]
- Valkonen, V.P.; Päivä, H.; Salonen, J.T.; Lakka, T.A.; Lehtimäki, T.; Laakso, J.; Laaksonen, R. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet 2001, 358, 2127–2128. [Google Scholar] [CrossRef]
- Burger, A.L.; Stojkovic, S.; Diedrich, A.; Demyanets, S.; Wojta, J.; Pezawas, T. Elevated plasma levels of asymmetric dimethylarginine and the risk for arrhythmic death in ischemic and non-ischemic, dilated cardiomyopathy—A prospective, controlled long-term study. Clin. Biochem. 2020, 83, 37–42. [Google Scholar] [CrossRef]
- Horowitz, J.D.; De Caterina, R.; Heresztyn, T.; Alexander, J.H.; Andersson, U.; Lopes, R.D.; Steg, P.G.; Hylek, E.M.; Mohan, P.; Hanna, M.; et al. Asymmetric and Symmetric Dimethylarginine Predict Outcomes in Patients With Atrial Fibrillation: An ARISTOTLE Substudy. J. Am. Coll. Cardiol. 2018, 72, 721–733. [Google Scholar] [CrossRef]
- Bonilla, I.M.; Sridhar, A.; Györke, S.; Cardounel, A.J.; Carnes, C.A. Nitric oxide synthases and atrial fibrillation. Front. Physiol. 2012, 3, 105. [Google Scholar] [CrossRef]
- Santos-Miranda, A.; Joviano-Santos, J.V.; Ribeiro, G.A.; Botelho, A.F.M.; Rocha, P.; Vieira, L.Q.; Cruz, J.S.; Roman-Campos, D. Reactive oxygen species and nitric oxide imbalances lead to in vivo and in vitro arrhythmogenic phenotype in acute phase of experimental Chagas disease. PLoS Pathog. 2020, 16, e1008379. [Google Scholar] [CrossRef] [PubMed]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wirleitner, B.; Rudzite, V.; Neurauter, G.; Murr, C.; Kalnins, U.; Erglis, A.; Trusinskis, K.; Fuchs, D. Immune activation and degradation of tryptophan in coronary heart disease. Eur. J. Clin. Invest 2003, 33, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Ruiz-Canela, M.; Guasch-Ferré, M.; Zheng, Y.; Toledo, E.; Clish, C.B.; Salas-Salvadó, J.; Liang, L.; Wang, D.D.; Corella, D.; et al. Increases in Plasma Tryptophan Are Inversely Associated with Incident Cardiovascular Disease in the Prevención con Dieta Mediterránea (PREDIMED) Study. J. Nutr. 2017, 147, 314–322. [Google Scholar] [CrossRef]
- Gostner, J.M.; Kurz, K.; Fuchs, D. The significance of tryptophan metabolism and vitamin B-6 status in cardiovascular disease. Am. J. Clin. Nutr. 2020, 111, 8–9. [Google Scholar] [CrossRef]
- Murr, C.; Grammer, T.B.; Kleber, M.E.; Meinitzer, A.; März, W.; Fuchs, D. Low serum tryptophan predicts higher mortality in cardiovascular disease. Eur. J. Clin. Investig. 2015, 45, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, I.; Hermanowicz, J.M.; Mysliwiec, M.; Pawlak, D. Oxidative Storm Induced by Tryptophan Metabolites: Missing Link between Atherosclerosis and Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Deng, Y.; Sun, L.; Lou, B.; Yuan, Z.; Wu, Y.; Zhou, B.; Liu, J.; She, J. Serum lipids profiling perturbances in patients with ischemic heart disease and ischemic cardiomyopathy. Lipids Health Dis. 2020, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef]
- Aitken-Buck, H.M.; Krause, J.; Zeller, T.; Jones, P.P.; Lamberts, R.R. Long-Chain Acylcarnitines and Cardiac Excitation-Contraction Coupling: Links to Arrhythmias. Front. Physiol. 2020, 11, 577856. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, X.F.; Huang, T.; Luo, H.H.; Chen, J.X.; Zeng, J.; Gu, M.; Li, J.; Sun, X.Y.; Sun, D.; et al. The Association Between Acylcarnitine Metabolites and Cardiovascular Disease in Chinese Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Volani, C.; Paglia, G.; Smarason, S.V.; Pramstaller, P.P.; Demetz, E.; Pfeifhofer-Obermair, C.; Weiss, G. Metabolic Signature of Dietary Iron Overload in a Mouse Model. Cells 2018, 7, 264. [Google Scholar] [CrossRef]
- Ferrari, R.; Merli, E.; Cicchitelli, G.; Mele, D.; Fucili, A.; Ceconi, C. Therapeutic effects of L-carnitine and propionyl-L-carnitine on cardiovascular diseases: A review. Ann. N. Y. Acad. Sci. 2004, 1033, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, W.; Wang, Y.; Pedram, P.; Cahill, F.; Zhai, G.; Randell, E.; Gulliver, W.; Sun, G. Serum metabolic biomarkers distinguish metabolically healthy peripherally obese from unhealthy centrally obese individuals. Nutr. Metab. 2016, 13, 33. [Google Scholar] [CrossRef]
- Xu, W.Y.; Shen, Y.; Zhu, H.; Gao, J.; Zhang, C.; Tang, L.; Lu, S.Y.; Shen, C.L.; Zhang, H.X.; Li, Z.; et al. 2-Aminoadipic acid protects against obesity and diabetes. J. Endocrinol. 2019, 243, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Stelzer, I.; Reininghaus, E.Z.; Weghuber, D.; Postolache, T.T.; Fuchs, D. Disturbed tryptophan metabolism in cardiovascular disease. Curr. Med. Chem. 2014, 21, 1931–1937. [Google Scholar] [CrossRef]
- Tomczyk, M.M.; Dolinsky, V.W. The Cardiac Lipidome in Models of Cardiovascular Disease. Metabolites 2020, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3. [Google Scholar] [CrossRef] [PubMed]
- Jewison, T.; Su, Y.; Disfany, F.M.; Liang, Y.; Knox, C.; Maciejewski, A.; Poelzer, J.; Huynh, J.; Zhou, Y.; Arndt, D.; et al. SMPDB 2.0: Big improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014, 42, D478–D484. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016, 55, 14.10.11–14.10.91. [Google Scholar] [CrossRef]
| ACM Patients (N = 36) | CTRL (N = 27) | p Value (Test) | |
|---|---|---|---|
| Male sex (%; n) | 88.89% (n = 32/36) | 74.07% (n = 20/27) | 0.18 (Fisher’s exact test) |
| Age (mean ± SD) | 45.31 ± 13.29 | 46.04 ± 14.19 | 0.83 (Student t-test) |
| Obesity (%; n) | 5.56% (n = 2/36) | 0.04% (n = 1/27) | 1.00 (Fisher’s exact test) |
| Athletic lifestyle (%; n) | 36.11% (n = 13/36) | 36.36% (n = 8/22) | 1.00 (Fisher’s exact test) |
| RV EF % (mean ± SD) | 44.43% ± 7.79 | ND | |
| LV EF % (mean ± SD) | 54.63% ± 13.64 | ND | |
| VT at presentation (%; n) | 63.89% (n = 23/36) | ND |
| Name | Log2 Fold Change | pBH |
|---|---|---|
| alpha-AAA | −2.2817718 | 0.00599521 |
| PC aa C32:2 | −0.5926965 | 0.01416184 |
| lysoPC a C18:2 | −0.5736359 | 0.00817355 |
| PC aa C34:4 | −0.519949 | 0.01416184 |
| PC aa C36:6 | −0.4945815 | 0.01972115 |
| C3 | −0.436115 | 0.02721582 |
| PC ae C34:3 | −0.4253616 | 0.01416184 |
| PC aa C30:0 | −0.3589317 | 0.03137787 |
| PC ae C40:1 | −0.3355771 | 0.02210991 |
| lysoPC a C17:0 | −0.322115 | 0.02210991 |
| PC aa C36:2 | −0.2985986 | 0.01241476 |
| PC ae C34:2 | −0.297975 | 0.01884033 |
| lysoPC a C28:1 | −0.2948432 | 0.01884033 |
| PC aa C34:2 | −0.2661214 | 0.01241476 |
| PC ae C36:2 | −0.2586741 | 0.03137787 |
| PC ae C36:3 | -0.2546263 | 0.03763856 |
| PC aa C36:3 | −0.2350526 | 0.03137787 |
| lysoPC a C16:0 | −0.2223977 | 0.04041611 |
| Trp | −0.2181965 | 0.01884033 |
| ADMA | 0.25872497 | 0.03833323 |
| C18:1 | 0.28073695 | 0.03750482 |
| ADMA/Arg | 0.48864835 | 0.00423649 |
| tADMASDMA/Arg | 0.43152368 | 0.0065969 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volani, C.; Rainer, J.; Hernandes, V.V.; Meraviglia, V.; Pramstaller, P.P.; Smárason, S.V.; Pompilio, G.; Casella, M.; Sommariva, E.; Paglia, G.; et al. Metabolic Signature of Arrhythmogenic Cardiomyopathy. Metabolites 2021, 11, 195. https://doi.org/10.3390/metabo11040195
Volani C, Rainer J, Hernandes VV, Meraviglia V, Pramstaller PP, Smárason SV, Pompilio G, Casella M, Sommariva E, Paglia G, et al. Metabolic Signature of Arrhythmogenic Cardiomyopathy. Metabolites. 2021; 11(4):195. https://doi.org/10.3390/metabo11040195
Chicago/Turabian StyleVolani, Chiara, Johannes Rainer, Vinicius Veri Hernandes, Viviana Meraviglia, Peter Paul Pramstaller, Sigurður Vidir Smárason, Giulio Pompilio, Michela Casella, Elena Sommariva, Giuseppe Paglia, and et al. 2021. "Metabolic Signature of Arrhythmogenic Cardiomyopathy" Metabolites 11, no. 4: 195. https://doi.org/10.3390/metabo11040195
APA StyleVolani, C., Rainer, J., Hernandes, V. V., Meraviglia, V., Pramstaller, P. P., Smárason, S. V., Pompilio, G., Casella, M., Sommariva, E., Paglia, G., & Rossini, A. (2021). Metabolic Signature of Arrhythmogenic Cardiomyopathy. Metabolites, 11(4), 195. https://doi.org/10.3390/metabo11040195













