Bariatric Surgery Improves the Atherogenic Profile of Circulating Methylarginines in Obese Patients: Results from a Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Obesity Increased the Serum Concentrations of Methylarginines
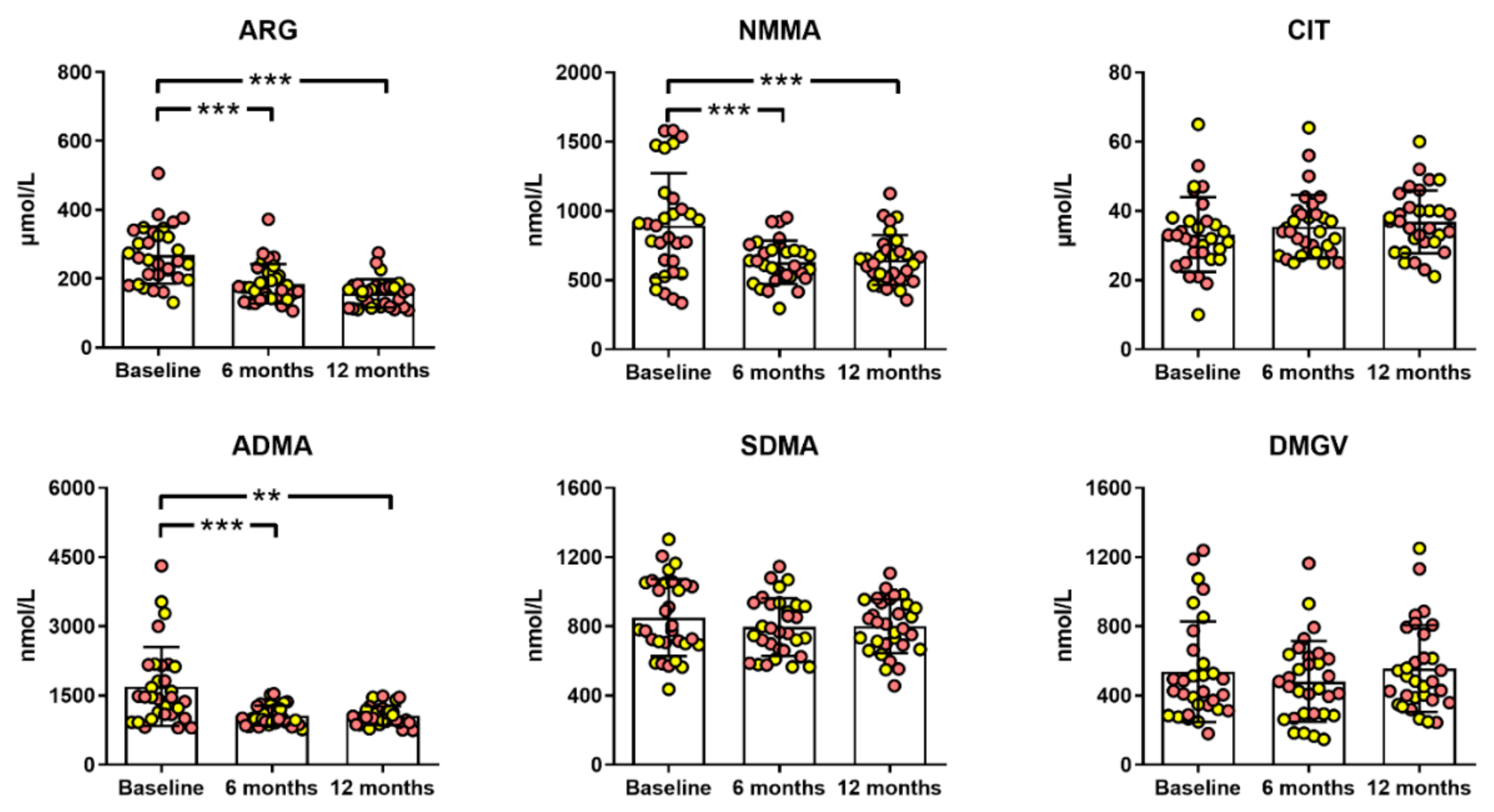
2.2. Bariatric Surgery Improved the Atherogenic Profile of Methylarginines
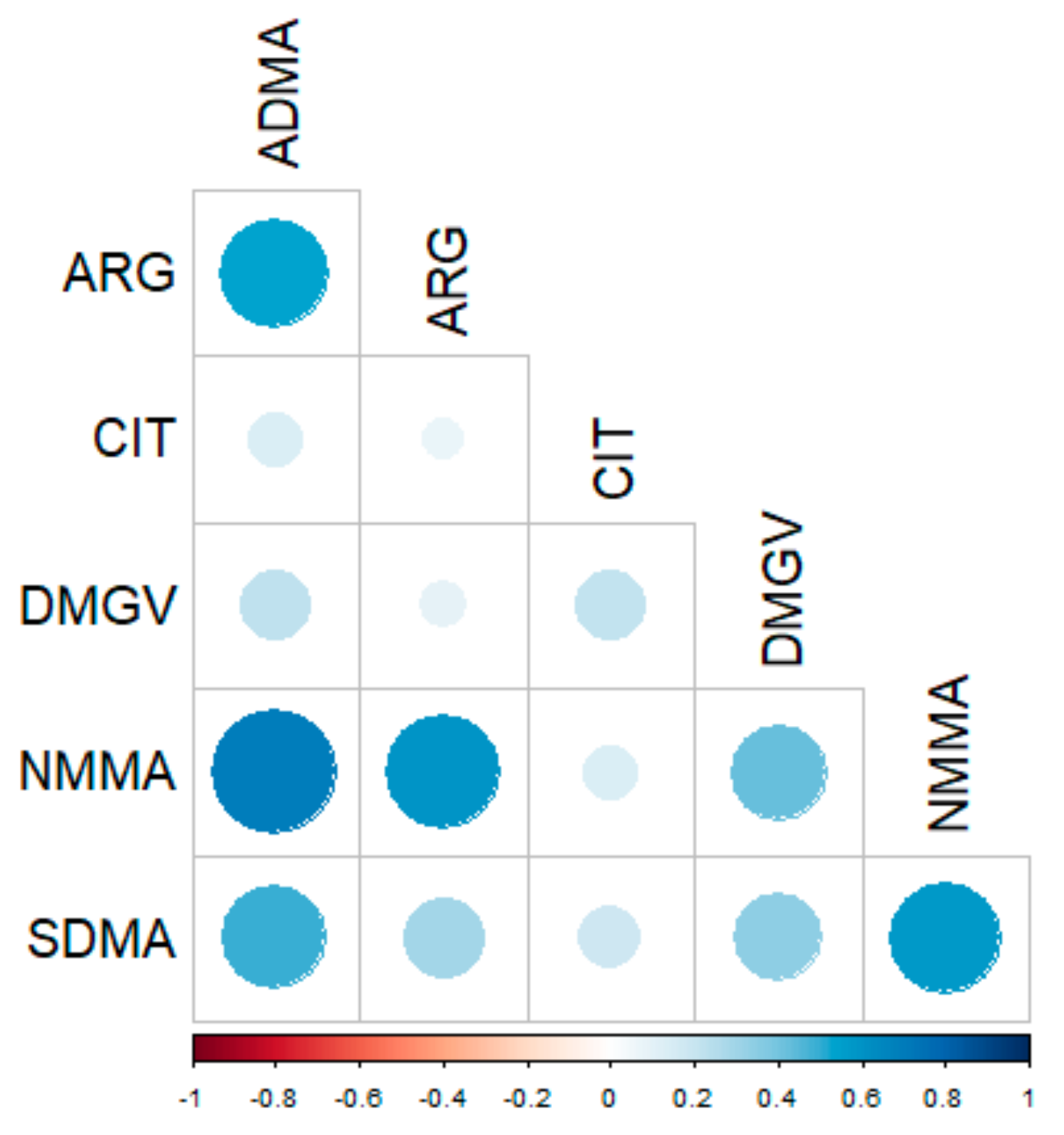
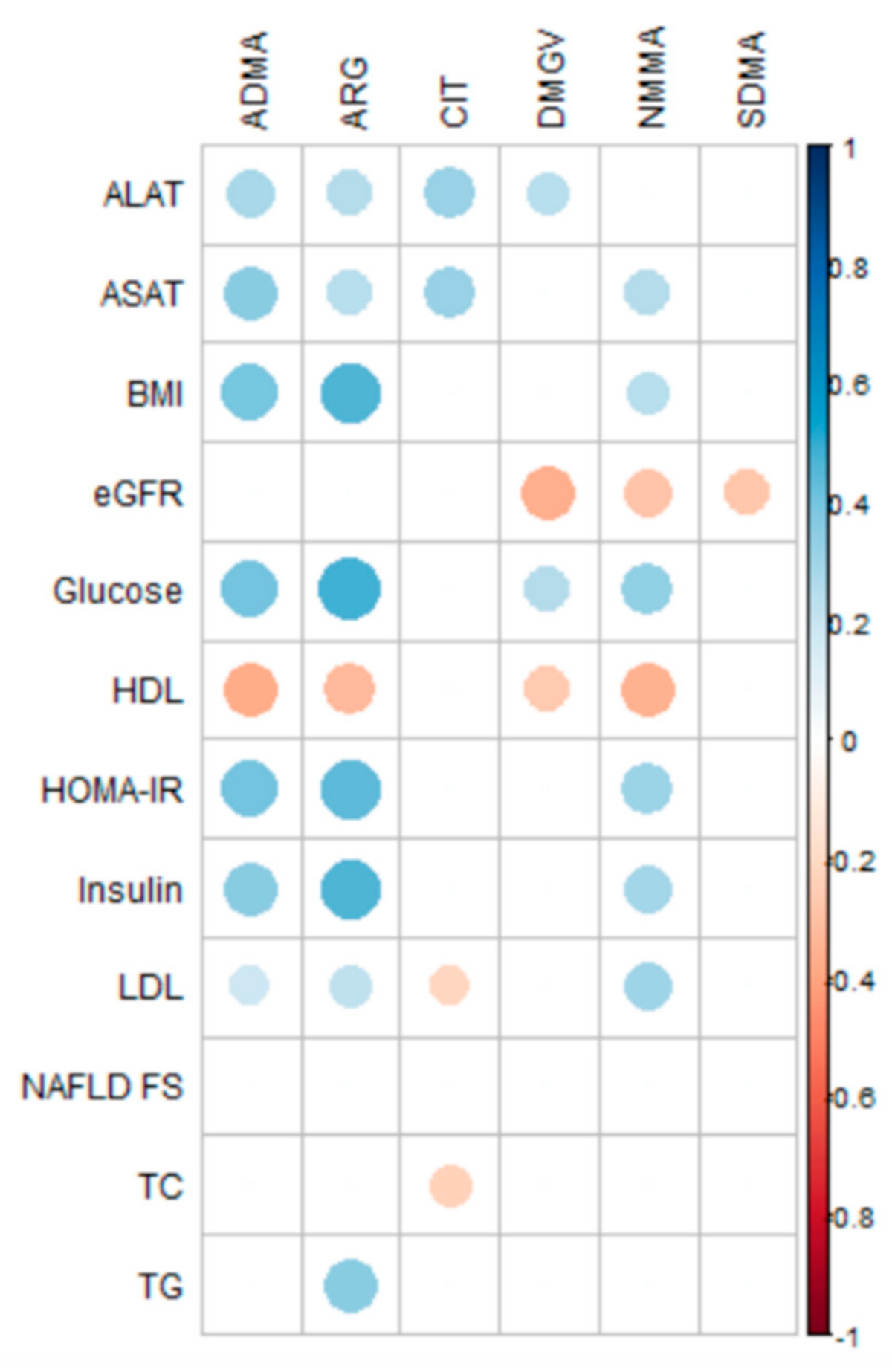
2.3. Correlations of Methylarginine Concentrations with Clinical Data
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Chemical and Reagents
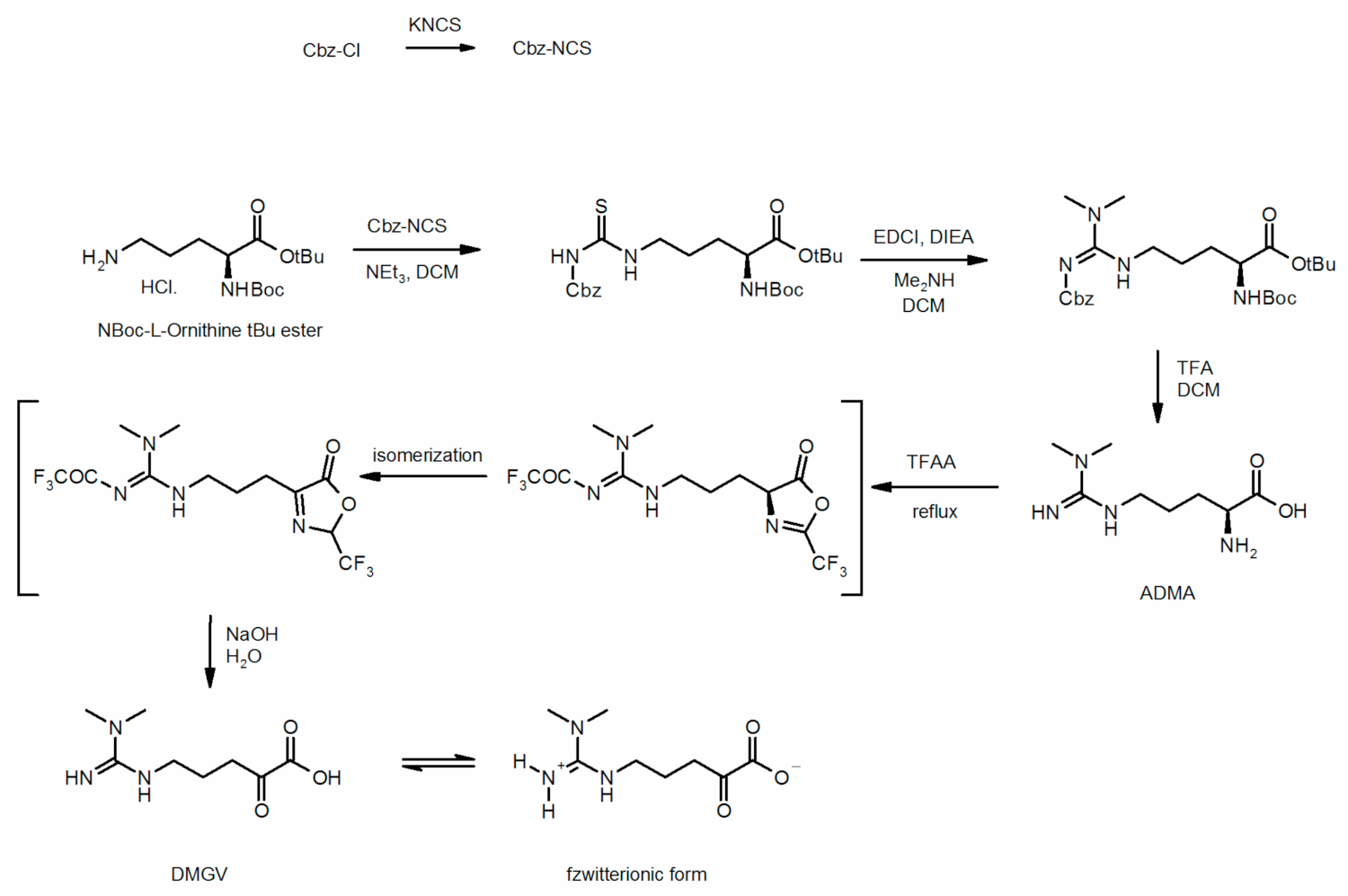
4.3. Chemical Synthesis of Dimethylguanidino Valerate
4.4. Biochemical Measurements
4.5. Quantification of Methylarginine Metabolites
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
References
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, D.B. Years of Life Lost Due to Obesity. JAMA 2003, 289, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The Diagnosis and Management of Non-Alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Tessari, R.; Zenari, L.; Day, C.; Arcaro, G. Prevalence of Nonalcoholic Fatty Liver Disease and Its Association with Cardiovascular Disease among Type 2 Diabetic Patients. Diabetes Care 2007, 30, 1212–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Zoppini, G.; Zenari, L.; Cigolini, M.; Falezza, G.; Arcaro, G. Relations between Carotid Artery Wall Thickness and Liver Histology in Subjects with Nonalcoholic Fatty Liver Disease. Diabetes Care 2006, 29, 1325–1330. [Google Scholar] [CrossRef] [Green Version]
- Blackett, P.R.; Sanghera, D.K. Genetic Determinants of Cardiometabolic Risk: A Proposed Model for Phenotype Association and Interaction. J. Clin. Lipidol. 2013, 7, 65–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinella, M.E. Nonalcoholic Fatty Liver Disease: A Systematic Review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.F.; Morningstar, J.E.; Yang, Q.; Zheng, B.; Gao, Y.; Jeanfavre, S.; Scott, J.; Fernandez, C.; Zheng, H.; O’Connor, S.; et al. Dimethylguanidino Valeric Acid Is a Marker of Liver Fat and Predicts Diabetes. J. Clin. Investig. 2017, 127, 4394–4402. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Oien, D.M. Metabolic/Bariatric Surgery Worldwide 2008. Obes. Surg. 2009, 19, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, H.; Buchwald, J.N. Metabolic (Bariatric and Nonbariatric) Surgery for Type 2 Diabetes: A Personal Perspective Review. Diabetes Care 2019, 42, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Netanel, C.; Goitein, D.; Rubin, M.; Kleinbaum, Y.; Katsherginsky, S.; Hermon, H.; Tsaraf, K.; Tachlytski, I.; Herman, A.; Safran, M.; et al. The Impact of Bariatric Surgery on Nonalcoholic Fatty Liver Disease as Measured Using Non-Invasive Tests. Am. J. Surg. 2021, 222, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lefere, S.; Onghena, L.; Vanlander, A.; van Nieuwenhove, Y.; Devisscher, L.; Geerts, A. Bariatric Surgery and the Liver-Mechanisms, Benefits, and Risks. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2021, 22, e13294. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric Surgery: A Systematic Review and Meta-Analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef]
- Di Gangi, I.M.; Chiandetti, L.; Gucciardi, A.; Moret, V.; Naturale, M.; Giordano, G. Simultaneous Quantitative Determination of N(G),N(G)-Dimethyl-L-Arginine or Asymmetric Dimethylarginine and Related Pathway’s Metabolites in Biological Fluids by Ultrahigh-Performance Liquid Chromatography/Electrospray Ionization-Tandem Mass Spectrometry. Anal. Chim. Acta 2010, 677, 140–148. [Google Scholar] [CrossRef]
- Amir, M.; Hassanein, S.I.; Abdel Rahman, M.F.; Gad, M.Z. AGXT2 and DDAH-1 Genetic Variants Are Highly Correlated with Serum ADMA and SDMA Levels and with Incidence of Coronary Artery Disease in Egyptians. Mol. Biol. Rep. 2018, 45, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Gluba-Brzózka, A.; Franczyk, B.; Jabłonowski, Z.; Ciałkowska-Rysz, A. Novel Biomarkers in the Diagnosis of Chronic Kidney Disease and the Prediction of Its Outcome. Int. J. Mol. Sci. 2017, 18, 1702. [Google Scholar] [CrossRef]
- Bulau, P.; Zakrzewicz, D.; Kitowska, K.; Leiper, J.; Gunther, A.; Grimminger, F.; Eickelberg, O. Analysis of Methylarginine Metabolism in the Cardiovascular System Identifies the Lung as a Major Source of ADMA. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 292, L18–L24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, J.R. Studies on the Catabolism of Ng-Methylarginine, Ng, Ng-Dimethylarginine and Ng, Ng-Dimethylarginine in the Rabbit. Biochem. J. 1976, 154, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawrys, J.; Gajecki, D.; Szahidewicz-Krupska, E.; Doroszko, A. Intraplatelet L-Arginine-Nitric Oxide Metabolic Pathway: From Discovery to Clinical Implications in Prevention and Treatment of Cardiovascular Disorders. Oxid. Med. Cell. Longev. 2020, 2020, 1015908. [Google Scholar] [CrossRef] [PubMed]
- Valkonen, V.-P.; Päivä, H.; Salonen, J.T.; Lakka, T.A.; Lehtimäki, T.; Laakso, J.; Laaksonen, R. Risk of Acute Coronary Events and Serum Concentration of Asymmetrical Dimethylarginine. Lancet 2001, 358, 2127–2128. [Google Scholar] [CrossRef]
- Gać, P.; Poręba, M.; Jurdziak, M.; Trzmielewska, E.; Gocławska, K.; Derkacz, A.; Mazur, G.; Szuba, A.; Poręba, R. Cardiovascular Risk Factors and the Concentration of Asymmetric Dimethylarginine. Adv. Clin. Exp. Med. 2020, 29, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Leong, T.; Zylberstein, D.; Graham, I.; Lissner, L.; Ward, D.; Fogarty, J.; Bengtsson, C.; Björkelund, C.; Thelle, D.; Swedish-Irish-Norwegian Collaboration. Asymmetric Dimethylarginine Independently Predicts Fatal and Nonfatal Myocardial Infarction and Stroke in Women: 24-Year Follow-up of the Population Study of Women in Gothenburg. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 961–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marliss, E.B.; Chevalier, S.; Gougeon, R.; Morais, J.A.; Lamarche, M.; Adegoke, O.A.J.; Wu, G. Elevations of Plasma Methylarginines in Obesity and Ageing Are Related to Insulin Sensitivity and Rates of Protein Turnover. Diabetologia 2006, 49, 351–359. [Google Scholar] [CrossRef] [Green Version]
- Rajapakse, N.W.; Giam, B.; Kuruppu, S.; Head, G.A.; Kaye, D.M. Impaired L-Arginine-Nitric Oxide Pathway Contributes to the Pathogenesis of Resistant Hypertension. Clin. Sci. Lond. Engl. 2019, 133, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Arlouskaya, Y.; Sawicka, A.; Głowala, M.; Giebułtowicz, J.; Korytowska, N.; Tałałaj, M.; Nowicka, G.; Wrzosek, M. Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) Concentrations in Patients with Obesity and the Risk of Obstructive Sleep Apnea (OSA). J. Clin. Med. 2019, 8, 897. [Google Scholar] [CrossRef] [Green Version]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W. Body-Mass Index and Mortality in a Prospective Cohort of U.S. Adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Jonsson, S.; Hedblad, B.; Engström, G.; Nilsson, P.; Berglund, G.; Janzon, L. Influence of Obesity on Cardiovascular Risk. Twenty-Three-Year Follow-up of 22,025 Men from an Urban Swedish Population. Int. J. Obes. 2002, 26, 1046–1053. [Google Scholar] [CrossRef] [Green Version]
- English, W.J.; Williams, D.B. Metabolic and Bariatric Surgery: An Effective Treatment Option for Obesity and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Adams, T.D.; Davidson, L.E.; Litwin, S.E.; Kim, J.; Kolotkin, R.L.; Nanjee, M.N.; Gutierrez, J.M.; Frogley, S.J.; Ibele, A.R.; Brinton, E.A.; et al. Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N. Engl. J. Med. 2017, 377, 1143–1155. [Google Scholar] [CrossRef]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Look AHEAD Research Group. Eight-Year Weight Losses with an Intensive Lifestyle Intervention: The Look AHEAD Study. Obesity 2014, 22, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric Surgery and Long-Term Cardiovascular Events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyzanowska, K.; Mittermayer, F.; Kopp, H.-P.; Wolzt, M.; Schernthaner, G. Weight Loss Reduces Circulating Asymmetrical Dimethylarginine Concentrations in Morbidly Obese Women. J. Clin. Endocrinol. Metab. 2004, 89, 6277–6281. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Kim, D.-I.; Jun, H.; Ma, Y.; Knights, A.J.; Park, M.-J.; Zhu, K.; Lipinski, J.H.; Liao, J.; Li, Y.; et al. Protein Arginine Methyltransferase 1 Interacts With PGC1α and Modulates Thermogenic Fat Activation. Endocrinology 2019, 160, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Jarzebska, N.; Mangoni, A.A.; Martens-Lobenhoffer, J.; Bode-Böger, S.M.; Rodionov, R.N. The Second Life of Methylarginines as Cardiovascular Targets. Int. J. Mol. Sci. 2019, 20, 4592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, F.; Maas, R.; Freese, R.; Schwedhelm, E.; Silberhorn, E.; Böger, R.H. Determination of a Reference Value for N(G), N(G)-Dimethyl-L-Arginine in 500 Subjects. Eur. J. Clin. Investig. 2005, 35, 622–626. [Google Scholar] [CrossRef]
- Martens-Lobenhoffer, J.; Bode-Böger, S.M. Fast and Efficient Determination of Arginine, Symmetric Dimethylarginine, and Asymmetric Dimethylarginine in Biological Fluids by Hydrophilic-Interaction Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Clin. Chem. 2006, 52, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Atzler, D.; Schwedhelm, E.; Nauck, M.; Ittermann, T.; Böger, R.H.; Friedrich, N. Serum Reference Intervals of Homoarginine, ADMA, and SDMA in the Study of Health in Pomerania. Clin. Chem. Lab. Med. 2014, 52, 1835–1842. [Google Scholar] [CrossRef]
- Short, K.R.; Chadwick, J.Q.; Teague, A.M.; Tullier, M.A.; Wolbert, L.; Coleman, C.; Copeland, K.C. Effect of Obesity and Exercise Training on Plasma Amino Acids and Amino Metabolites in American Indian Adolescents. J. Clin. Endocrinol. Metab. 2019, 104, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Wali, J.A.; Koay, Y.C.; Chami, J.; Wood, C.; Corcilius, L.; Payne, R.J.; Rodionov, R.N.; Birkenfeld, A.L.; Samocha-Bonet, D.; Simpson, S.J.; et al. Nutritional and Metabolic Regulation of the Metabolite Dimethylguanidino Valeric Acid: An Early Marker of Cardiometabolic Disease. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E509–E518. [Google Scholar] [CrossRef]
- Kittel, A.; Maas, R.; König, J.; Mieth, M.; Weiss, N.; Jarzebska, N.; Hohenstein, B.; Martens-Lobenhoffer, J.; Bode-Böger, S.M.; Rodionov, R.N. In Vivo Evidence That Agxt2 Can Regulate Plasma Levels of Dimethylarginines in Mice. Biochem. Biophys. Res. Commun. 2013, 430, 84–89. [Google Scholar] [CrossRef]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric Acid Induces Browning of White Fat and Hepatic β-Oxidation and Is Inversely Correlated with Cardiometabolic Risk Factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begriche, K.; Massart, J.; Abbey-Toby, A.; Igoudjil, A.; Lettéron, P.; Fromenty, B. Beta-Aminoisobutyric Acid Prevents Diet-Induced Obesity in Mice with Partial Leptin Deficiency. Obesity 2008, 16, 2053–2067. [Google Scholar] [CrossRef]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost All about Citrulline in Mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qiu, L.; Xiao, Q.; Wang, Y.; Meng, X.; Xu, R.; Wang, S.; Na, R. Obesity and Diabetes Related Plasma Amino Acid Alterations. Clin. Biochem. 2013, 46, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Verdam, F.J.; Greve, J.W.M.; Roosta, S.; van Eijk, H.; Bouvy, N.; Buurman, W.A.; Rensen, S.S. Small Intestinal Alterations in Severely Obese Hyperglycemic Subjects. J. Clin. Endocrinol. Metab. 2011, 96, E379–E383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottosson, F.; Ericson, U.; Almgren, P.; Smith, E.; Brunkwall, L.; Hellstrand, S.; Nilsson, P.M.; Orho-Melander, M.; Fernandez, C.; Melander, O. Dimethylguanidino Valerate: A Lifestyle-Related Metabolite Associated With Future Coronary Artery Disease and Cardiovascular Mortality. J. Am. Heart Assoc. 2019, 8, e012846. [Google Scholar] [CrossRef]
- Robbins, J.M.; Herzig, M.; Morningstar, J.; Sarzynski, M.A.; Cruz, D.E.; Wang, T.J.; Gao, Y.; Wilson, J.G.; Bouchard, C.; Rankinen, T.; et al. Association of Dimethylguanidino Valeric Acid With Partial Resistance to Metabolic Health Benefits of Regular Exercise. JAMA Cardiol. 2019, 4, 636–643. [Google Scholar] [CrossRef]
- Mattar, S.G.; Velcu, L.M.; Rabinovitz, M.; Demetris, A.J.; Krasinskas, A.M.; Barinas-Mitchell, E.; Eid, G.M.; Ramanathan, R.; Taylor, D.S.; Schauer, P.R. Surgically-Induced Weight Loss Significantly Improves Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome. Ann. Surg. 2005, 242, 610–617; discussion 618–620. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.B.; Suzuki, T.; Hooker, J.C.; Covarrubias, Y.; Schlein, A.; Liu, S.; Schwimmer, J.B.; Reeder, S.B.; Funk, L.M.; Greenberg, J.A.; et al. How Bariatric Surgery Affects Liver Volume and Fat Density in NAFLD Patients. Surg. Endosc. 2018, 32, 1675–1682. [Google Scholar] [CrossRef]
- Li, Q.; Dhyani, M.; Grajo, J.R.; Sirlin, C.; Samir, A.E. Current Status of Imaging in Nonalcoholic Fatty Liver Disease. World J. Hepatol. 2018, 10, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Feng, R.; Zhao, C.; Wang, Y.; Wang, J.; Liu, S.; Cao, J.; Wang, H.; Wang, T.; Guo, Y.; et al. Dimethylarginine Dimethylaminohydrolase 1 Protects Against High-Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Antioxid. Redox Signal. 2017, 26, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Haute Autorité de Santé—Obésité: Prise En Charge Chirurgicale Chez L’adulte. Available online: https://www.has-sante.fr/jcms/c_765529/fr/obesite-prise-en-charge-chirurgicale-chez-l-adulte (accessed on 15 October 2019).
- Association, A.D. Standards of Medical Care in Diabetes—2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar] [CrossRef] [Green Version]
- Linton, B.R.; Carr, A.J.; Orner, B.P.; Hamilton, A.D. A Versatile One-Pot Synthesis of 1,3-Substituted Guanidines from Carbamoyl Isothiocyanates. J. Org. Chem. 2000, 65, 1566–1568. [Google Scholar] [CrossRef] [PubMed]
- Schade, D.; Kotthaus, J.; Clement, B. Efficient Synthesis of Optically Pure Nω-Alkylated l-Arginines. Synthesis 2008, 2008, 2391–2397. [Google Scholar]
- Klein, C.; Schulz, G.; Steglich, W. Umwandlung von ω-Guanidino- und ω-Ureido-α-aminosäuren in α-Ketosäuren und deren heterocyclische Folgeprodukte. Liebigs Ann. Chem. 1983, 1983, 1623–1637. [Google Scholar] [CrossRef]
- Treeprasertsuk, S.; Björnsson, E.; Enders, F.; Suwanwalaikorn, S.; Lindor, K.D. NAFLD Fibrosis Score: A Prognostic Predictor for Mortality and Liver Complications among NAFLD Patients. World J. Gastroenterol. 2013, 19, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable Selection—A Review and Recommendations for the Practicing Statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Healthy Controls | Obese Patients at Baseline | p-Value 1 | |
|---|---|---|---|
| n | 31 | 31 | - |
| Male/Female | 9/22 | 9/22 | 0.713 |
| Age, years | 40 ± 10 | 41 ± 9 | 0.652 |
| BMI, kg/m2 | 22 ± 3 | 45 ± 6 | 0.003 |
| TC, mg/dL | 187 ± 31 | 189 ± 31 | 0.888 |
| TG, mg/dL | 81 [45–176] | 108 [59–625] | 0.003 |
| HDL-C, mg/dL | 73 [50–90] | 0.003 | |
| LDL-C, mg/dL | 95 ± 34 | 120 ± 21 | 0.003 |
| Glucose, mg/dL | 77 [58–103] | 93 [72–341] | 0.007 |
| Insulin, mIU/L | 15 [3–48] | 19 [6–55] | 0.031 |
| HOMA-IR | 2.9 [0.7–12.1] | 4.9 [2.9–23.9] | 0.006 |
| ASAT, IU/L | 14 [3–44] | 19 [11–64] | 0.027 |
| ALAT, IU/L | 22 [13–98] | 28 [5–114] | 0.005 |
| eGFR, mL/min/1.73 m2 | Not available | 99 ± 19 | - |
| NAFLD FS | Not available | −0.662 ± 1.259 | - |
| 6 Months | 12 Months | |||
|---|---|---|---|---|
| Parameter | % of Change | p-Value 1 | % of Change | p-Value 1 |
| BMI | −23.8 | 0.002 | −28.7 | 0.002 |
| TC | −9.6 | 0.002 | −11.4 | 0.002 |
| TG | −26.4 | 0.004 | −36.2 | 0.004 |
| HDL-C | +10.6 | 0.999 | +31.1 | 0.027 |
| LDL-C | −11.7 | 0.038 | −18.9 | 0.008 |
| Glucose | −17.9 | 0.036 | −21.8 | 0.011 |
| Insulin | −55.4 | 0.002 | −63.7 | 0.003 |
| HOMA-IR | −62.0 | 0.002 | −71.4 | 0.003 |
| ASAT | −16.4 | 0.043 | −15.3 | 0.031 |
| ALAT | −30.8 | 0.009 | −30.9 | 0.004 |
| eGFR | +11.1 | 0.002 | +5.9 | 0.004 |
| NAFLD FS | −66.7 | 0.011 | −92.4 | 0.002 |
| Parameter | Healthy Controls | Obese Patients (12 Months Post-Surgery) | p-Value 1 |
|---|---|---|---|
| n | 31 | 31 | - |
| Male/Female | 9/22 | 9/22 | 0.785 |
| Age, years | 40 ± 10 | 41 ± 9 | 0.698 |
| BMI, kg/m2 | 22 ± 3 | 32 ± 5 | 0.003 |
| TC, mg/dL | 187 ± 31 | 167 ± 30 | 0.020 |
| TG, mg/dL | 81 [45–176] | 68 [36–125] | 0.020 |
| HDL-C, mg/dL | 73 [50–121] | 54 [37–79] | 0.003 |
| LDL-C, mg/dL | 95 ± 34 | 97 ± 24 | 0.815 |
| Glucose, mg/dL | 77 [58–103] | 83 [70–106] | 0.089 |
| Insulin, mIU/L | 15 [3–48] | 8 [2–14] | 0.003 |
| HOMA-IR | 2.9 [0.7–12.1] | 1.7 [0.4–2.9] | 0.038 |
| ASAT, IU/L | 14 [3–44] | 16 [11–37] | 0.831 |
| ALAT, IU/L | 22 [13–98] | 0.020 | |
| eGFR, mL/min/1.73 m2 | Not available | - | |
| ARG, µmol/L | 157 ± 24 | 16 [5–36] | 0.972 |
| NMMA, nmol/L | 466 ± 118 | 105 ± 16 | 0.003 |
| CIT, µmol/L | 33 ± 5 | 37 ± 9 | 0.089 |
| ADMA, nmol/L | 820 ± 176 | 1066 ± 202 | 0.003 |
| SDMA, nmol/L | 685 ± 103 | 800 ± 156 | 0.003 |
| DMGV, nmol/L | 324 [87–891] | 483 [245–1251] | 0.003 |
| Compound | MRM Transition (m/z) | Cone/Collision (V) |
|---|---|---|
| ARG | 231.1 → 69.9 | 30/20 |
| 13C-ARG (IS) | 232.1 → 69.9 | 30/20 |
| CIT | 232.1 → 112.9 | 30/20 |
| 2H7-CIT (IS) | 239.1 → 119.9 | 30/20 |
| NMMA | 245.1 → 69.9 | 30/20 |
| ADMA | 259.1 → 214.1 | 30/20 |
| SDMA | 259.1 → 228.1 | 30/20 |
| DMGV | 258.2 → 113.9 | 30/25 |
| 2H7-ADMA (IS) | 266.2 → 221.1 | 30/20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poirier, J.; Cloteau, C.; Aguesse, A.; Billot, X.; Thévenot, E.; Krempf, M.; Valéro, R.; Maraninchi, M.; Croyal, M. Bariatric Surgery Improves the Atherogenic Profile of Circulating Methylarginines in Obese Patients: Results from a Pilot Study. Metabolites 2021, 11, 759. https://doi.org/10.3390/metabo11110759
Poirier J, Cloteau C, Aguesse A, Billot X, Thévenot E, Krempf M, Valéro R, Maraninchi M, Croyal M. Bariatric Surgery Improves the Atherogenic Profile of Circulating Methylarginines in Obese Patients: Results from a Pilot Study. Metabolites. 2021; 11(11):759. https://doi.org/10.3390/metabo11110759
Chicago/Turabian StylePoirier, Julie, Chloé Cloteau, Audrey Aguesse, Xavier Billot, Etienne Thévenot, Michel Krempf, René Valéro, Marie Maraninchi, and Mikaël Croyal. 2021. "Bariatric Surgery Improves the Atherogenic Profile of Circulating Methylarginines in Obese Patients: Results from a Pilot Study" Metabolites 11, no. 11: 759. https://doi.org/10.3390/metabo11110759