Probing Hepatic Glucose Metabolism via 13C NMR Spectroscopy in Perfused Livers—Applications to Drug Development
Abstract
1. Introduction
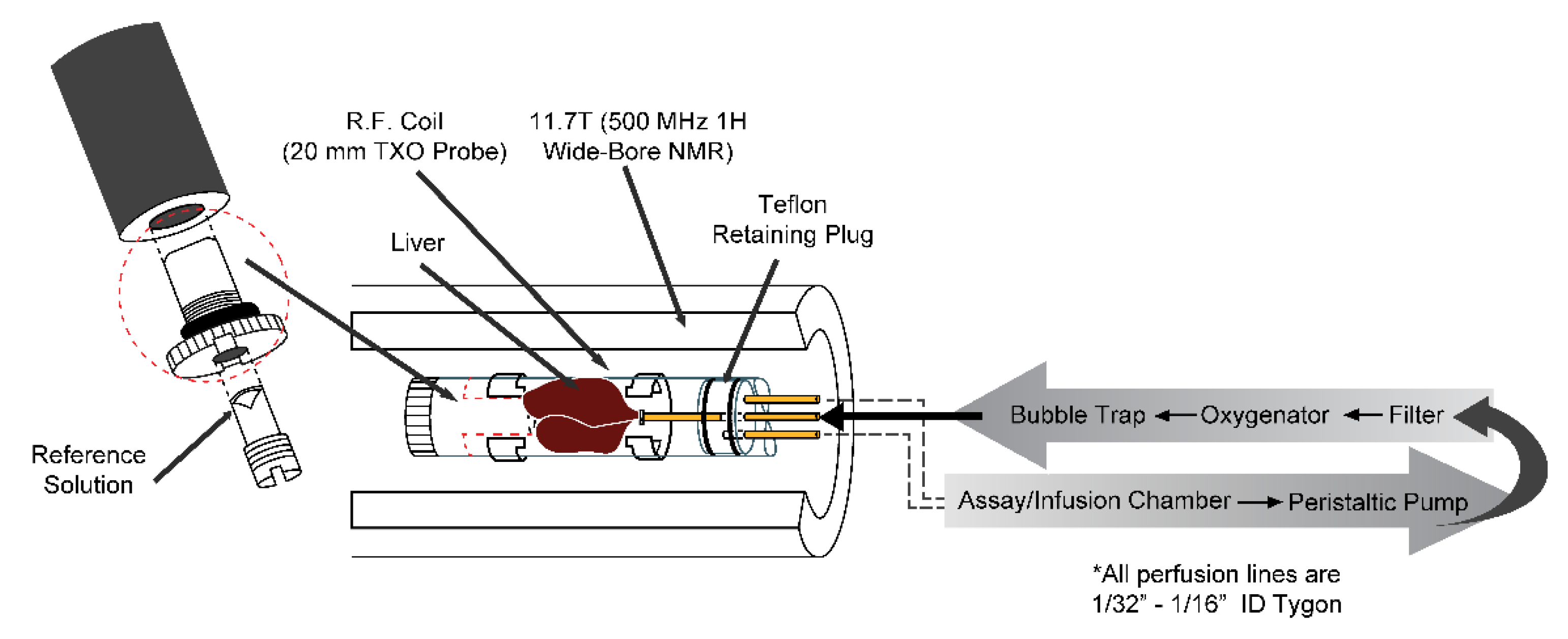
2. Perfused Liver Methods
3. Glycogen Metabolism
3.1. Glycogen Metabolism from [2-13C] Pyruvate
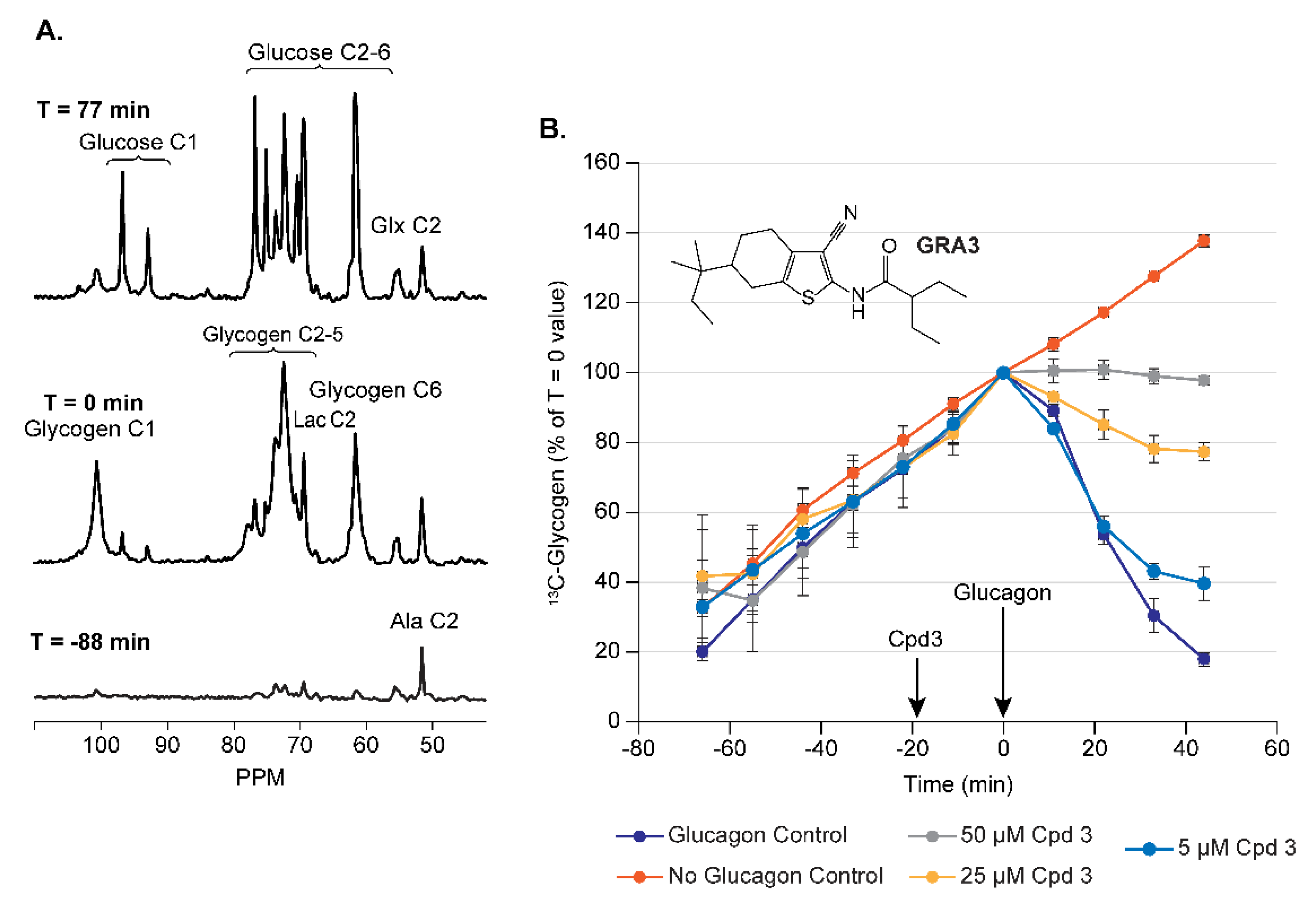
3.1.1. Assay Development for Glucagon Receptor Antagonists (GRA)
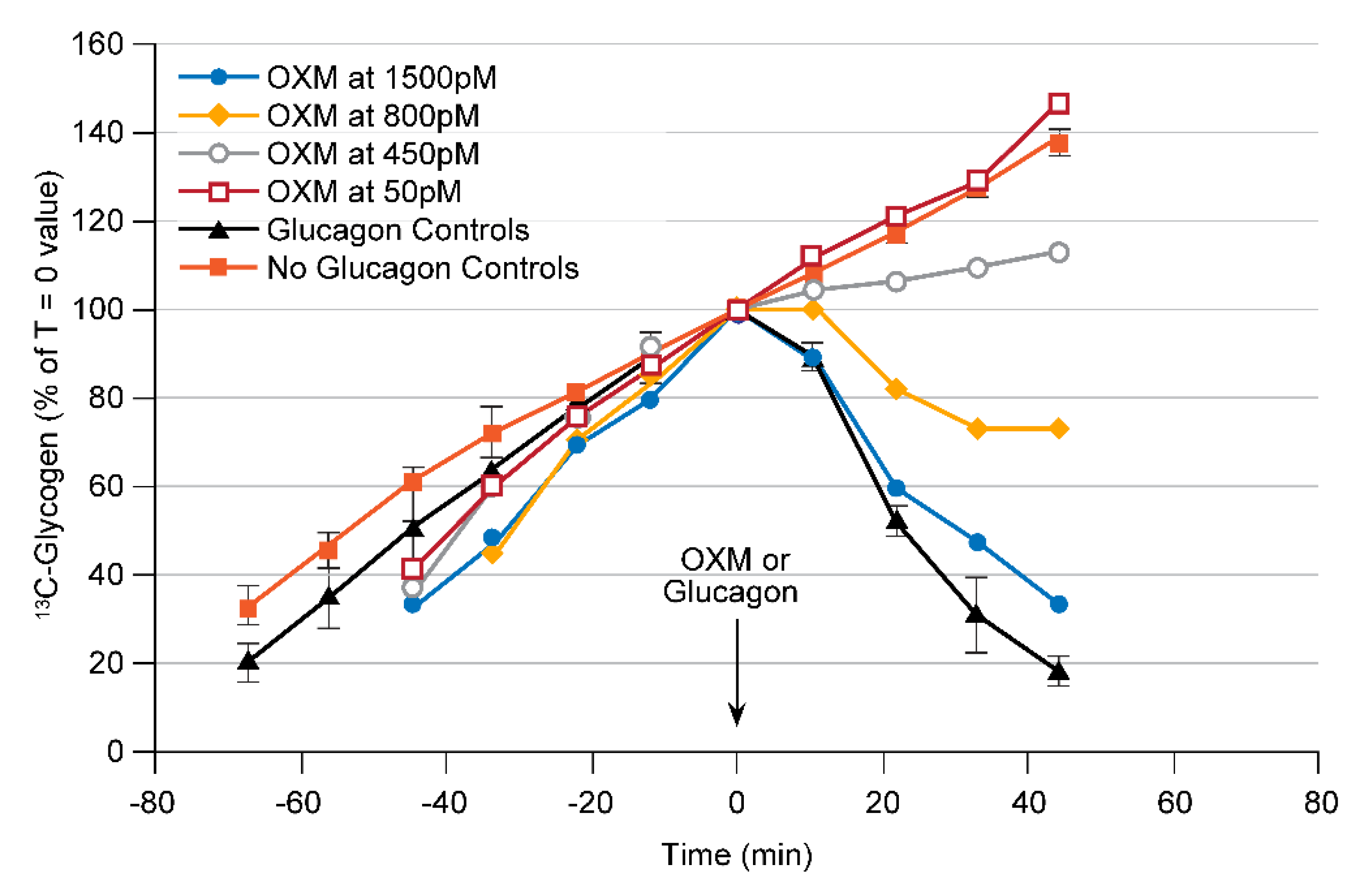
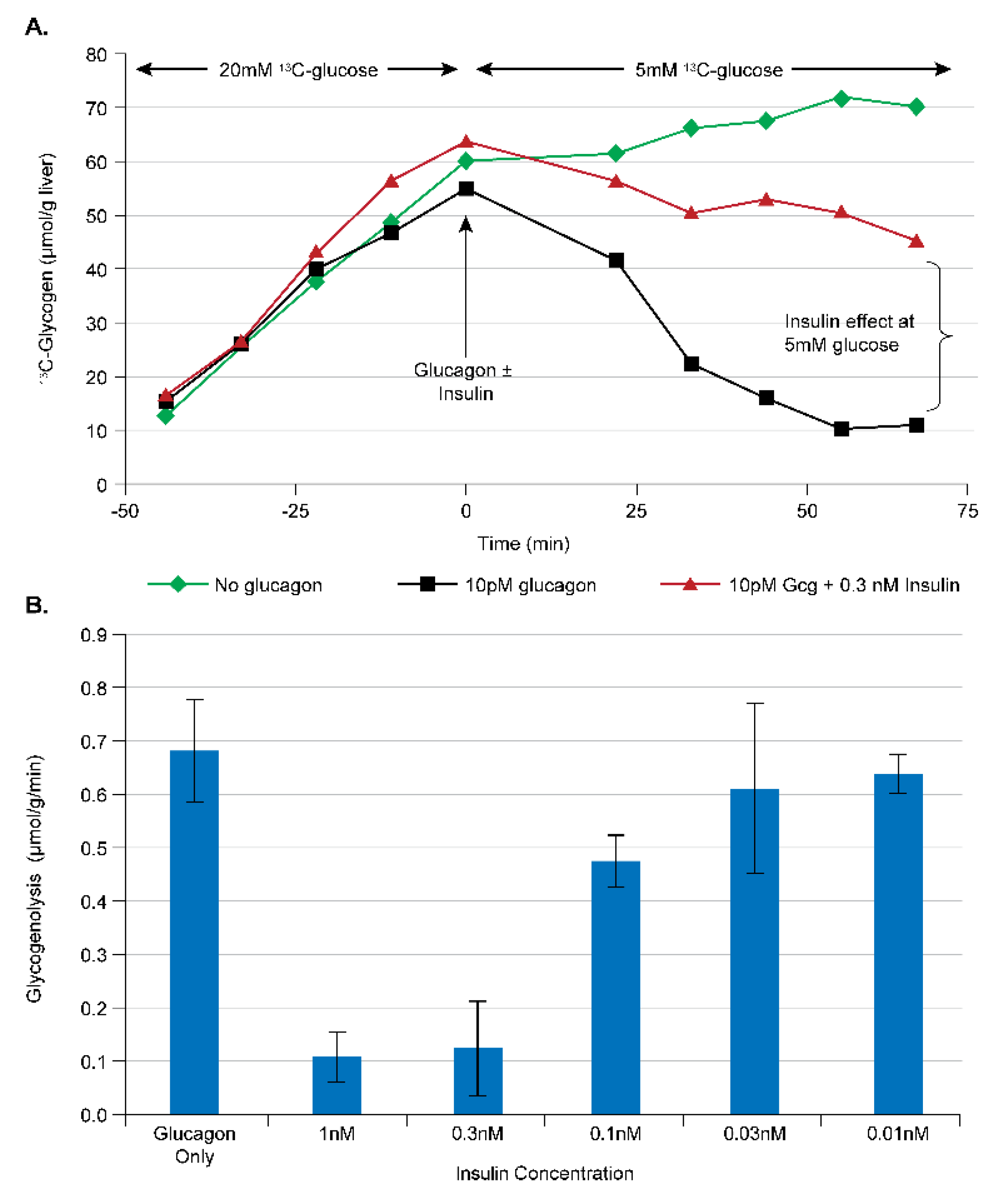
3.1.2. Assay Development for GLP1/Glucagon Dual Receptor Agonists
3.2. Glycogen Metabolism from [1-13C] Glucose
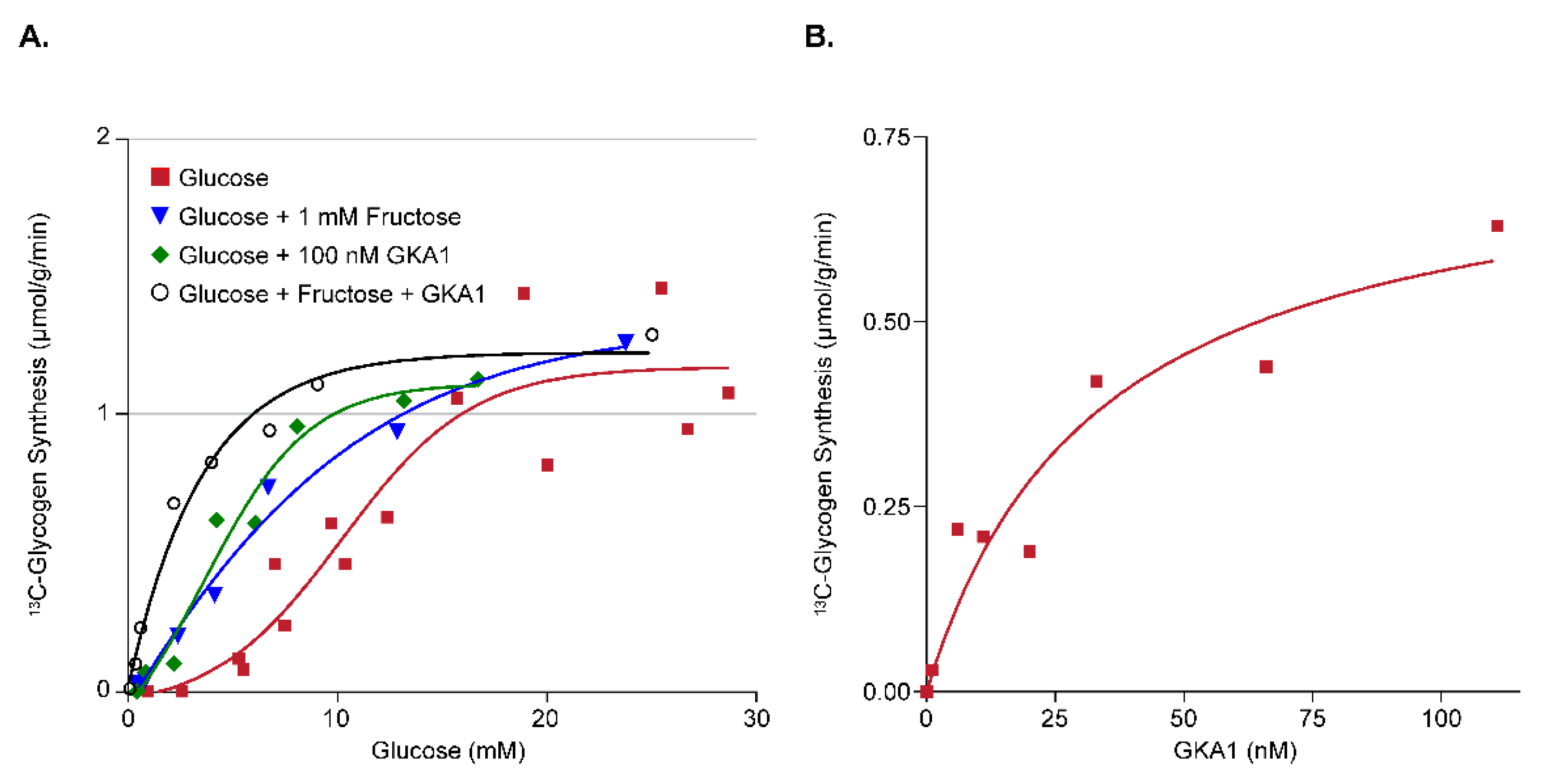
3.2.1. Assay Development for Glucokinase Activators (GKA)
3.2.2. Assay Development for Insulin Analogs and Insulin Receptor Agonists
4. Gluconeogenesis
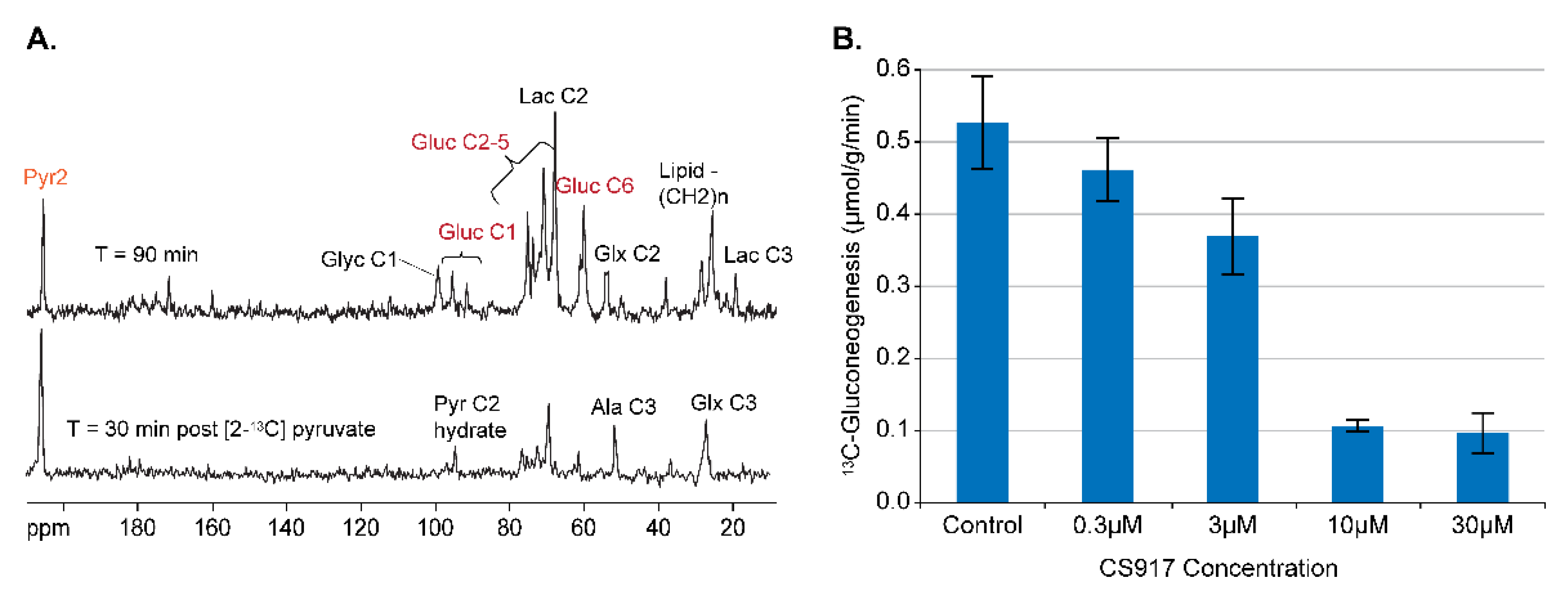
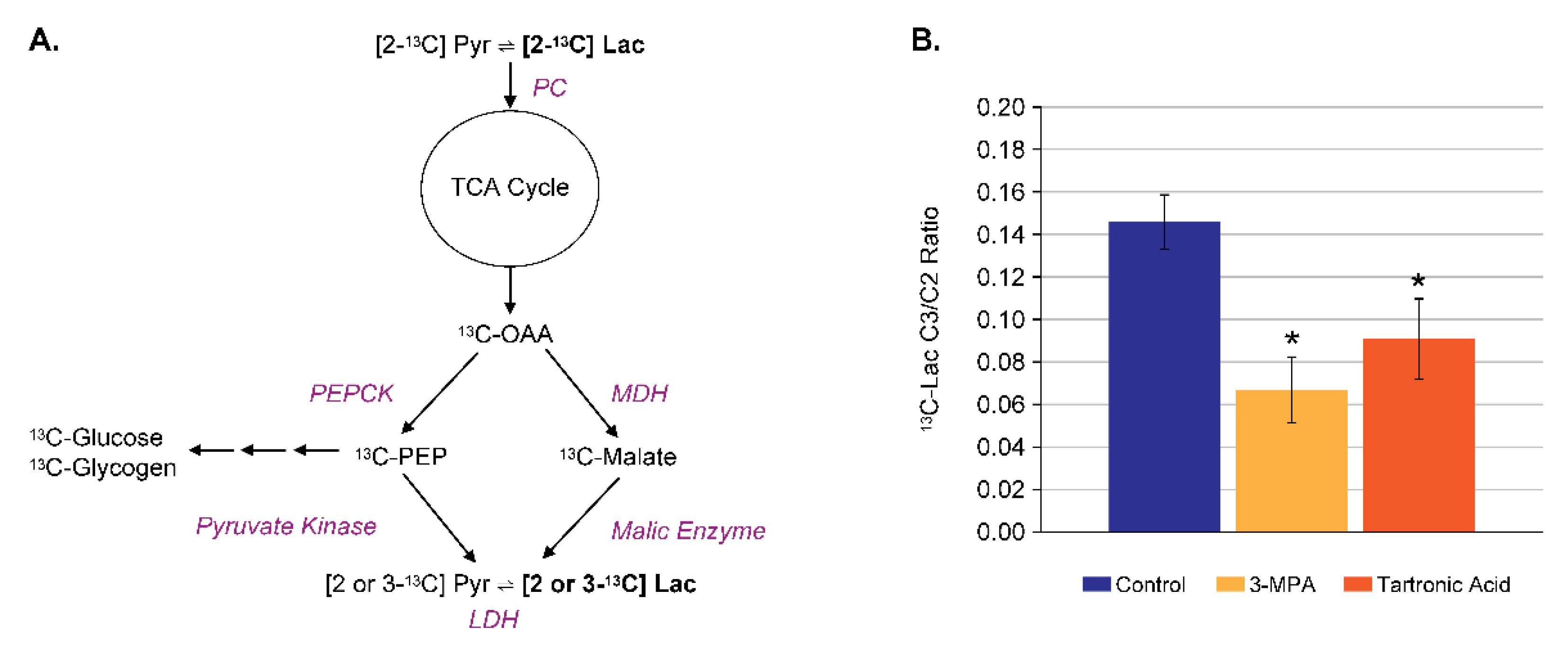
4.1. Gluconeogeneiss from [2-13C] Pyrzuvate
4.2. Assay Development for Inhibitors of GNG
4.3. Assay Development for Inhibitors of Malic Enzyme
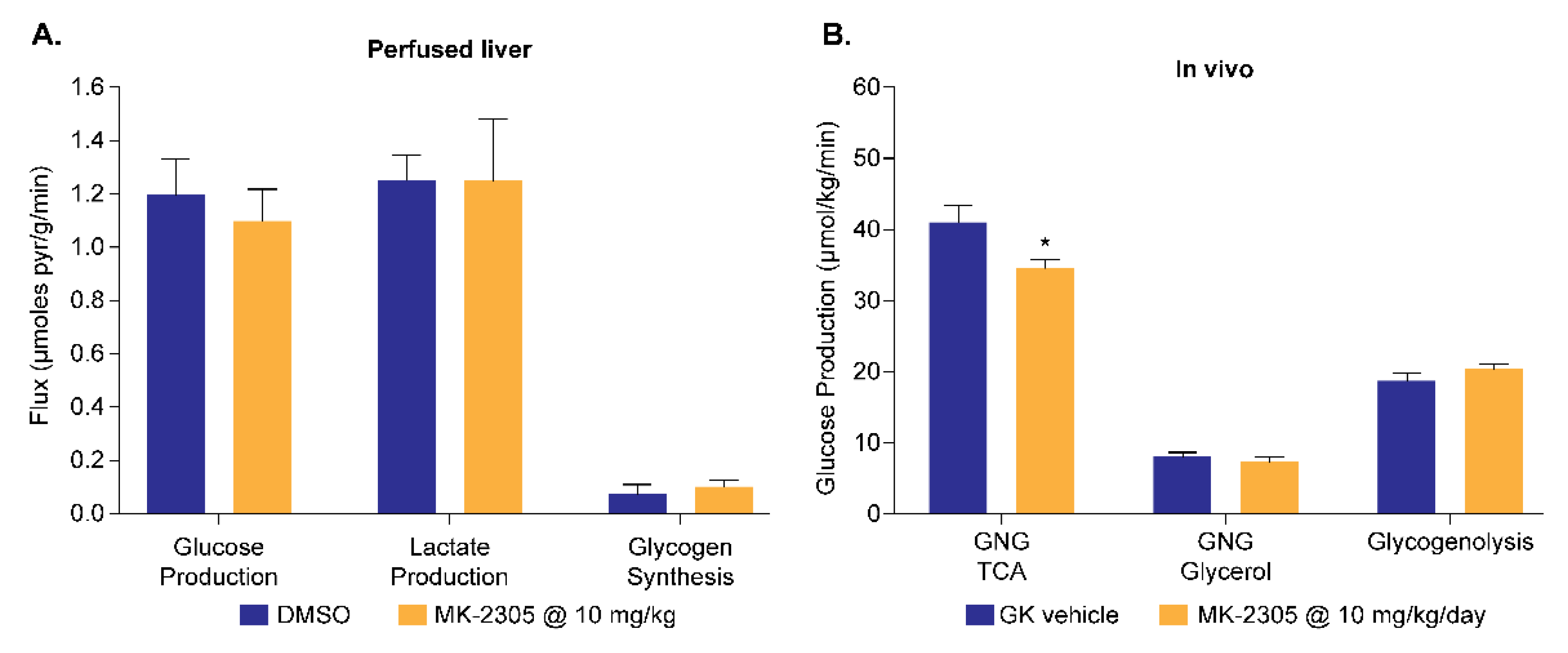
4.4. Application to GPR40 Agonists
5. Interaction of Glucose and Lipid Metabolism
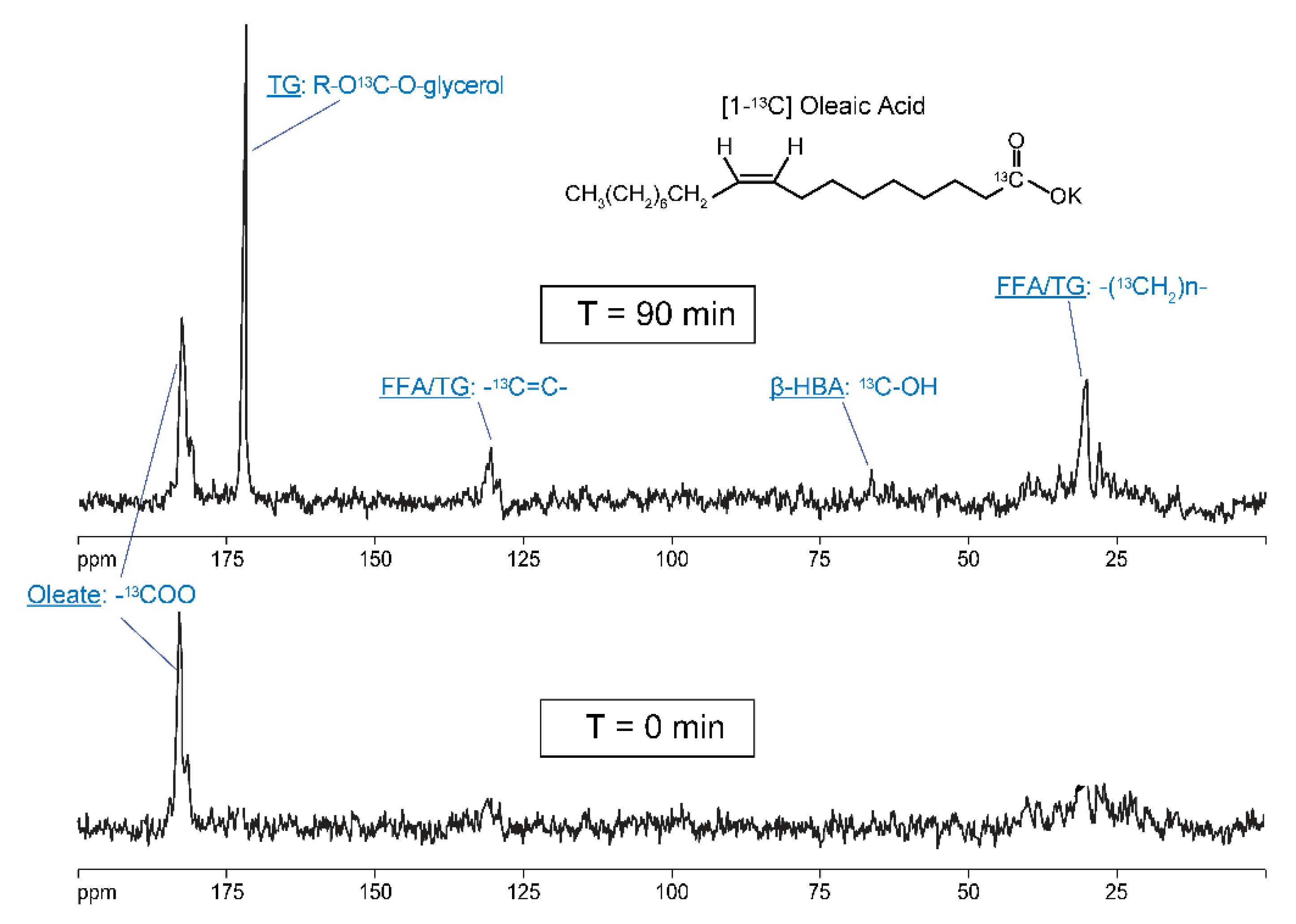
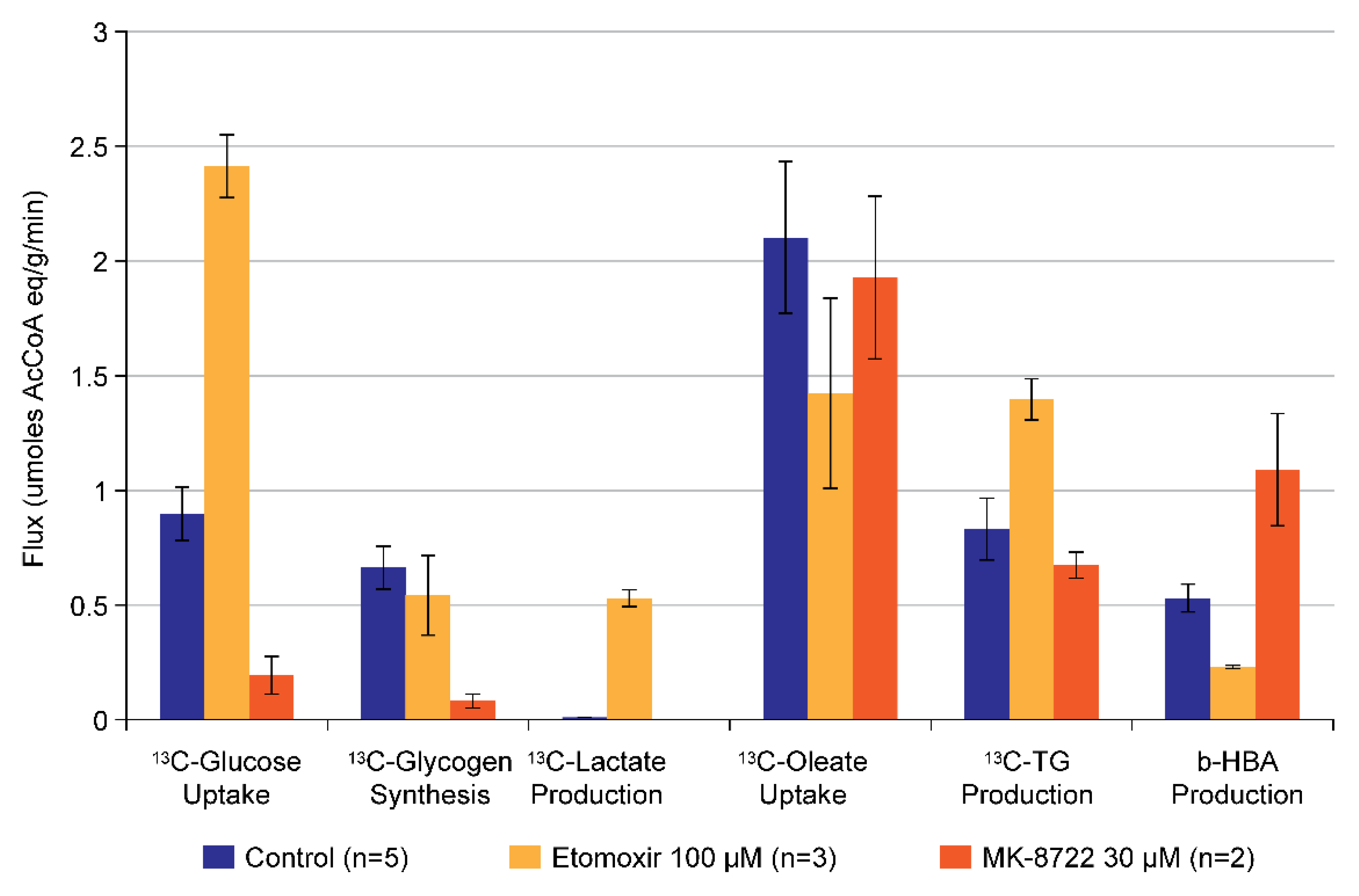
5.1. Metabolism of [1-13C] Oleate
5.2. Combined Metabolism of [1-13C] Oleate and [1-13C] Glucose
6. Summary and Other Applications
Author Contributions
Funding
Conflicts of Interest
References
- Hauner, H. The mode of action of thiazolidinediones. Diabetes Metab. Res. Rev. 2002, 18 (Suppl. S2), S10–S15. [Google Scholar] [CrossRef]
- Chao, E.C. SGLT-2 inhibitors: A New mechanism for glycemic control. Clin. Diabetes 2014, 32, 4–11. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Stott, N.L.; Marino, J.S. High fat rodent models of type 2 diabetes: From rodent to human. Nutrients 2020, 12, 3650. [Google Scholar] [CrossRef]
- Vos, M.B.; Lavine, J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013, 57, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Regnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: Two sides of the same coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.; Rehman, K.; Chen, S. Goto-kakizaki rats: Its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr. Diabetes Rev. 2013, 9, 387–396. [Google Scholar] [CrossRef]
- Wu, K.K.; Huan, Y. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2008, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M. 13C and 31P NMR study of gluconeogenesis: Utilization of 13C-labeled substrates by perfused liver from streptozotocin-diabetic and untreated rats. Biochemistry 1987, 26, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.O.; Cao, J.; Zhu, H.; Chen, L.M.; Wilson, G.; Kennan, R.; Gore, J.C. 13C MRS studies of the control of hepatic glycogen metabolism at high magnetic fields. Front. Phys. 2017, 5. [Google Scholar] [CrossRef]
- Villar-Palasi, C.; Guinovart, J.J. The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J. 1997, 11, 544–558. [Google Scholar] [CrossRef]
- Cohen, S.M.; Duffy, J.L.; Miller, C.; Kirk, B.A.; Candelore, M.R.; Ding, V.D.; Kaczorowski, G.; Tota, L.M.; Werrmann, J.G.; Wright, M.; et al. Direct observation (NMR) of the efficacy of glucagon receptor antagonists in murine liver expressing the human glucagon receptor. Bioorg. Med. Chem. 2006, 14, 1506–1517. [Google Scholar] [CrossRef]
- Duffy, J.L.; Kirk, B.A.; Konteatis, Z.; Campbell, E.L.; Liang, R.; Brady, E.J.; Candelore, M.R.; Ding, V.D.; Jiang, G.; Liu, F.; et al. Discovery and investigation of a novel class of thiophene-derived antagonists of the human glucagon receptor. Bioorg. Med. Chem. Lett. 2005, 15, 1401–1405. [Google Scholar] [CrossRef]
- Shiao, L.L.; Cascieri, M.A.; Trumbauer, M.; Chen, H.; Sullivan, K.A. Generation of mice expressing the human glucagon receptor with a direct replacement vector. Transgenic Res. 1999, 8, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Cherrington, A.D. Banting lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes 1999, 48, 1198–1214. [Google Scholar] [CrossRef] [PubMed]
- Druce, M.R.; Bloom, S.R. Oxyntomodulin: A novel potential treatment for obesity. Treat. Endocrinol. 2006, 5, 265–272. [Google Scholar] [CrossRef]
- Pyke, C.; Heller, R.S.; Kirk, R.K.; Orskov, C.; Reedtz-Runge, S.; Kaastrup, P.; Hvelplund, A.; Bardram, L.; Calatayud, D.; Knudsen, L.B. GLP-1 receptor localization in monkey and human tissue: Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014, 155, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, J.R.; Hubert, J.; Carrington, P.E.; Chicchi, G.G.; Mu, J.; Miller, C.; Cao, J.; Bianchi, E.; Pessi, A.; Sinharoy, R.; et al. The glucagon receptor is involved in mediating the body weight-lowering effects of oxyntomodulin. Obesity 2012, 20, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Matschinsky, F.M.; Wilson, D.F. The central role of glucokinase in glucose homeostasis: A perspective 50 years after demonstrating the presence of the enzyme in islets of langerhans. Front. Physiol. 2019, 10, 148. [Google Scholar] [CrossRef]
- van Schaftingen, E.; Vandercammen, A.; Detheux, M.; Davies, D.R. The regulatory protein of liver glucokinase. Adv. Enzym. Regul. 1992, 32, 133–148. [Google Scholar] [CrossRef]
- Winnick, J.J.; An, Z.; Ramnanan, C.J.; Smith, M.; Irimia, J.M.; Neal, D.W.; Moore, M.C.; Roach, P.J.; Cherrington, A.D. Hepatic glycogen supercompensation activates AMP-activated protein kinase, impairs insulin signaling, and reduces glycogen deposition in the liver. Diabetes 2011, 60, 398–407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.; Nowotny, P.; Dalla Man, C.; Cobelli, C.; Cline, G.W.; Shulman, G.I.; Waldhausl, W.; et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Kwon, A.H.; Teshima, S.; Nakanishi, H.; Kitano, T.; Kamiyama, Y.; Okumura, T. Hepatocyte growth factor inhibits insulin-stimulated glycogen synthesis in primary cultured hepatocytes. J. Hepatol. 2003, 38, 407–413. [Google Scholar] [CrossRef]
- Pilar Lopez, M.; Gomez-Lechon, M.J.; Castell, J.V. Role of glucose, insulin, and glucagon in glycogen mobilization in human hepatocytes. Diabetes 1991, 40, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Krause, U.; Bertrand, L.; Maisin, L.; Rosa, M.; Hue, L. Signalling pathways and combinatory effects of insulin and amino acids in isolated rat hepatocytes. Eur. J. Biochem. 2002, 269, 3742–3750. [Google Scholar] [CrossRef]
- Erion, M.D.; van Poelje, P.D.; Dang, Q.; Kasibhatla, S.R.; Potter, S.C.; Reddy, M.R.; Reddy, K.R.; Jiang, T.; Lipscomb, W.N. MB06322 (CS-917): A potent and selective inhibitor of fructose 1,6-bisphosphatase for controlling gluconeogenesis in type 2 diabetes. Proc. Natl. Acad. Sci. USA 2005, 102, 7970–7975. [Google Scholar] [CrossRef]
- Yang, X.; Deignan, J.L.; Qi, H.; Zhu, J.; Qian, S.; Zhong, J.; Torosyan, G.; Majid, S.; Falkard, B.; Kleinhanz, R.R.; et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat. Genet. 2009, 41, 415–423. [Google Scholar] [CrossRef]
- Miller, C.; Liu, H.; Balogh, L.; Cao, J.; Tota, M.R.; Patel, R. A novel 13C MRS-based marker of pyruvate cycling in perfused mouse liver using [2-13C] pyruvate and 13C MRS. In Proceedings of the ISMRM 17th Meeting and Exhibition, Honolulu, HI, USA, 18–24 April 2009. [Google Scholar]
- Petersen, K.F.; Cline, G.W.; Blair, J.B.; Shulman, G.I. Substrate cycling between pyruvate and oxaloacetate in awake normal and 3,3′-5-triiodo-l-thyronine-treated rats. Am. J. Physiol. 1994, 267, E273–E277. [Google Scholar] [CrossRef]
- Kendrick, A.; Ratledge, C. Desaturation of polyunsaturated fatty acids in Mucor circinelloides and the involvement of a novel membrane-bound malic enzyme. Eur. J. Biochem. 1992, 209, 667–673. [Google Scholar] [CrossRef]
- Ito, R.; Tsujihata, Y.; Matsuda-Nagasumi, K.; Mori, I.; Negoro, N.; Takeuchi, K. TAK-875, a GPR40/FFAR1 agonist, in combination with metformin prevents progression of diabetes and beta-cell dysfunction in Zucker diabetic fatty rats. Br. J. Pharmacol. 2013, 170, 568–580. [Google Scholar] [CrossRef]
- Mohammad, S. GPR40 agonists for the treatment of type 2 diabetes mellitus: Benefits and challenges. Curr. Drug Targets 2016, 17, 1292–1300. [Google Scholar] [CrossRef]
- Miller, C.; Pachanski, M.J.; Kirkland, M.E.; Kosinski, D.T.; Mane, J.; Bunzel, M.; Cao, J.; Souza, S.; Thomas-Fowlkes, B.; Di Salvo, J.; et al. GPR40 partial agonist MK-2305 lower fasting glucose in the Goto Kakizaki rat via suppression of endogenous glucose production. PLoS ONE 2017, 12, e0176182. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.C.; Hausler, N.; Merritt, M.; Jeffrey, F.M.; Storey, C.; Milde, A.; Koshy, S.; Lindner, J.; Magnuson, M.A.; Malloy, C.R.; et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 2004, 279, 48941–48949. [Google Scholar] [CrossRef] [PubMed]
- Randle, P.J.; Garland, P.B.; Hales, C.N.; Newsholme, E.A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Alves, T.C.; Befroy, D.E.; Kibbey, R.G.; Kahn, M.; Codella, R.; Carvalho, R.A.; Falk Petersen, K.; Shulman, G.I. Regulation of hepatic fat and glucose oxidation in rats with lipid-induced hepatic insulin resistance. Hepatology 2011, 53, 1175–1181. [Google Scholar] [CrossRef]
- Krssak, M.; Falk Petersen, K.; Dresner, A.; DiPietro, L.; Vogel, S.M.; Rothman, D.L.; Roden, M.; Shulman, G.I. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef]
- Roden, M.; Krssak, M.; Stingl, H.; Gruber, S.; Hofer, A.; Furnsinn, C.; Moser, E.; Waldhausl, W. Rapid impairment of skeletal muscle glucose transport/phosphorylation by free fatty acids in humans. Diabetes 1999, 48, 358–364. [Google Scholar] [CrossRef]
- Bryson, J.M.; Cooney, G.J.; Wensley, V.R.; Phuyal, J.L.; Caterson, I.D. The effects of the inhibition of fatty acid oxidation on pyruvate dehydrogenase complex activity in tissues of lean and obese mice. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 738–744. [Google Scholar]
- Timmers, S.; Nabben, M.; Bosma, M.; van Bree, B.; Lenaers, E.; van Beurden, D.; Schaart, G.; Westerterp-Plantenga, M.S.; Langhans, W.; Hesselink, M.K.; et al. Augmenting muscle diacylglycerol and triacylglycerol content by blocking fatty acid oxidation does not impede insulin sensitivity. Proc. Natl. Acad. Sci. USA 2012, 109, 11711–11716. [Google Scholar] [CrossRef]
- O’Donnell, J.M.; Zampino, M.; Alpert, N.M.; Fasano, M.J.; Geenen, D.L.; Lewandowski, E.D. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E448–E455. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Biftu, T.; Romero, F.A.; Kekec, A.; Dropinski, J.; Kassick, A.; Xu, S.; Kurtz, M.M.; Gollapudi, A.; Shao, Q.; et al. Discovery of MK-8722: A systemic, direct pan-activator of AMP-activated protein kinase. ACS Med. Chem. Lett. 2018, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.W.; Guan, H.P.; Ehrhart, J.; Petrov, A.; Prahalada, S.; Tozzo, E.; Yang, X.; Kurtz, M.M.; Trujillo, M.; Gonzalez Trotter, D.; et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science 2017, 357, 507–511. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, C.O.; Cao, J. Probing Hepatic Glucose Metabolism via 13C NMR Spectroscopy in Perfused Livers—Applications to Drug Development. Metabolites 2021, 11, 712. https://doi.org/10.3390/metabo11110712
Miller CO, Cao J. Probing Hepatic Glucose Metabolism via 13C NMR Spectroscopy in Perfused Livers—Applications to Drug Development. Metabolites. 2021; 11(11):712. https://doi.org/10.3390/metabo11110712
Chicago/Turabian StyleMiller, Corin O., and Jin Cao. 2021. "Probing Hepatic Glucose Metabolism via 13C NMR Spectroscopy in Perfused Livers—Applications to Drug Development" Metabolites 11, no. 11: 712. https://doi.org/10.3390/metabo11110712
APA StyleMiller, C. O., & Cao, J. (2021). Probing Hepatic Glucose Metabolism via 13C NMR Spectroscopy in Perfused Livers—Applications to Drug Development. Metabolites, 11(11), 712. https://doi.org/10.3390/metabo11110712





