Identification of Circulating Endocan-1 and Ether Phospholipids as Biomarkers for Complications in Thalassemia Patients
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Female vs. Male TDT Patients
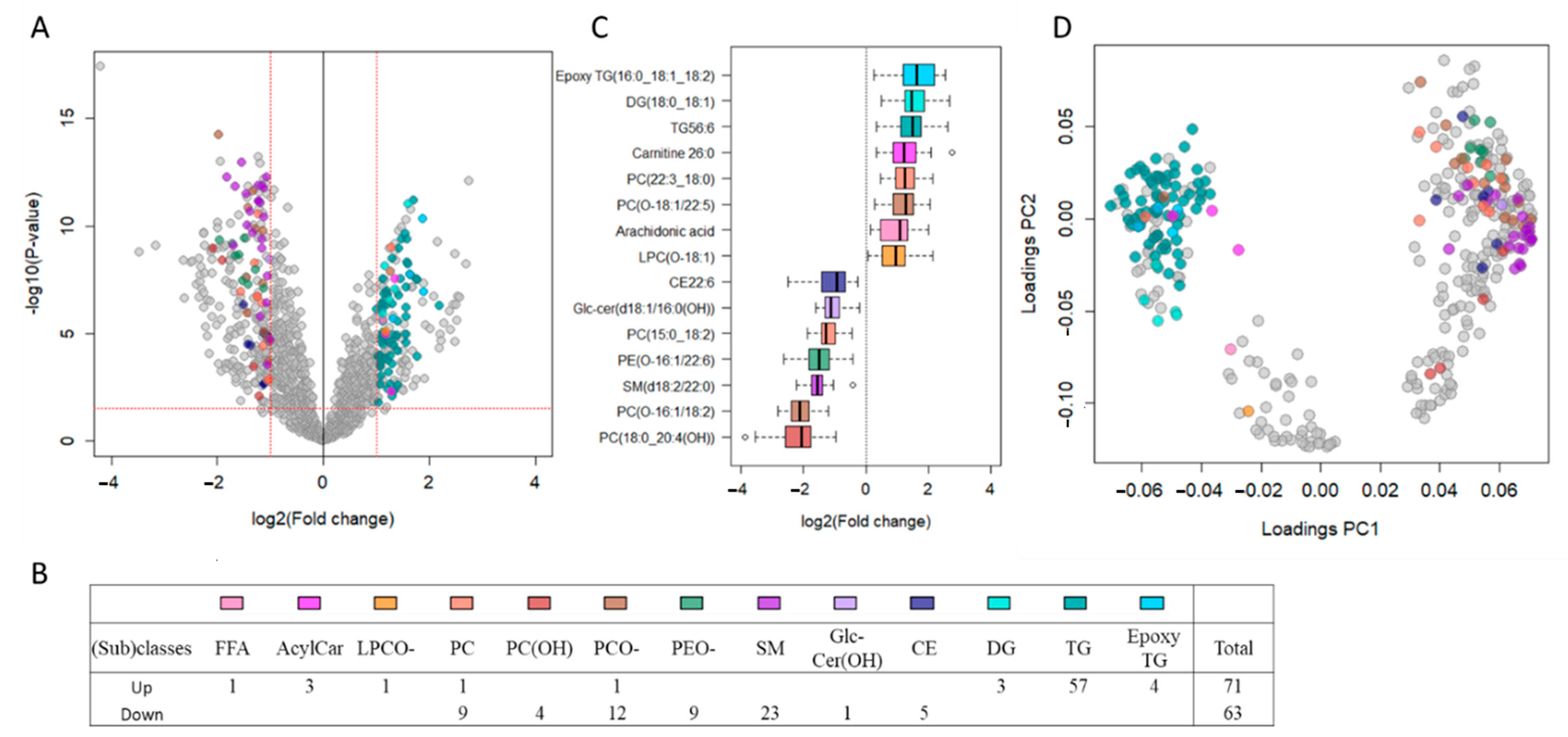
2.2. TDT Patients Show Major Circulating Lipidomic Profile Alterations Compared to Healthy Controls
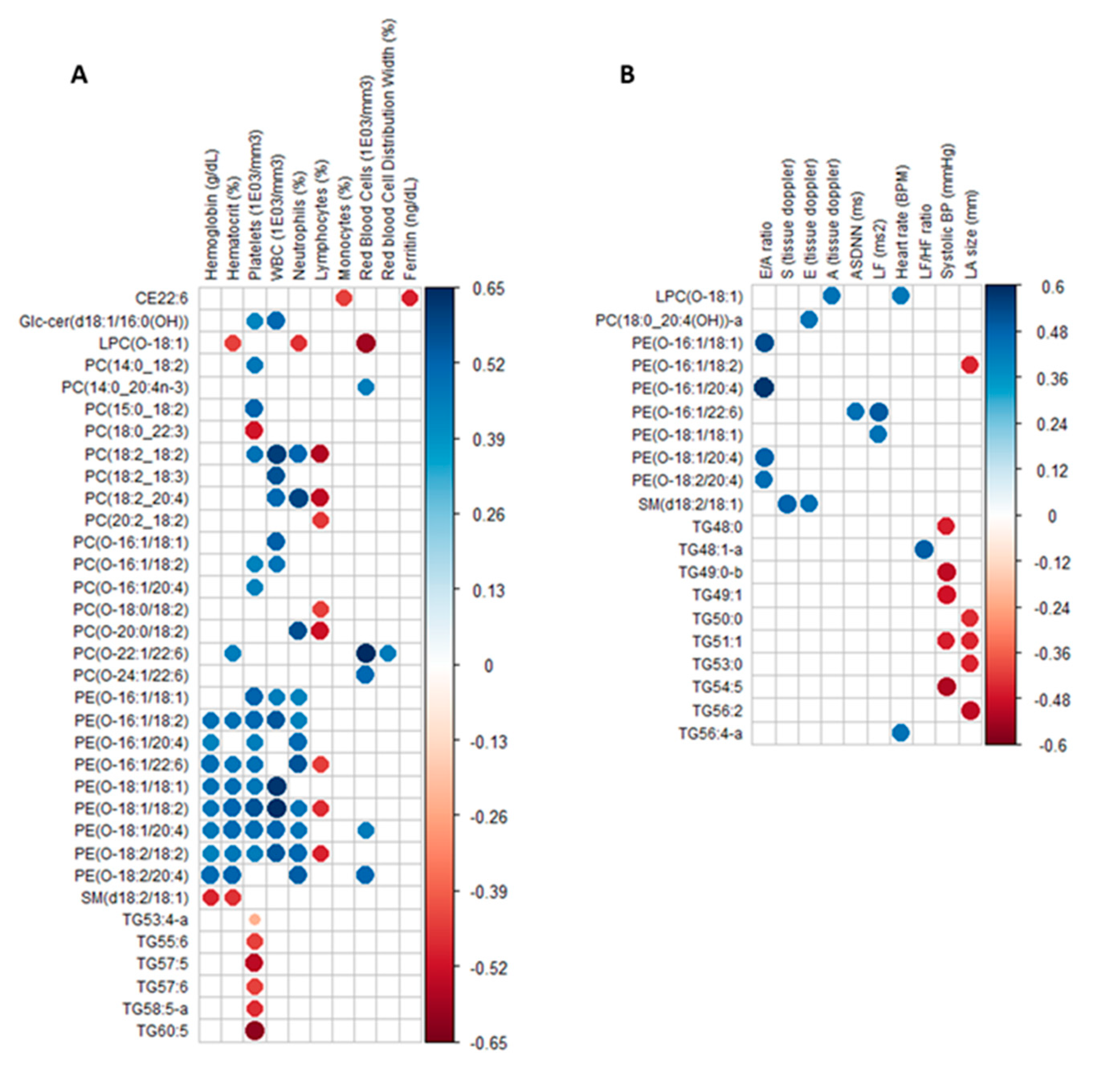
2.3. Circulating Lipids Discriminating Female TDT Patients from Controls Are Differentially Correlated to Clinical Parameters
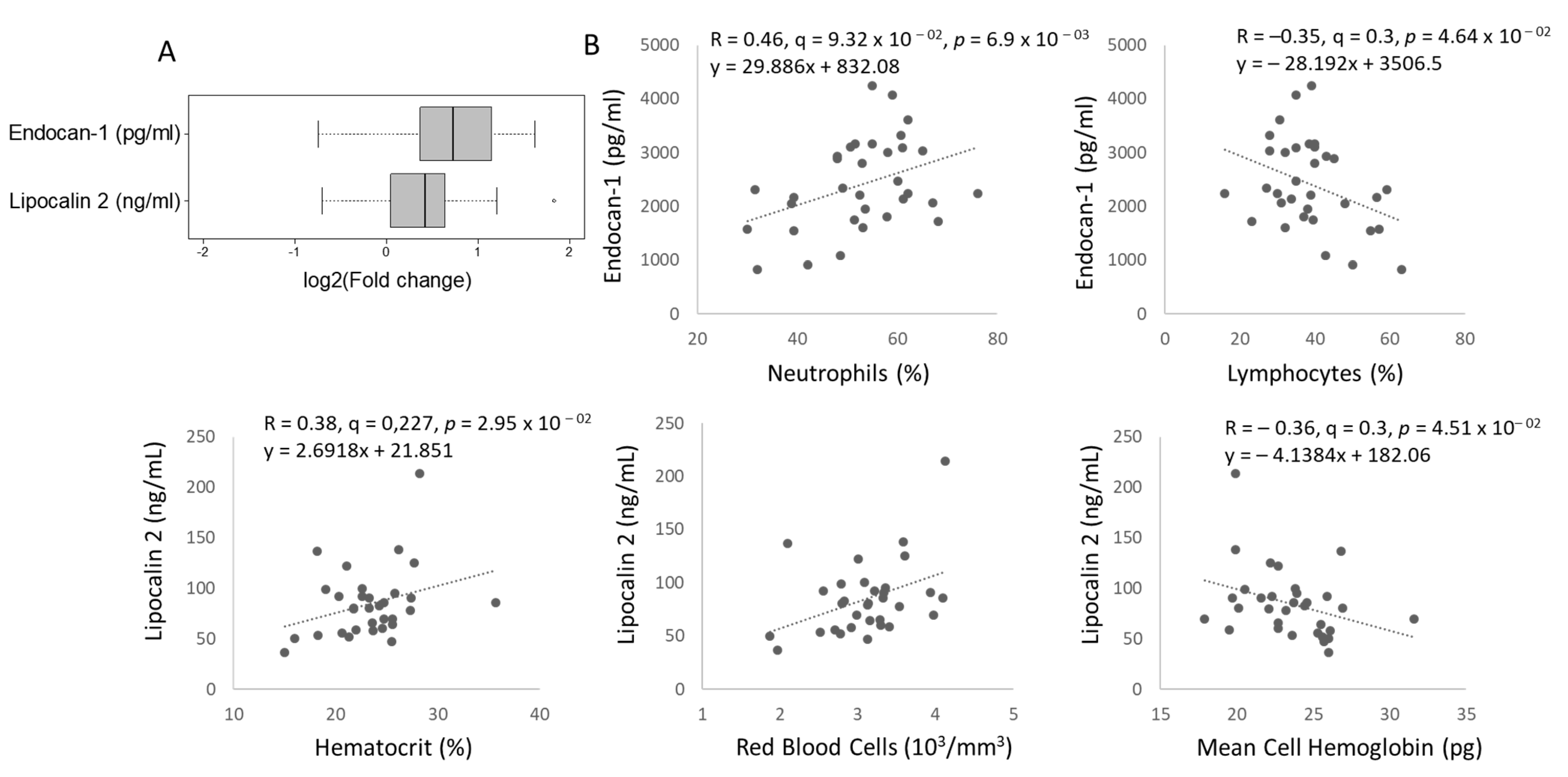
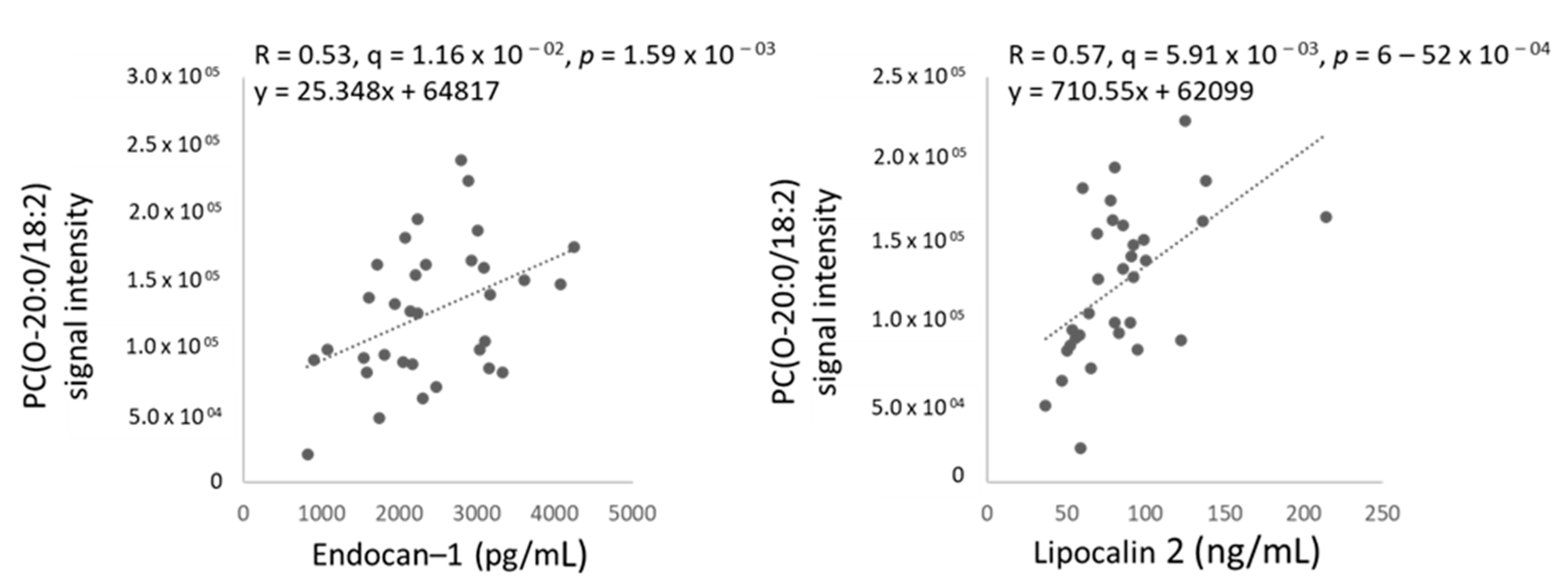
2.4. Two Circulating Protein Biomarkers Related to Cardiometabolic Disease Are Elevated in Female TDT Patients Versus Controls and Correlated with Blood Parameters and Circulating Ether PCs
3. Discussion
4. Materials and Methods
4.1. Human Cohort of TDT Patients and Control Healthy Subjects
4.2. Sample Processing and Untargeted Lipidomic Analysis by LC-MS
4.3. Biomarker and NTBI Analyses
4.4. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sankaran, V.G.; Orkin, S.H. The switch from fetal to adult hemoglobin. Cold Spring Harb. Perspect. Med. 2013, 3, a011643. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Thein, S.L. The emerging role of fetal hemoglobin induction in non-transfusion-dependent thalassemia. Blood Rev. 2012, 26, S35–S39. [Google Scholar] [CrossRef]
- Galanello, R.; Origa, R. Beta-thalassemia. Orphanet J. Rare Dis. 2010, 5, 11. [Google Scholar] [CrossRef]
- Al-Allawi, N.A.; Hassan, K.; Sheikha, A.K.; Nerweiy, F.F.; Dawood, R.S.; Jubrael, J. β-Thalassemia Mutations among Transfusion-Dependent Thalassemia Major Patients in Northern Iraq. Mol. Biol. Int. 2010, 2010, 4792820. [Google Scholar] [CrossRef]
- Taher, A.T.; Cappellini, M.D. How I manage medical complications of β-thalassemia in adults. Blood J. Am. Soc. Hematol. 2018, 132, 1781–1791. [Google Scholar] [CrossRef]
- Taher, A.T.; Saliba, A.N. Iron overload in thalassemia: Different organs at different rates. Hematology Am. Soc. Hematol. Educ. Program Book 2017, 2017, 265–271. [Google Scholar] [CrossRef]
- Borgna-Pignatti, C.; Rugolotto, S.; De Stefano, P.; Zhao, H.; Cappellini, M.D.; Del Vecchio, G.C.; Romeo, M.A.; Forni, G.L.; Gamberini, M.R.; Ghilardi, R. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004, 89, 1187–1193. [Google Scholar]
- Thompson, A.A.; Walters, M.C.; Kwiatkowski, J.; Rasko, J.E.; Ribeil, J.-A.; Hongeng, S.; Magrin, E.; Schiller, G.J.; Payen, E.; Semeraro, M. Gene therapy in patients with transfusion-dependent β-thalassemia. N. Engl. J. Med. 2018, 378, 1479–1493. [Google Scholar] [CrossRef]
- Berdoukas, V.; Coates, T.D.; Cabantchik, Z.I. Iron and oxidative stress in cardiomyopathy in thalassemia. Free. Radic. Biol. Med. 2015, 88, 3–9. [Google Scholar] [CrossRef]
- Kremastinos, D.; Toutouzas, P.; Vyssoulis, G.; Venetis, C.; Avgoustakis, D. Iron overload and left ventricular performance in beta thalassemia. Acta Cardiol. 1984, 39, 29–40. [Google Scholar] [PubMed]
- Cohen, A.R.; Glimm, E.; Porter, J.B. Effect of transfusional iron intake on response to chelation therapy in β-thalassemia major. Blood J. Am. Soc. Hematol. 2008, 111, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Roudkenar, M.H.; Halabian, R.; Oodi, A.; Roushandeh, A.M.; Yaghmai, P.; Najar, M.R.; Amirizadeh, N.; Shokrgozar, M.A. Upregulation of neutrophil gelatinase-associated lipocalin, NGAL/Lcn2, in β-thalassemia patients. Arch. Med. Res. 2008, 39, 402–407. [Google Scholar] [CrossRef]
- Pennell, D.J.; Udelson, J.E.; Arai, A.E.; Bozkurt, B.; Cohen, A.R.; Galanello, R.; Hoffman, T.M.; Kiernan, M.S.; Lerakis, S.; Piga, A. Cardiovascular function and treatment in β-thalassemia major: A consensus statement from the American Heart Association. Circulation 2013, 128, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Smoleńska, Ż.; Zdrojewski, Z. Metabolomics and its potential in diagnosis, prognosis and treatment of rheumatic diseases. Reumatologia 2015, 53, 152. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Siddiqui, A.J.; Ansari, S.H.; Musharraf, S.G. Reflection of treatment proficiency of hydroxyurea treated β-thalassemia serum samples through nuclear magnetic resonance based metabonomics. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Ansari, S.H.; Parveen, S.; Khan, I.A.; Siddiqui, A.J.; Musharraf, S.G. Hydroxyurea treated β-thalassemia children demonstrate a shift in metabolism towards healthy pattern. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Cooper, L.T., Jr.; Blauwet, L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Kitaoka, H.; Okawa, M.; Hirota, T.; Hayato, K.; Yamasaki, N.; Matsumura, Y.; Yabe, T.; Doi, Y.L. Gender-specific differences in the clinical features of hypertrophic cardiomyopathy in a community-based Japanese population: Results from Kochi RYOMA study. J. Cardiol. 2010, 56, 314–319. [Google Scholar] [CrossRef]
- Marsella, M.; Borgna-Pignatti, C.; Meloni, A.; Caldarelli, V.; Dell’Amico, M.C.; Spasiano, A.; Pitrolo, L.; Cracolici, E.; Valeri, G.; Positano, V. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: A T2* magnetic resonance imaging study. Haematologica 2011, 96, 515–520. [Google Scholar] [CrossRef]
- Marsella, M.; Pepe, A.; Borgna-Pignatti, C. Better survival and less cardiac morbidity in female patients with thalassemia major: A review of the literature. Ann. N. Y. Acad. Sci. 2010, 1202, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, A.; Savva, S.C.; Savvides, I.; Pangalou, E.; Ioannou, Y.S.; Christou, S.; Skordis, N. Gender differences in the prevalence and severity of bone disease in thalassaemia. Pediatric Endocrinol. Rev. PER 2008, 6, 116–122. [Google Scholar]
- Forest, A.; Ruiz, M.; Bouchard, B.; Boucher, G.; Gingras, O.; Daneault, C.; Robillard Frayne, I.; Rhainds, D.; Tardif, J.C.; Rioux, J.D.; et al. Comprehensive and Reproducible Untargeted Lipidomic Workflow Using LC-QTOF Validated for Human Plasma Analysis. J. Proteome Res. 2018, 17, 3657–3670. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Cuillerier, A.; Daneault, C.; Deschênes, S.; Frayne, I.R.; Bouchard, B.; Forest, A.; Legault, J.T.; Vaz, F.M.; Rioux, J.D. Lipidomics unveils lipid dyshomeostasis and low circulating plasmalogens as biomarkers in a monogenic mitochondrial disorder. JCI Insight 2019, 4, e123231. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Barlow, C.K.; Kingwell, B.A. Lipidomics: Potential role in risk prediction and therapeutic monitoring for diabetes and cardiovascular disease. Pharmacol. Ther. 2014, 143, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Y.; Vaziri, N.D.; Lin, R.-C. Lipidomics: New insight into kidney disease. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; Volume 68, pp. 153–175. [Google Scholar]
- Kohno, S.; Keenan, A.L.; Ntambi, J.M.; Miyazaki, M. Lipidomic insight into cardiovascular diseases. Biochem. Biophys. Res. Commun. 2018, 504, 590–595. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Waterham, H.R.; Ferdinandusse, S. Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum. Front. Cell Dev. Biol. 2016, 3. [Google Scholar] [CrossRef]
- Boonyawat, B.; Monsereenusorn, C.; Traivaree, C. Molecular analysis of beta-globin gene mutations among Thai beta-thalassemia children: Results from a single center study. Appl. Clin. Genet. 2014, 7, 253. [Google Scholar]
- Pirastru, M.; Mereu, P.; Nguyen, C.Q.; Nguyen, N.V.; Nguyen, T.D.; Manca, L. A Novel-72 (T→ A) β-Promoter Mutation Causing Slightly Elevated HbA2 in a Vietnamese Heterozygote. BioMed Res. Int. 2017, 2017, 4537409. [Google Scholar] [CrossRef]
- Hassan, T.; Zakaria, M.; Fathy, M.; Arafa, M.; El Gebaly, S.; Emam, A.; Wahab, A.A.; Shehab, M.; Salah, H.; Malek, M. Association between genotype and disease complications in Egyptian patients with beta thalassemia: A Cross-sectional study. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Afshinnia, F.; Rajendiran, T.M.; Wernisch, S.; Soni, T.; Jadoon, A.; Karnovsky, A.; Michailidis, G.; Pennathur, S. Lipidomics and biomarker discovery in kidney disease. Semin. Nephrol. 2018, 38, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.; Han, X. Unlocking the complexity of lipids: Using lipidomics to identify disease mechanisms, biomarkers and treatment efficacy. Future Lipidol. 2006, 1, 539–547. [Google Scholar] [CrossRef]
- Musharraf, S.G.; Iqbal, A.; Ansari, S.H.; Parveen, S.; Khan, I.A.; Siddiqui, A.J. Β-thalassemia patients revealed a significant change of untargeted metabolites in comparison to healthy individuals. Sci. Rep. 2017, 7, 42249. [Google Scholar] [CrossRef] [PubMed]
- Monni, G.; Murgia, F.; Corda, V.; Peddes, C.; Iuculano, A.; Tronci, L.; Balsamo, A.; Atzori, L. Metabolomic Investigation of β-Thalassemia in Chorionic Villi Samples. J. Clin. Med. 2019, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Sanghani, S.; Haldankar, V.; Shalia, K.; Bichlle, S. Comparative analysis of RBC membrane lipids in thalassemia, and iron deficiency anemia in relation to hypochromia and oxidant injury. Indian J. Clin. Biochem. 2001, 16, 116–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Honsho, M.; Fujiki, Y. Plasmalogen homeostasis—Regulation of plasmalogen biosynthesis and its physiological consequence in mammals. FEBS Lett. 2017, 591, 2720–2729. [Google Scholar] [CrossRef]
- Jenkins, C.M.; Yang, K.; Liu, G.; Moon, S.H.; Dilthey, B.G.; Gross, R.W. Cytochrome c is an oxidative stress–activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J. Biol. Chem. 2018, 293, 8693–8709. [Google Scholar] [CrossRef]
- Lal, A.; Gomez, E.; Calloway, C. Increased mitochondrial DNA deletions and copy number in transfusion-dependent thalassemia. JCI Insight 2016, 1, e88150. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.P.; Cheng, S.; Larson, M.G.; Walford, G.A.; Lewis, G.D.; McCabe, E.; Yang, E.; Farrell, L.; Fox, C.S.; O’Donnell, C.J.; et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J. Clin. Investig. 2011, 121, 1402–1411. [Google Scholar] [CrossRef]
- Görski, J.; Dobrzyn, A.; ZENDZIAN-PIOTROWSKA, M. The sphingomyelin-signaling pathway in skeletal muscles and its role in regulation of glucose uptake. Ann. N. Y. Acad. Sci. 2002, 967, 236–248. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Jayadev, S. The sphingomyelin cycle: The flip side of the lipid signaling paradigm. In Advances in Lipobiology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 2, pp. 143–166. [Google Scholar]
- Kalofoutis, A.; Stratakis, N.; Diskakis, E.; Koutselinis, A. Erythrocyte phospholipid fatty acid fluctuations in patients with β-thalassemia minor. Clin. Biochem. 1980, 13, 273–276. [Google Scholar] [CrossRef]
- Aslan, M.; Kıraç, E.; Kaya, S.; Özcan, F.; Salim, O.; Küpesiz, O.A. Decreased serum levels of sphingomyelins and ceramides in sickle cell disease patients. Lipids 2018, 53, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lancaster, G.I.; Meikle, P.J. Plasmalogens: A potential therapeutic target for neurodegenerative and cardiometabolic disease. Prog. Lipid Res. 2019, 74, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kimura, A.K.; Ren, M.; Monteiro, V.; Xu, Y.; Berno, B.; Schlame, M.; Epand, R.M. Plasmalogen loss caused by remodeling deficiency in mitochondria. Life Sci. Alliance 2019, 2. [Google Scholar] [CrossRef] [PubMed]
- Orešič, M.; Hyötyläinen, T.; Kotronen, A.; Gopalacharyulu, P.; Nygren, H.; Arola, J.; Castillo, S.; Mattila, I.; Hakkarainen, A.; Borra, R.J.; et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia 2013, 56, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Macias, R.I.R.; Muñoz-Bellvís, L.; Sánchez-Martín, A.; Arretxe, E.; Martínez-Arranz, I.; Lapitz, A.; Gutiérrez, M.L.; La Casta, A.; Alonso, C. A Novel Serum Metabolomic Profile for the Differential Diagnosis of Distal Cholangiocarcinoma and Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 1433. [Google Scholar] [CrossRef] [PubMed]
- Voskou, S.; Aslan, M.; Fanis, P.; Phylactides, M.; Kleanthous, M. Oxidative stress in β-thalassaemia and sickle cell disease. Redox Biol. 2015, 6, 226–239. [Google Scholar] [CrossRef]
- Walter, P.B.; Fung, E.B.; Killilea, D.W.; Jiang, Q.; Hudes, M.; Madden, J.; Porter, J.; Evans, P.; Vichinsky, E.; Harmatz, P. Oxidative stress and inflammation in iron-overloaded patients with β-thalassaemia or sickle cell disease. Br. J. Haematol. 2006, 135, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585, 603–608. [Google Scholar] [CrossRef]
- Rishi, G.; Bhatia, M.; Secondes, E.S.; Melino, M.; Crane, D.I.; Subramaniam, V.N. Hepatocyte-specific deletion of peroxisomal protein PEX13 results in disrupted iron homeostasis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165882. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, J.H.; Wang, Y.; Sweeney, G. Emerging clinical and experimental evidence for the role of lipocalin-2 in metabolic syndrome. Clin. Exp. Pharmacol. Physiol. 2012, 39, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Taube, A.; Schlich, R.; Sell, H.; Eckardt, K.; Eckel, J. Inflammation and metabolic dysfunction: Links to cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2148–H2165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Lee, J.S.; Kim, E.J.; Baik, S.H.; Seo, H.S.; Choi, D.S.; Oh, D.J.; Park, C.G. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur. J. Endocrinol. 2008, 158, 203–207. [Google Scholar] [CrossRef]
- Law, I.K.; Xu, A.; Lam, K.S.; Berger, T.; Mak, T.W.; Vanhoutte, P.M.; Liu, J.T.; Sweeney, G.; Zhou, M.; Yang, B.; et al. Lipocalin-2 deficiency attenuates insulin resistance associated with aging and obesity. Diabetes 2010, 59, 872–882. [Google Scholar] [CrossRef]
- Guo, H.; Jin, D.; Zhang, Y.; Wright, W.; Bazuine, M.; Brockman, D.A.; Bernlohr, D.A.; Chen, X. Lipocalin-2 deficiency impairs thermogenesis and potentiates diet-induced insulin resistance in mice. Diabetes 2010, 59, 1376–1385. [Google Scholar] [CrossRef]
- Yan, Q.W.; Yang, Q.; Mody, N.; Graham, T.E.; Hsu, C.H.; Xu, Z.; Houstis, N.E.; Kahn, B.B.; Rosen, E.D. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes 2007, 56, 2533–2540. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B. Lipocalins and insulin resistance: Etiological role of retinol-binding protein 4 and lipocalin-2? Clin. Chem. 2007, 53, 5–7. [Google Scholar] [CrossRef]
- Catalan, V.; Gomez-Ambrosi, J.; Rodriguez, A.; Ramirez, B.; Silva, C.; Rotellar, F.; Gil, M.J.; Cienfuegos, J.A.; Salvador, J.; Fruhbeck, G. Increased adipose tissue expression of lipocalin-2 in obesity is related to inflammation and matrix metalloproteinase-2 and metalloproteinase-9 activities in humans. J. Mol. Med. 2009, 87, 803–813. [Google Scholar] [CrossRef]
- Haase-Fielitz, A.; Haase, M.; Devarajan, P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: A critical evaluation of current status. Ann. Clin. Biochem. 2014, 51, 335–351. [Google Scholar] [CrossRef]
- Ito, M.; Doi, K.; Takahashi, M.; Koyama, K.; Myojo, M.; Hosoya, Y.; Kiyosue, A.; Ando, J.; Noiri, E.; Yahagi, N.; et al. Plasma neutrophil gelatinase-associated lipocalin predicts major adverse cardiovascular events after cardiac care unit discharge. J. Cardiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, H.; Fang, Q.; Jiang, S.; Zhang, L.; Zhang, J.; Hou, X.; Lu, J.; Bao, Y.; Xu, A.; et al. Elevated circulating lipocalin-2 levels independently predict incident cardiovascular events in men in a population-based cohort. Arter. Thromb. Vasc. Biol. 2014, 34, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Latouche, C.; El Moghrabi, S.; Messaoudi, S.; Nguyen Dinh Cat, A.; Hernandez-Diaz, I.; Alvarez de la Rosa, D.; Perret, C.; Lopez Andres, N.; Rossignol, P.; Zannad, F.; et al. Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension 2012, 59, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Hemdahl, A.L.; Gabrielsen, A.; Zhu, C.; Eriksson, P.; Hedin, U.; Kastrup, J.; Thoren, P.; Hansson, G.K. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arter. Thromb. Vasc. Biol. 2006, 26, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Patsaoura, A.; Tatsi, E.; Margeli, A.; Kanavaki, I.; Delaporta, P.; Kyriakopoulou, D.; Kouraklis-Symeonidis, A.; Kattamis, A.; Papassotiriou, I. Plasma neutrophil gelatinase-associated lipocalin levels are markedly increased in patients with non-transfusion-dependent thalassemia: Lack of association with markers of erythropoiesis, iron metabolism and renal function. Clin. Biochem. 2014, 47, 1060–1064. [Google Scholar] [CrossRef]
- Karaman, K.; Şahin, S.; Geylan, H.; Yaşar, A.Ş.; Çetin, M.; Kömüroğlu, A.U.; Öner, A.F. Evaluation of Renal Function Disorder With Urinary Neutrophil Gelatinase–associated Lipocalin Level in Patients With β-Thalassemia Major. J. Pediatric Hematol. Oncol. 2019, 41, 507–510. [Google Scholar] [CrossRef]
- Zhao, T.; Kecheng, Y.; Zhao, X.; Hu, X.; Zhu, J.; Wang, Y.; Ni, J. The higher serum endocan levels may be a risk factor for the onset of cardiovascular disease: A meta-analysis. Medicine 2018, 97, e13407. [Google Scholar] [CrossRef]
- Balta, S.; Mikhailidis, D.P.; Demirkol, S.; Ozturk, C.; Celik, T.; Iyisoy, A. Endocan: A novel inflammatory indicator in cardiovascular disease? Atherosclerosis 2015, 243, 339–343. [Google Scholar] [CrossRef]
- Poon, P.Y.-K.; Ng, J.K.-C.; Fung, W.W.-S.; Chow, K.-M.; Kwan, B.C.-H.; Li, P.K.-T.; Szeto, C.-C. Relationship between Plasma Endocan Level and Clinical Outcome of Chinese Peritoneal Dialysis Patients. Kidney Blood Press. Res. 2019, 44, 1259–1270. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, L.; Zeng, X.; Liao, J.; Ouyang, D. Ginsenoside compound K alleviates sodium valproate-induced hepatotoxicity in rats via antioxidant effect, regulation of peroxisome pathway and iron homeostasis. Toxicol. Appl. Pharmacol. 2020, 386, 114829. [Google Scholar] [CrossRef]
- Dubreuil, M.M.; Morgens, D.W.; Okumoto, K.; Honsho, M.; Contrepois, K.; Lee-McMullen, B.; Traber, G.M.; Sood, R.S.; Dixon, S.J.; Snyder, M.P. Systematic identification of regulators of oxidative stress reveals non-canonical roles for peroxisomal import and the pentose phosphate pathway. Cell Rep. 2020, 30, 1417–1433.e1417. [Google Scholar] [CrossRef] [PubMed]
- Pattanakuhar, S.; Phrommintikul, A.; Tantiworawit, A.; Konginn, S.; Srichairattanakool, S.; Chattipakorn, S.C.; Chattipakorn, N. Increased sympathovagal imbalance evaluated by heart rate variability is associated with decreased T2* MRI and left ventricular function in transfusion-dependent thalassemia patients. Biosci. Rep. 2018, 38, BSR20171266. [Google Scholar] [CrossRef] [PubMed]
- Godzien, J.; Ciborowski, M.; Martínez-Alcázar, M.a.P.; Samczuk, P.; Kretowski, A.; Barbas, C. Rapid and reliable identification of phospholipids for untargeted Metabolomics with LC–ESI–QTOF–MS/MS. J. Proteome Res. 2015, 14, 3204–3216. [Google Scholar] [CrossRef] [PubMed]
- Sumneang, N.; Kumfu, S.; Khamseekaew, J.; Siri-Angkul, N.; Fucharoen, S.; Chattipakorn, S.C.; Chattipakorn, N. Combined iron chelator with N-acetylcysteine exerts the greatest effect on improving cardiac calcium homeostasis in iron-overloaded thalassemic mice. Toxicology 2019, 427, 152289. [Google Scholar] [CrossRef] [PubMed]
| Male | Female | p–Value | |||
|---|---|---|---|---|---|
| Mean/Median | Deviation | Mean/Median | Deviation | ||
| Number | 28 | 33 | |||
| Age (years) | 27 | (18–45) | 27 | (19–49) | 0.6155 |
| Body weight (kg) | 49 | 7 | 46 | 7 | 0.1960 |
| Height (cm) | 160 | 8 | 154 | 7 | 0.0012 |
| BMI | 19 | 2 | 20 | 2 | 0.1667 |
| Blood Parameters | |||||
| Hemoglobin (g/dL) | 7.0 | 1.1 | 7.1 | 1.0 | 0.5875 |
| Hematocrit (%) | 23 | 4 | 23 | 4 | 0.9719 |
| Platelets (106/mm3) | 527 | 255 | 435 | 276 | 0.1951 |
| WBC (106/mm3) | 14 | (6–33) | 12 | (3–30) | 0.1459 |
| Neutrophils (%) | 46 | 12 | 53 | 11 | 0.0184 |
| Eosinophils (%) | 3.4 | (0.7–50) | 1.5 | (0–8) | 0.0392 |
| Basophils (%) | 1.7 | (0.2–8.4) | 0.60 | (0–4) | 0.0119 |
| Lymphocytes (%) | 38 | 16 | 39 | 11 | 0.8875 |
| Monocytes (%) | 7.6 | 3.9 | 5.6 | 2.1 | 0.0203 |
| Red Blood Cells (103/mm3) | 3.1 | 0.7 | 3.1 | 0.5 | 0.8987 |
| Mean Corpuscular Volume (μm3) | 79 | (57–91) | 74 | (27–87) | 0.3822 |
| Mean Cell Hemoglobin (pg) | 23 | 3 | 23 | 3 | 0.4947 |
| Mean Corpuscular Hemoglobin Concentration (g/dL) | 30 | (25–34) | 31 | (18–34) | 0.5327 |
| Red blood Cell Distribution Width (%) | 25 | 4 | 25 | 6 | 0.6800 |
| Mean Platelet Volume (μm3) | 8.1 | 1.4 | 8.2 | 1.4 | 0.8374 |
| Iron Status | |||||
| NTBI (μM) | 6.5 | 2.31 | 7.2 | 1.7 | 0.2277 |
| Ferritin (ng/dL) | 1375 | (434–6079) | 1499 | (274–7371) | 0.8686 |
| CMR T2* (ms) | 37 | 10 | 40 | 15 | 0.4430 |
| Heart Function | |||||
| Heart rate (BPM) | 91 | 14 | 96 | 12 | 0.1851 |
| Systolic BP (mmHg) | 111 | 13 | 109 | 11 | 0.4455 |
| Diastolic BP (mmHg) | 60 | (43–99) | 64 | (54–88) | 0.1572 |
| LV diastolic volume (ml) | 102 | 21 | 79 | 17 | <0.0001 |
| LA size (mm) | 3.8 | 0.39 | 3.4 | 0.45 | 0.0008 |
| LV EF (%) | 69 | 4.4 | 69 | 3.5 | 0.4861 |
| TRV max | 259 | (191–452) | 236 | (189–536) | 0.3482 |
| E/A ratio | 1.6 | (0.9–2.8) | 1.7 | (0.8–3.5) | 0.6379 |
| S (tissue doppler) | 9.2 | (6.9–11.7) | 8.9 | (5.2–11.1) | 0.0475 |
| E (tissue doppler) | 11 | 2.0 | 11 | 2.6 | 0.3499 |
| A (tissue doppler) | 8.3 | 2.1 | 7.7 | 2.0 | 0.2789 |
| SDNN (ms) | 100 | 27 | 94 | 29 | 0.4206 |
| SDANN (ms) | 92 | 27 | 88 | 30 | 0.6033 |
| ASDNN (ms) | 39 | 11 | 33 | 11 | 0.0628 |
| rMSSD (ms) | 21 | (9–46) | 17 | (9–43) | 0.1506 |
| LF (ms2) | 13 | (5–21) | 10.0 | (4–24) | 0.0808 |
| HF (ms2) | 8.5 | (2.7–18.6) | 6.3 | (2.1–17.2) | 0.2540 |
| LF/HF ratio | 1.6 | 0.46 | 1.5 | 0.32 | 0.2295 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botta, A.; Forest, A.; Daneault, C.; Pantopoulos, K.; Tantiworawit, A.; Phrommintikul, A.; Chattipakorn, S.; Chattipakorn, N.; Des Rosiers, C.; Sweeney, G. Identification of Circulating Endocan-1 and Ether Phospholipids as Biomarkers for Complications in Thalassemia Patients. Metabolites 2021, 11, 70. https://doi.org/10.3390/metabo11020070
Botta A, Forest A, Daneault C, Pantopoulos K, Tantiworawit A, Phrommintikul A, Chattipakorn S, Chattipakorn N, Des Rosiers C, Sweeney G. Identification of Circulating Endocan-1 and Ether Phospholipids as Biomarkers for Complications in Thalassemia Patients. Metabolites. 2021; 11(2):70. https://doi.org/10.3390/metabo11020070
Chicago/Turabian StyleBotta, Amy, Anik Forest, Caroline Daneault, Kostas Pantopoulos, Adisak Tantiworawit, Arintaya Phrommintikul, Siriporn Chattipakorn, Nipon Chattipakorn, Christine Des Rosiers, and Gary Sweeney. 2021. "Identification of Circulating Endocan-1 and Ether Phospholipids as Biomarkers for Complications in Thalassemia Patients" Metabolites 11, no. 2: 70. https://doi.org/10.3390/metabo11020070
APA StyleBotta, A., Forest, A., Daneault, C., Pantopoulos, K., Tantiworawit, A., Phrommintikul, A., Chattipakorn, S., Chattipakorn, N., Des Rosiers, C., & Sweeney, G. (2021). Identification of Circulating Endocan-1 and Ether Phospholipids as Biomarkers for Complications in Thalassemia Patients. Metabolites, 11(2), 70. https://doi.org/10.3390/metabo11020070






