The Interaction of the Flavonoid Fisetin with Human Glutathione Transferase A1-1
Abstract
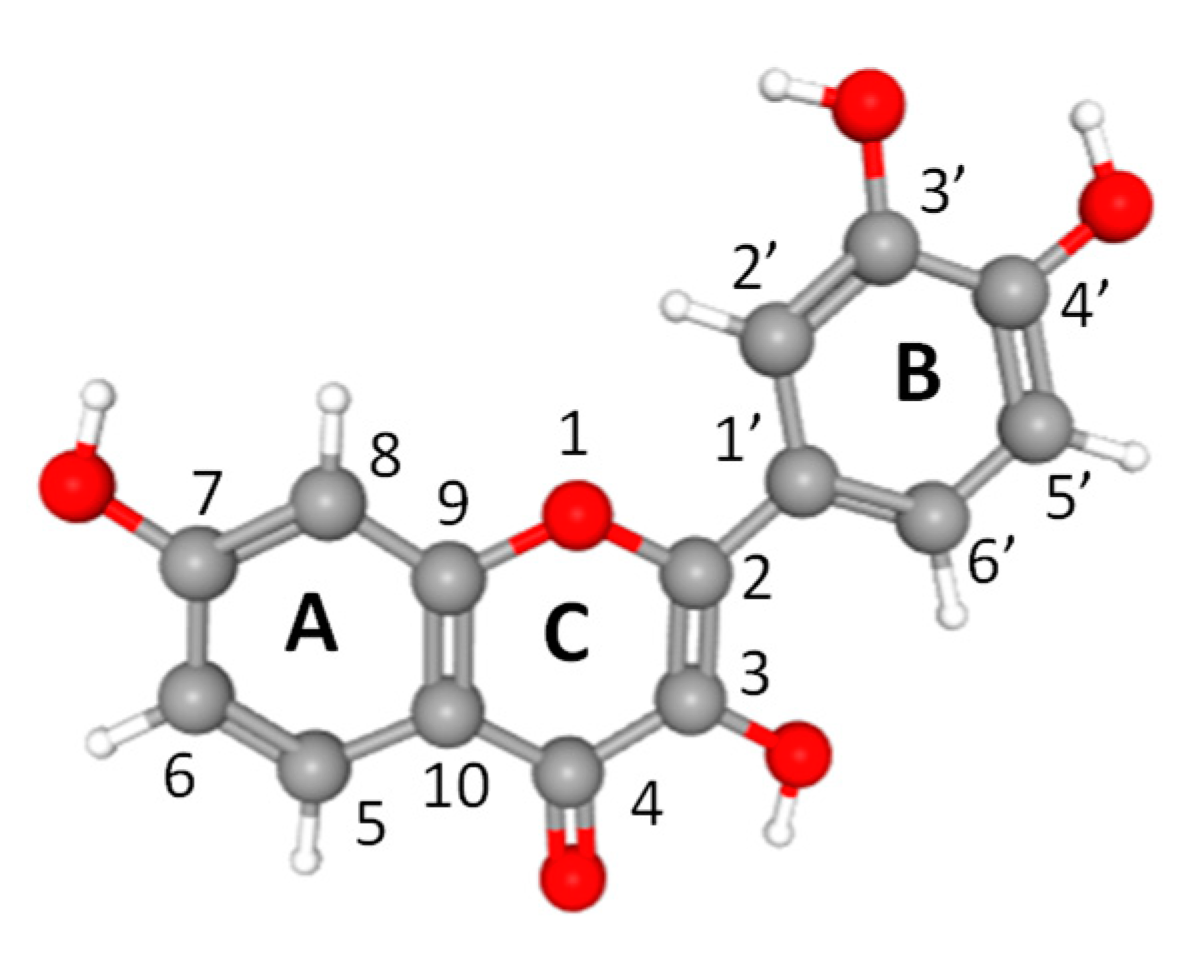
1. Introduction
2. Results and Discussion
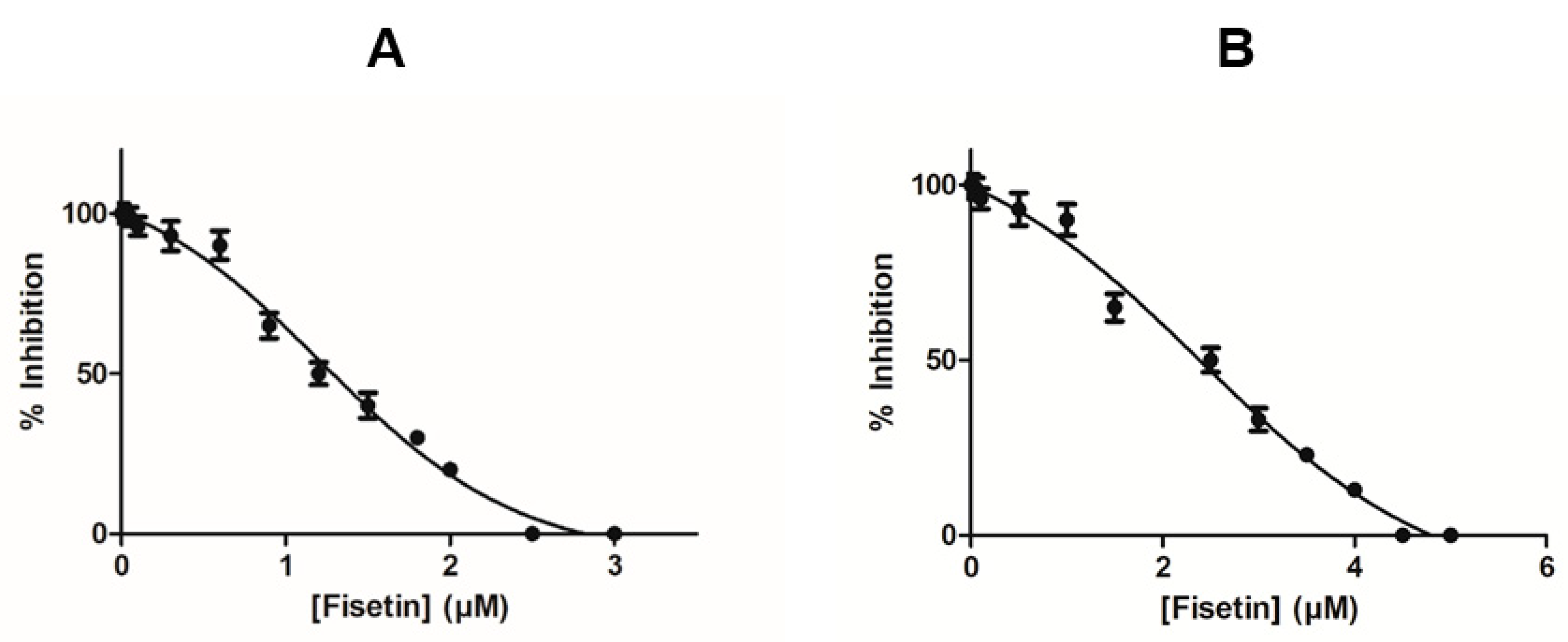
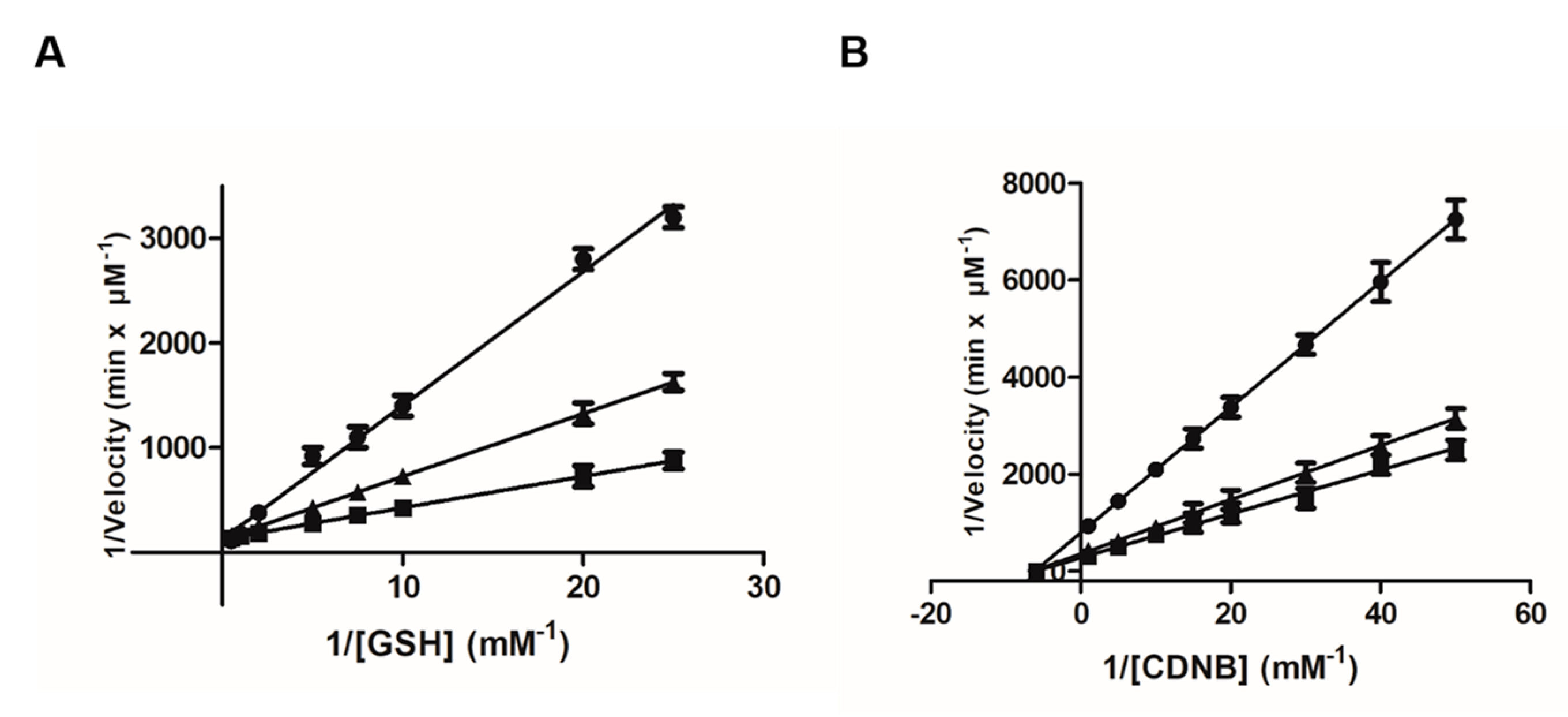
2.1. The Inhibition of Recombinant hGSTA1-1 by Fisetin
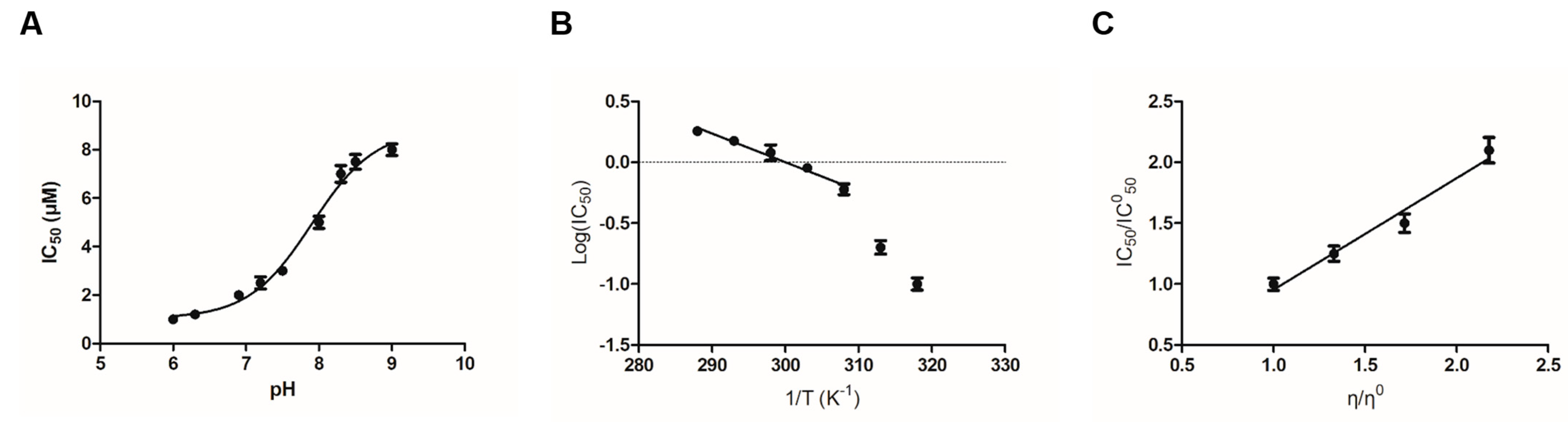
2.2. The Effect of pH, Temperature and Viscosity on IC50
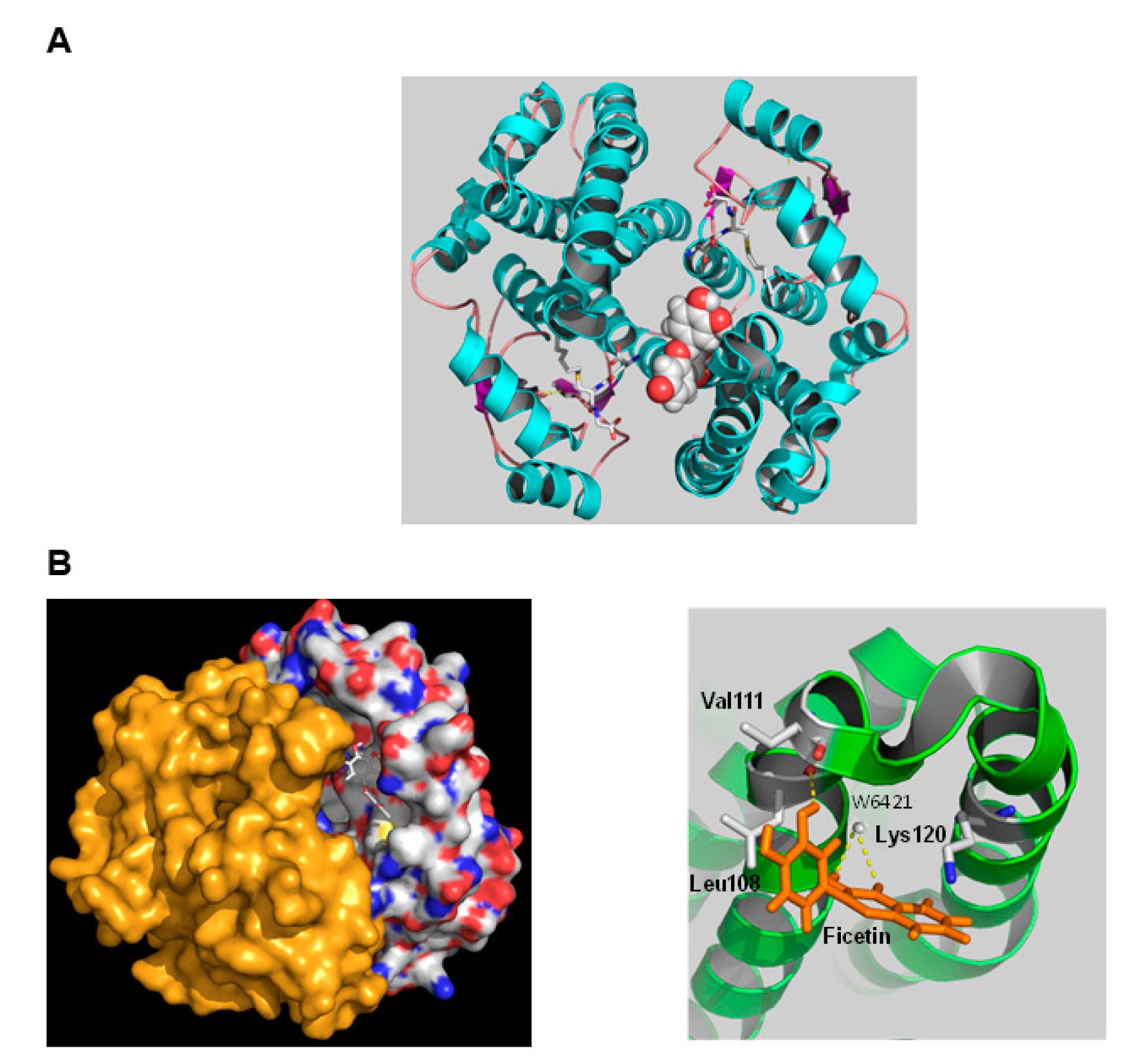
2.3. The Interaction of hGSTA1-1 and Fisetin by In Silico Molecular Docking
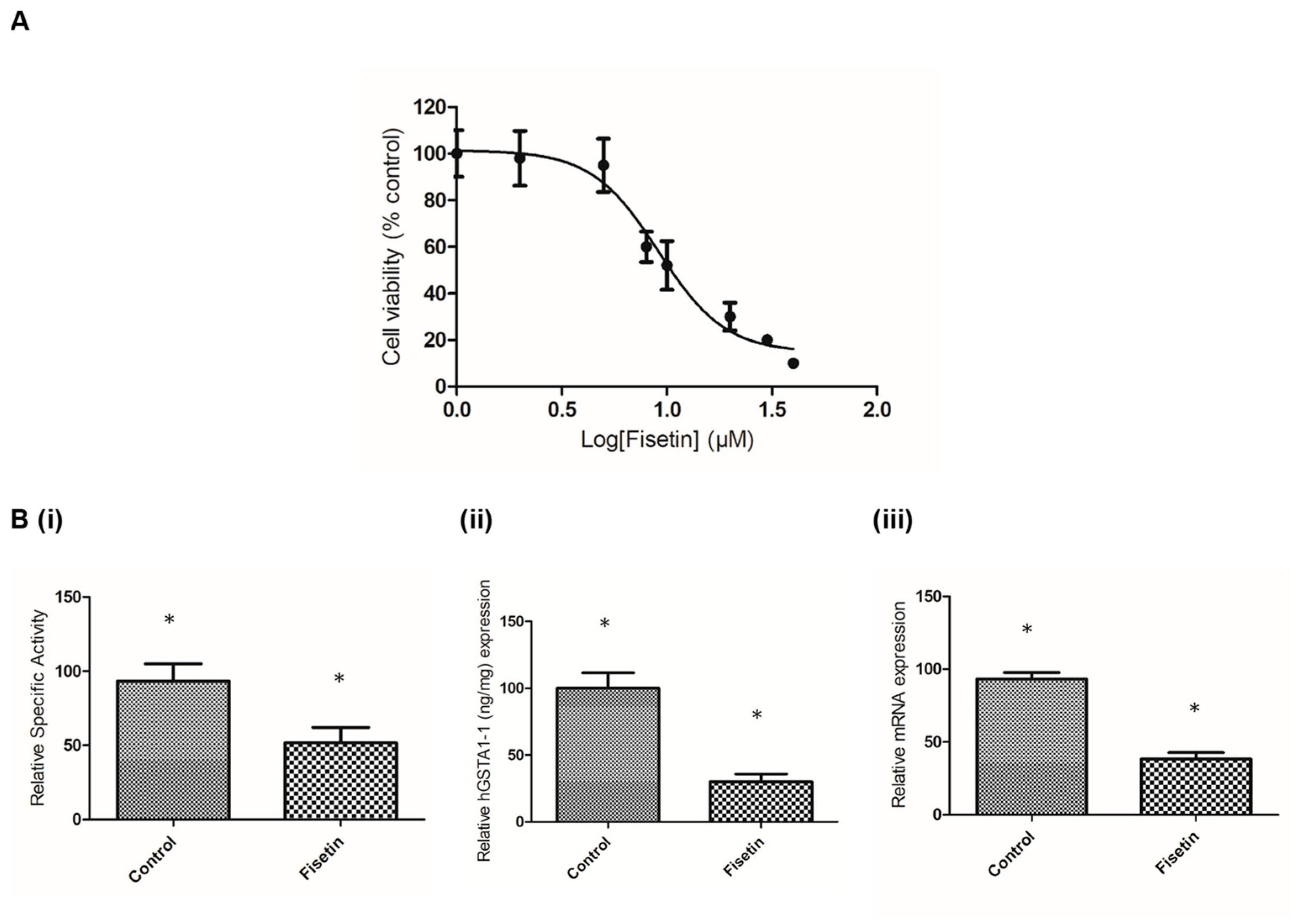
2.4. The Effect of Fisetin on hGSTA1-1 Expression and Inhibition in Caco-2 Cells
3. Materials and Methods
3.1. Materials
3.2. Heterologous Expression and Purification of Recombinant hGSTA1-1
3.3. Assay of GST Activity and Inhibition Analysis by Fisetin
3.4. Kinetic Measurements
3.5. The Dependence of IC50 on Viscosity, pH and Temperature
3.6. Molecular Modeling
3.7. Cultivation of the CaCo-2 Cell Line
3.8. Treatment of CaCo-2 Cells with Fisetin and GST Activity Measurements
3.9. RNA Extraction and RT-qPCR
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, A.; Gupta, S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018, 433, 33–42. [Google Scholar] [CrossRef]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef]
- Laborde, E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010, 17, 1373–1380. [Google Scholar] [CrossRef]
- Oakley, A. Glutathione transferases: A structural perspective. Drug Meta. Rev. 2011, 43, 138–151. [Google Scholar] [CrossRef]
- Wu, B.; Dong, D. Human cytosolic glutathione transferases: Structure, function, and drug discovery. Trends Pharmacol. Sci. 2012, 33, 656–668. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Pljesa-Ercegovac, M.; Savic-Radojevic, A.; Matic, M.; Coric, V.; Djukic, T.; Radic, T.; Simic, T. Glutathione Transferases: Potential Targets to Overcome Chemoresistance in Solid Tumors. Int. J. Mol. Sci. 2018, 19, 3785. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.-C.; Sha, H.-H.; Xu, X.-Y.; Hu, T.-M.; Lou, R.; Li, H.; Wu, J.-Z.; Dan, C.; Feng, J. Glutathione S-transferase π: A potential role in antitumor therapy. Drug. Des. Dev. Ther. 2018, 12, 3535–3547. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Arifoglu, P.; Ronai, Z.; Tew, K.D. Glutathione S-transferase P1-1 (GSTP1-1) inhibits c-Jun N-terminal kinase (JNK1) signaling through interaction with the C terminus. J. Biol. Chem. 2001, 276, 20999–21003. [Google Scholar] [CrossRef] [PubMed]
- Lawson, R.; Staatz, C.E.; Fraser, C.J.; Hennig, S. Review of the Pharmacokinetics and Pharmacodynamics of Intravenous Busulfan in Paediatric Patients. Clin. Pharmacokinet. 2020, (in press). [CrossRef]
- Lawson, R.; Staatz, C.E.; Fraser, C.J.; Hennig, S. Drug metabolizing enzymes and their inhibitors’ role in cancer resistance. Biomed. Pharmacother. 2018, 105, 53–65. [Google Scholar]
- Wang, K.; Zhang, F.-L.; Jia, W. Glutathione S-transferase ω 1 promotes the proliferation, migration and invasion, and inhibits the apoptosis of non small cell lung cancer cells, via the JAK/STAT3 signaling pathway. Mol. Med. Rep. 2021, 23, 71. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Liu, H.; Song, Z.; Chen, W. Phytochemical compounds targeting on Nrf2 for chemoprevention in colorectal cancer. Eur. J. Pharmacol. 2020, 887, 173588. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D. ; Glutathione-associated Enzymes in Anticancer Drug Resistance. Cancer. Res. 1994, 54, 4313–4320. [Google Scholar] [CrossRef]
- Lin, J.-H.; Tu, S.-H.; Chen, L.-C.; Huang, C.-C.; Chang, H.-L.; Cheng, T.-C.; Chang, H.-W.; Wu, C.-H.; Wu, H.-C.; Ho, Y.-S. Oestrogen receptor-regulated glutathione S-transferase mu 3 expression attenuates hydrogen peroxide-induced cytotoxicity, which confers tamoxifen resistance on breast cancer cells. Breast. Cancer. Res. Treat 2018, 172, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Patrick, B.; Li, J.; Sharma, R.; Jeyabal, P.V.; Reddy, P.M.; Awasthi, S.; Awasthi, Y.C. Glutathione S-transferases as antioxidant enzymes: Small cell lung cancer (H69) cells transfected with hGSTA1 resist doxorubicin-induced apoptosis. Arch. Biochem. Biophys. 2006, 452, 165–173. [Google Scholar] [CrossRef]
- Zou, M.; Hu, X.; Xu, B.; Tong, T.; Jing, Y.; Xi, L.; Zhou, W.; Lu, J.; Wang, X.; Yang, X.; et al. Glutathione S transferase isozyme alpha 1 is predominantly involved in the cisplatin resistance of common types of solid cancer. Oncol. Rep. 2019, 41, 989–998. [Google Scholar] [CrossRef]
- Zompra, A.N.; Georgakis, E.; Pappa, T.; Thireou, E.; Eliopoulos, N.; Labrou, C.P.; Clonis, Y. Glutathione analogues as substrates or inhibitors that discriminate between allozymes of the MDR-involved human glutathione transferase P1-1. Pept. Sci. 2016, 106, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Zoi, O.G.; Thireou, T.N.; Rinotas, V.E.; Tsoungas, P.G.; Eliopoulos, E.E.; Douni, E.K.; Labrou, N.E.; Clonis, Y.D. Designer xanthone: An inhibitor scaffold for MDR-involved human glutathione transferase isoenzyme A1-1. J. Biomol. Screen. 2013, 18, 1092–1102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, H.-B.; Jin, X.-L.; Zheng, J.-F.; Wang, F.; Dai, F.; Zhou, B. Developing piperlongumine-directed glutathione S-transferase inhibitors by an electrophilicity-based strategy. Eur. J. Med. Chem. 2017, 126, 517–525. [Google Scholar] [CrossRef]
- Georgakis, N.D.; Karagiannopoulos, D.A.; Thireou, T.N.; Eliopoulos, E.E.; Labrou, N.E.; Tsoungas, P.G.; Koutsilieris, M.N.; Clonis, Y.D. Concluding the trilogy: The interaction of 2,2’-dihydroxy-benzophenones and their carbonyl N-analogues with human glutathione transferase M1-1 face to face with the P1-1 and A1-1 isoenzymes involved in MDR. Chem Biol. Drug. Des. 2017, 90, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Premetis, G.; Marugas, P.; Fanos, G.; Vlachakis, D.; Chronopoulou, E.G.; Perperopoulou, F.; Dubey, K.K.; Shukla, P.; Foudah, A.I.; Muharram, M.M.; et al. The Interaction of the Microtubule Targeting Anticancer Drug Colchicine with Human Glutathione Transferases. Curr. Pharm. Des. 2020, 26, 5205–5212. [Google Scholar] [CrossRef] [PubMed]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current prospects of nutraceuticals: A review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Mulat, M.; Pandita, A.; Khan, F. Medicinal plant compounds for combating the multidrug resistant pathogenic bacteria: A review. Curr. Pharm. Biotechnol. 2019, 20, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Nabil-Adam, A.; Shreadah, M.A.; Abd El-Moneam, N.M.; El-Assar, S.A. Marine algae of the genus gracilaria as multi products source for different biotechnological and medical applications. Recent. Pat. Biotechnol. 2020, 14, 203–228. [Google Scholar] [CrossRef]
- Li, C.; Gao, L.; Zhang, Y.; Simpson, B.K. Preparation of quercetin loaded microparticles and their antitumor activity against human lung cancer cells (A549) in vitro. Curr. Pharm. Biotechnol. 2019, 20, 945–954. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Wu, Y.; Shen, X.; Xu, S.; Guo, Z.; Zhang, R.; Xing, D. The physiologic activity and mechanism of quercetin-like natural plant flavonoids. Curr. Pharm. Biotechnol. 2020, 21, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Syed, D.N.; Adhami, V.M.; Khan, N.; Khan, M.I.; Mukhtar, H. Exploring the molecular targets of dietary flavonoid fisetin in cancer. Semin. Cancer Biol. 2016, 40–41, 130–140. [Google Scholar] [CrossRef]
- Pouliou, F.M.; Thireou, T.N.; Eliopoulos, E.E.; Tsoungas, P.G.; Labrou, N.E.; Clonis, Y.D. Isoenzyme- and allozyme-specific inhibitors: 2,2’-dihydroxybenzophenones and their carbonyl N-analogues that discriminate between human glutathione transferase A1-1 and P1-1 allozymes. Chem. Biol. Drug. Des. 2015, 86, 1055–1063. [Google Scholar] [CrossRef]
- Koutsoumpli, G.E.; Dimaki, V.D.; Thireou, T.N.; Eliopoulos, E.E.; Labrou, N.E.; Varvounis, G.I.; Clonis, Y.D. Synthesis and study of 2-(pyrrolesulfonylmethyl)-N-arylimines: A new class of inhibitors for human glutathione transferase A1-1. J. Med. Chem. 2012, 55, 6802–6813. [Google Scholar] [CrossRef][Green Version]
- Atkins, W.M.; Wang, R.W.; Bird, A.W.; Newton, D.J.; Lu, A.Y.H. The catalytic mechanism of glutathione S-transferase (GST). J. Biol. Chem. 1993, 268, 19188–19191. [Google Scholar] [CrossRef]
- Allardyce, C.S.; McDonagh, P.D.; Lian, L.Y.; Wolf, C.R.; Roberts, G.C. The role of tyrosine-9 and the C-terminal helix in the catalytic mechanism of Alpha-class glutathione S-transferases. Biochem. J. 1999, 343, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Meza, J.M.; Sampedro, J.G. Trehalose Mediated Inhibition of Lactate Dehydrogenase from Rabbit Muscle. The Application of Kramers’ Theory in Enzyme Catalysis. J. Phys. Chem. B. 2018, 122, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Gadda, G.; Sobrado, P. Kinetic Solvent Viscosity Effects as Probes for Studying the Mechanisms of Enzyme Action. Biochemistry 2018, 57, 3445–3453. [Google Scholar] [CrossRef] [PubMed]
- Grosdidier, A.; Zoete, V.; Michielin, O. EADock: Docking of small molecules into protein active sites with a multiobjective evolutionary optimization. Proteins 2007, 67, 1010–1025. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Z.; Zang, L.; Wang, G.; Zhou, S.; Jin, G.; Yang, Z.; Pan, X. Downregulation of Glutathione S-transferase A1 suppressed tumor growth and induced cell apoptosis in A549 cell line. Oncol. Lett. 2018, 16, 467–474. [Google Scholar] [CrossRef]
- Lněničková, K.; Šadibolová, M.; Matoušková, P.; Szotáková, B.; Skálová, L.; Boušová, I. The Modulation of Phase II Drug-Metabolizing Enzymes in Proliferating and Differentiated CaCo-2 Cells by Hop-Derived Prenylflavonoids. Nutrients 2020, 12, 2138. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuzniak, V.; Paluszczak, J.; Baer-Dubowska, W. Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol. Vitr. 2013, 27, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Pichler, C.; Ferk, F.; Al-Serori, H.; Huber, W.; Jager, W.; Waldherr, M.; Misik, M.; Kundi, M.; Nersesyan, A.; Herbacek, I.; et al. Xanthohumol Prevents DNA Damage by Dietary Carcinogens: Results of a Human Intervention Trial. Cancer Prev. Res. 2017, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Dietz, B.M.; Hagos, G.K.; Eskra, J.N.; Wijewickrama, G.T.; Anderson, J.R.; Nikolic, D.; Guo, J.; Wright, B.; Chen, S.-N.; Pauli, G.F.; et al. Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (Humulus lupulus) in vitro and in vivo. Mol. Nutr. Food Res. 2013, 57, 1055–1066. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Sundarraj, K.; Raghunath, A.; Perumal, E. A review on the chemotherapeutic potential of fisetin: In vitro evidences. Biomed. Pharmacother. 2018, 97, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Grynkiewicz, G.; Demchuk, O.M. New Perspectives for Fisetin. Front Chem. 2019, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Axarli, I.; Labrou, N.E.; Petrou, C.; Rassias, N.; Cordopatis, P.; Clonis, Y.D. Sulphonamide-based bombesin prodrug analogues for glutathione transferase, useful in targeted cancer chemotherapy. Eur. J. Med. Chem. 2009, 44, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.V.; Brown, M.G.; Prentiss, P.G. Handbook of Chemistry and Physics; Weast, R.C., Astle, M.J., Beyer, W.H., Eds.; CRC Press: Boca Raton, FL, USA, 1985; pp. D-219–D-269. [Google Scholar]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqarni, M.H.; Foudah, A.I.; Muharram, M.M.; Labrou, N.E. The Interaction of the Flavonoid Fisetin with Human Glutathione Transferase A1-1. Metabolites 2021, 11, 190. https://doi.org/10.3390/metabo11030190
Alqarni MH, Foudah AI, Muharram MM, Labrou NE. The Interaction of the Flavonoid Fisetin with Human Glutathione Transferase A1-1. Metabolites. 2021; 11(3):190. https://doi.org/10.3390/metabo11030190
Chicago/Turabian StyleAlqarni, Mohammed Hamed, Ahmed Ibrahim Foudah, Magdy Mohamed Muharram, and Nikolaos E. Labrou. 2021. "The Interaction of the Flavonoid Fisetin with Human Glutathione Transferase A1-1" Metabolites 11, no. 3: 190. https://doi.org/10.3390/metabo11030190
APA StyleAlqarni, M. H., Foudah, A. I., Muharram, M. M., & Labrou, N. E. (2021). The Interaction of the Flavonoid Fisetin with Human Glutathione Transferase A1-1. Metabolites, 11(3), 190. https://doi.org/10.3390/metabo11030190






