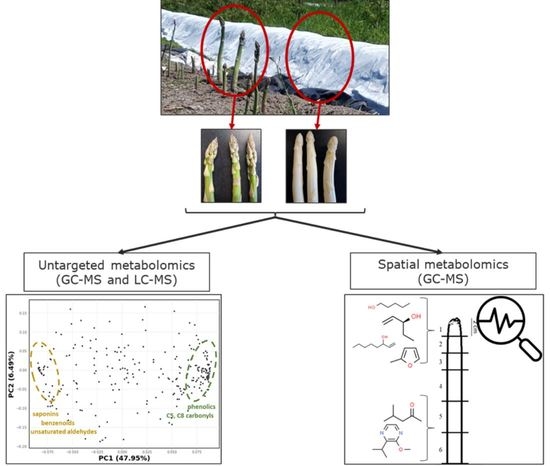
Metabolomics Reveals Heterogeneity in the Chemical Composition of Green and White Spears of Asparagus (A. officinalis)
Abstract
:1. Introduction
2. Results
2.1. Untargeted Metabolomics of Green and White Asparagus Spears
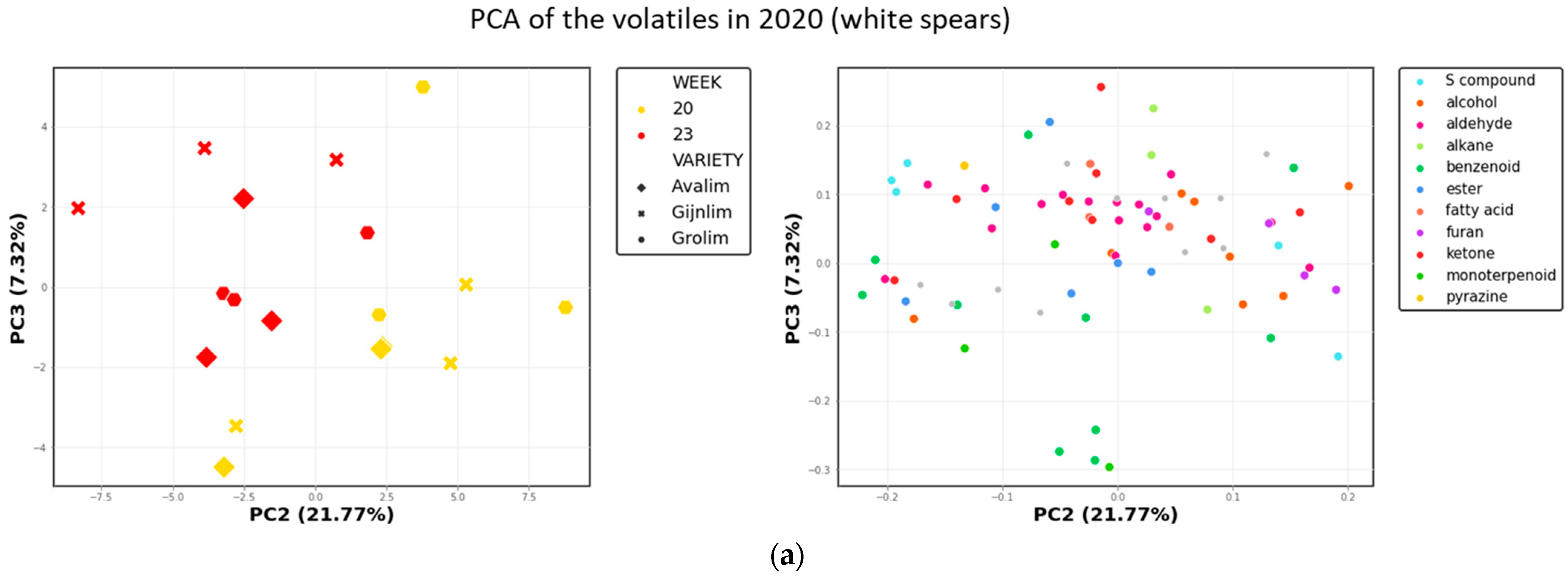
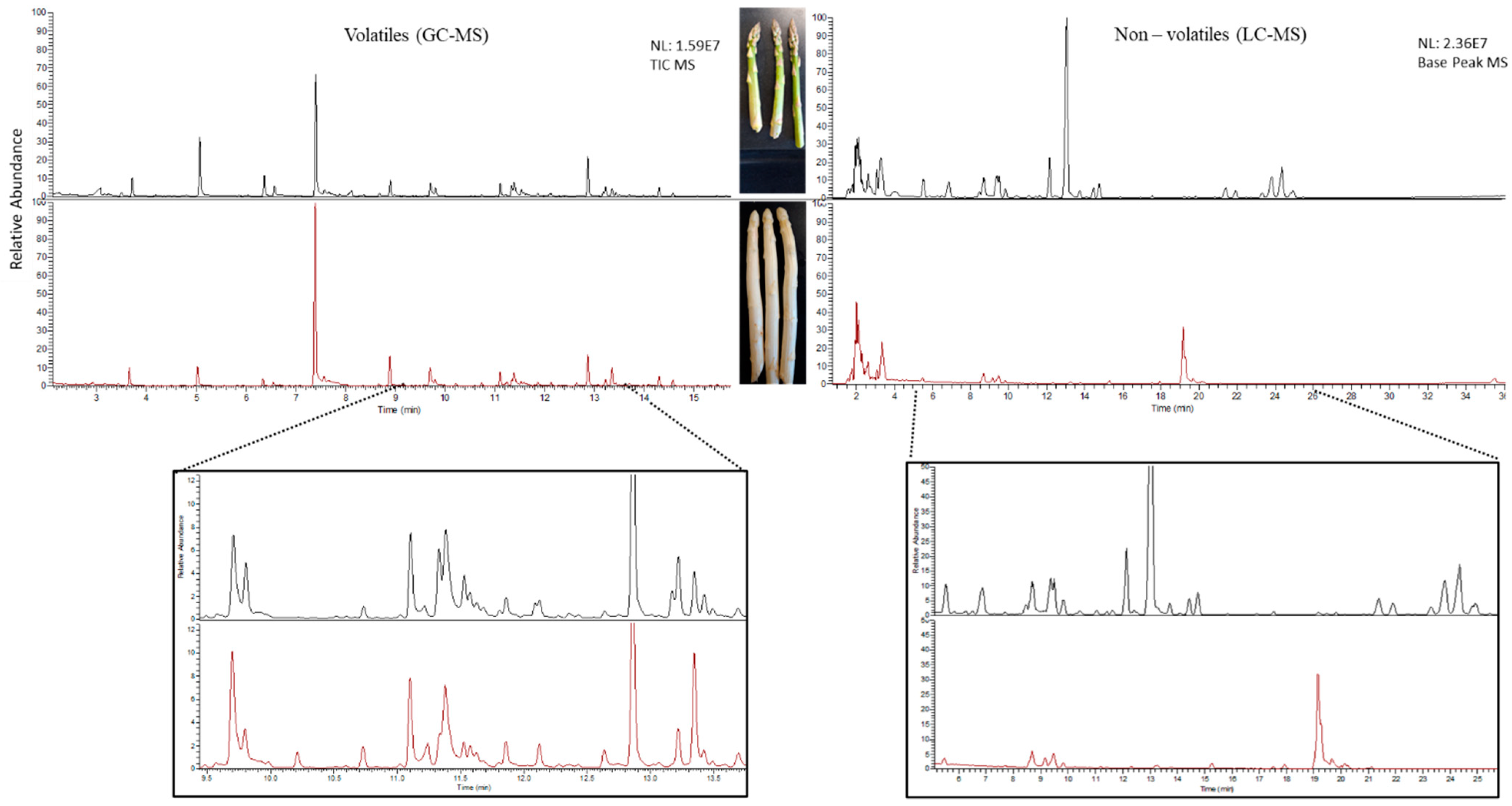
2.1.1. Volatile Secondary Metabolites in Green and White Asparagus
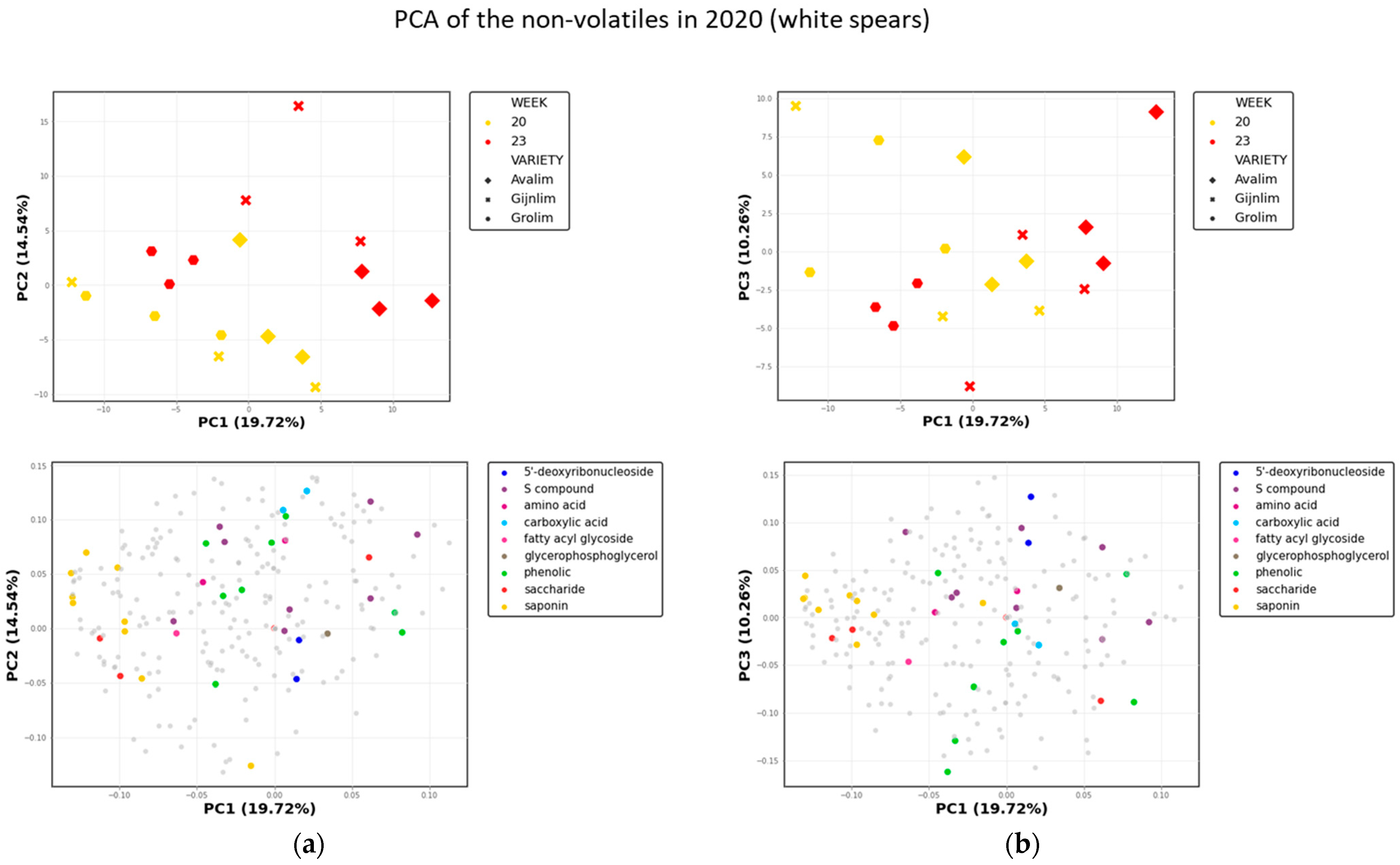
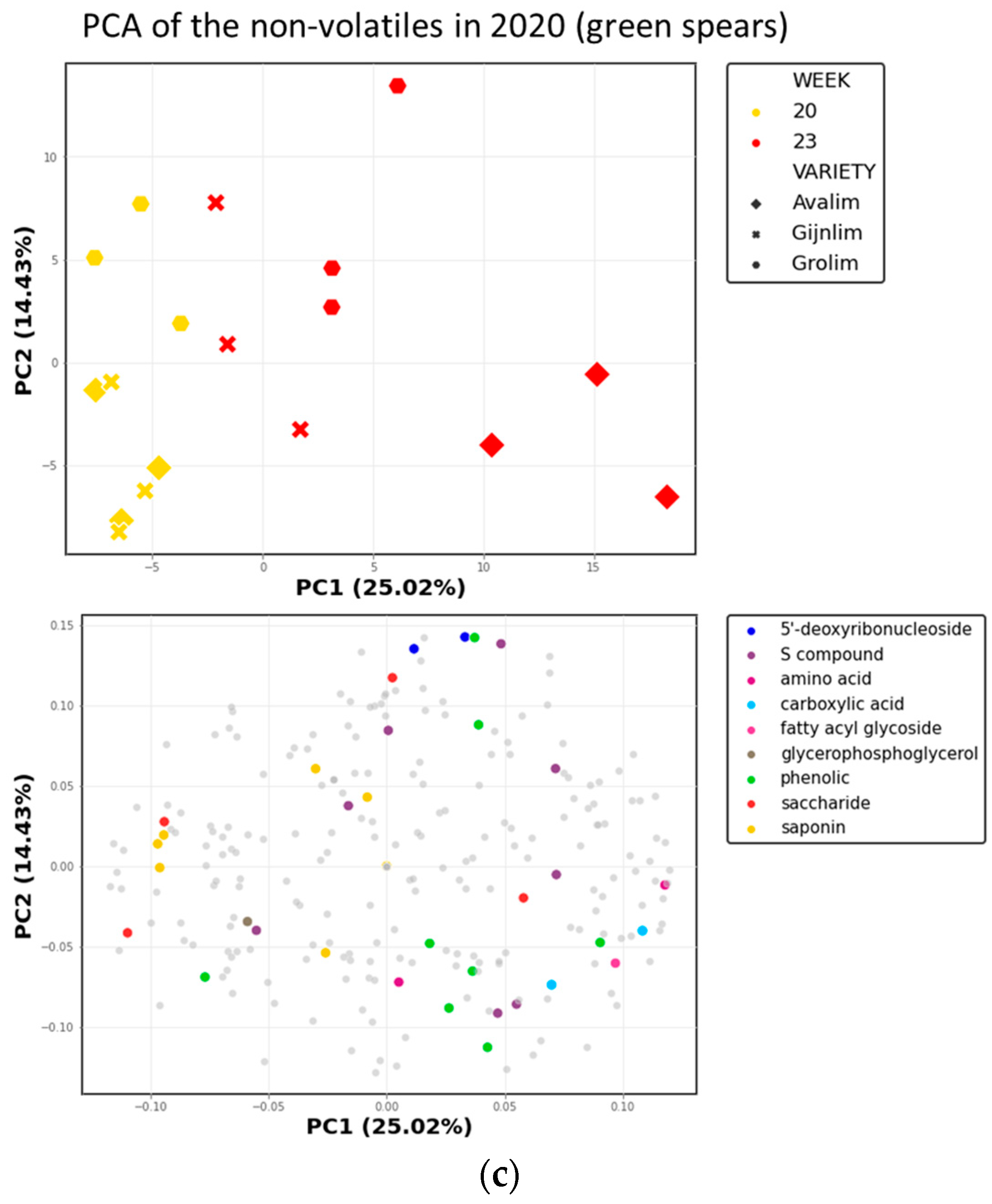
2.1.2. Non-Volatile Secondary Metabolites in Green and White Asparagus
2.2. Comparison of the Chemical Composition of Green and White Asparagus in 2019
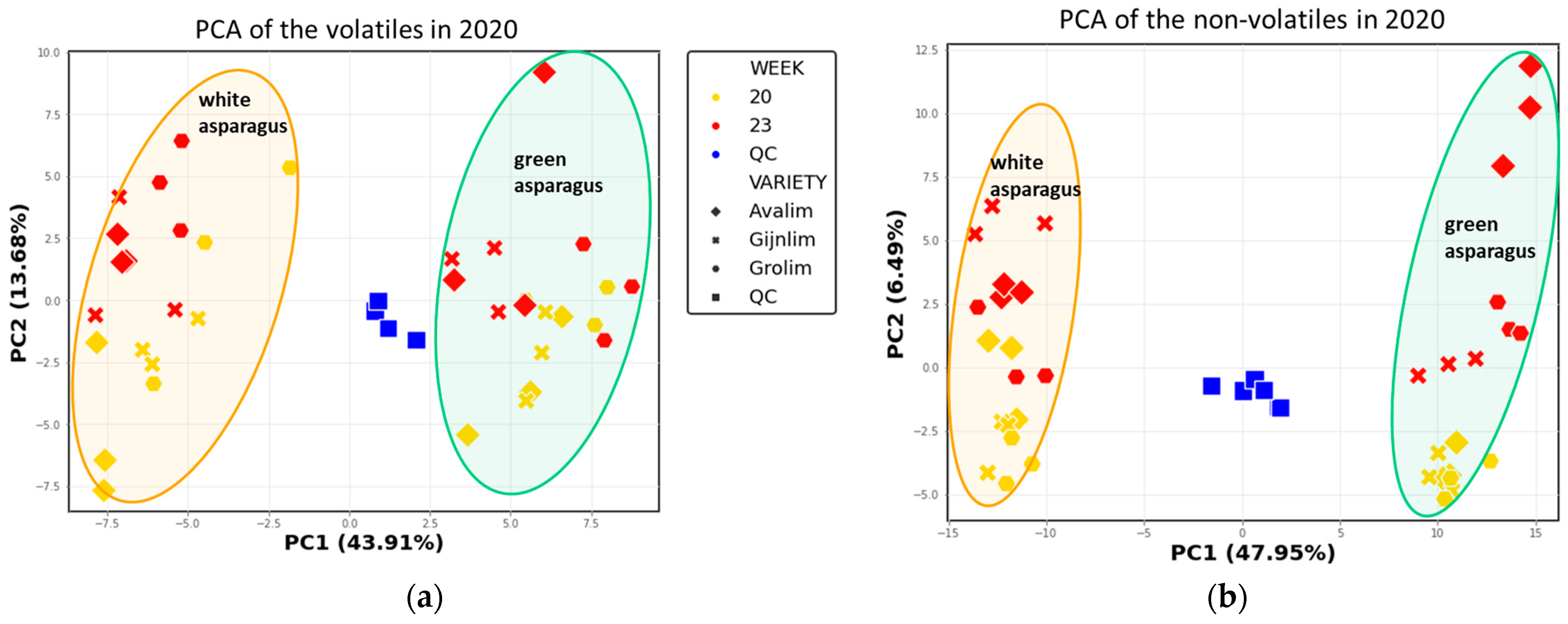
2.3. Comparison of the Chemical Composition of Green and White Asparagus in 2020
2.4. Non-Discriminatory Compounds in Green and White Asparagus
2.5. Spatial Metabolomics of the Asparagus Spear
3. Discussion
3.1. Asparagus Metabolome and Differences in Chemical Compositions of Green and White Spears
3.2. Non-Discriminatory Asparagus Compounds in Green and White Spears
3.3. Variety and Harvest Time-Point Effects on the Asparagus Metabolome
3.4. Metabolite Distribution along the Asparagus Spear
4. Materials and Methods
4.1. Asparagus Plant Materials
4.2. Chemicals and Analytical Standards
4.3. Sample Preparation for Volatile Analysis
4.4. Extraction of Volatiles and Analysis with HS-SPME GC-MS
4.5. Extraction of Semi-Polar Non-Volatiles and Analysis with LC-MS
4.6. Processing of Mass Spectrometry Data
4.7. Statistical Analysis and Visualisation Tools
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Pegiou, E.; Mumm, R.; Acharya, P.; de Vos, R.C.H.; Hall, R.D. Green and white asparagus (Asparagus officinalis): A source of developmental, chemical and urinary intrigue. Metabolites 2020, 10, 17. [Google Scholar] [CrossRef]
- Schweingruber, F.H.; Börner, A. Primary, secondary and tertiary meristems. In The Plant Stem; Springer: Cham, Switzerland, 2018; pp. 43–79. [Google Scholar]
- Maung, M.; Myint, K.T.; Thu, M.K. Effects of Different Explant Types, Plant Growth Regulators and Shoot Density on In vitro Regeneration of Asparagus (Asparagus officinallis L.). J. Agric. Res. 2019, 6, 109–115. [Google Scholar]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [Green Version]
- Gigot, C.; Ongena, M.; Fauconnier, M.-L.; Wathelet, J.-P.; Jardin, P.; Thonart, P. The Lipoxygenase Metabolic Pathway in Plants: Potential for Industrial Production of Natural Green Leaf Volatiles [La Voie métabolique de la Lipoxygénase chez les Plantes: Un Potentiel pour la Production d’arômes Naturels á Notes Vertes.]. Biotechnol. Agron. Soc. Environ. 2010, 14, 451–460. Available online: http://www.scopus.com/inward/record.url?eid=2-s2.0-78049336630&partnerID=40&md5=e73052983771bbc86b5eaf097a857cd7 (accessed on 21 March 2019).
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.; Tadmor, Y.; Gepstein, S.; Giovannoni, J.J.; Katzir, N.; et al. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar] [CrossRef] [Green Version]
- Mortzfeld, F.B.; Hashem, C.; Vranková, K.; Winkler, M.; Rudroff, F. Pyrazines: Synthesis and Industrial Application of these Valuable Flavor and Fragrance Compounds. Biotechnol. J. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Tressl, R.; Holzer, M.; Apetz, M. Formation of Flavor Components in Asparagus. 1. Biosynthesis of Sulfur-Containing Acids in Asparagus. J. Agric. Food Chem. 1977, 25, 455–459. [Google Scholar] [CrossRef]
- Tressl, R.; Holzer, M.; Apetz, M. Formation of Flavor Components in Asparagus. 2. Formation of Flavor Components in Cooked Asparagus. J. Agric. Food Chem. 1977, 25, 459–463. [Google Scholar] [CrossRef]
- Ulrich, D.; Hoberg, E.; Bittner, T.; Engewald, W.; Meilchen, K. Contribution of volatile compounds to the flavor of cooked asparagus. Eur. Food Res. Technol. 2001, 213, 200–204. [Google Scholar] [CrossRef]
- Hoberg, E.; Ulrich, D.; Wonneberger, C. Proposal for a Flavour Standard—Sensory Profiles of European White Asparagus officinalis L. Cultivars. Acta Hortic. 2008, 776, 239–245. [Google Scholar] [CrossRef]
- Ulrich, D.; Hoberg, E. Flavor analysis in asparagus breeding. Acta Hortic. 2002, 589, 281–285. [Google Scholar] [CrossRef]
- Hoberg, E.; Ulrich, D.; Standhardt, D.; Kecke, S. Evaluation of Asparagus officinalis L. flavour quality for breeding purposes. Acta Hortic. 1999, 479, 135–140. [Google Scholar] [CrossRef]
- Amaro-López, M.; Zurera-Cosano, G.; Moreno-Rojas, R. Trends and nutritional significance of mineral content in fresh white asparagus spears. Int. J. Food Sci. Nutr. 1998, 49, 353–363. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Fan, R.; Yuan, F.; Wang, N.; Gao, Y.; Huang, Y. Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J. Food Sci. Technol. 2016, 52, 2690–2700. [Google Scholar] [CrossRef] [Green Version]
- Onlom, C.; Nuengchamnong, N.; Phrompittayarat, W.; Putalun, W.; Waranuch, N.; Ingkaninan, K. Quantification of saponins in Asparagus racemosus by HPLC-Q-TOF-MS/MS. Nat. Prod. Commun. 2017, 12, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Kong, L. Steroidal saponins from roots of Asparagus officinalis. Steroids 2006, 71, 171–176. [Google Scholar] [CrossRef]
- Makus, D.J. Mineral nutrient composition of green and white asparagus spears. HortScience 1994, 29, 1468–1469. [Google Scholar] [CrossRef]
- Cuppett, S.; Deleon, A.; Parkhurst, A.; Hodges, L. Factors affecting asparagus sensory evaluation. J. Food Qual. 1997, 20, 127–144. [Google Scholar] [CrossRef]
- Maeda, T.; Kakuta, H.; Sonoda, T.; Motoki, S.; Ueno, R.; Suzuki, T.; Oosawa, K. Antioxidation capacities of extracts from green, purple, and white asparagus spears related to polyphenol concentration. HortScience 2005, 40, 1221–1224. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Yoo, K.S.; Patil, B.S. Development of a Rapid HPLC-UV Method for Simultaneous Quantification of Protodioscin and Rutin in White and Green Asparagus Spears. J. Food Sci. 2010, 75. [Google Scholar] [CrossRef]
- Siccama, J.W.; Pegiou, E.; Zhang, L.; Mumm, R.; Hall, R.D.; Boom, R.M.; Schutyser, M.A. Maltodextrin improves physical properties and volatile compound retention of spray- dried asparagus concentrate. LWT-Food Sci. Technol. 2021, 142, 111058. [Google Scholar] [CrossRef]
- Asparagus. KnapSack. 2021. Available online: http://www.knapsackfamily.com/knapsack_jsp/result.jsp?sname=all&word=asparagus (accessed on 26 January 2021).
- Asparagus. Dictionary of Natural Products. 2019. Available online: http://dnp.chemnetbase.com/faces/chemical/ChemicalSearchResults.xhtml (accessed on 26 September 2019).
- mzCloud. Available online: https://www.mzcloud.org/ (accessed on 17 April 2021).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Qin, W.; Ma, L.; Xu, F.; Jin, P.; Zheng, Y. Effect of high pressure processing and thermal treatment on physicochemical parameters, antioxidant activity and volatile compounds of green asparagus juice. LWT-Food Sci. Technol. 2015, 62, 927–933. [Google Scholar] [CrossRef]
- Sun, R.; Wang, Y.; Chin, C.K.; Garrison, S.A. Volatile compounds in Asparagus officinalis L. Acta Hortic. 2002, 589, 257–266. [Google Scholar] [CrossRef]
- FooDB. 2021. Available online: www.foodb.ca (accessed on 12 February 2021).
- Zhang, H.; Zhang, L.; Yu, X.; Xu, Y. The Biosynthesis Mechanism Involving 2,3-Pentanedione and Aminoacetone Describes the Production of 2-Ethyl-3,5-dimethylpyrazine and 2-Ethyl-3,6-dimethylpyrazine by Bacillus subtilis. J. Agric. Food Chem. 2020, 68, 3558–3567. [Google Scholar] [CrossRef]
- Rodrĩguez-Arcos, R.C.; Smith, A.C.; Waldron, K.W. Ferulic acid crosslinks in asparagus cell walls in relation to texture. J. Agric. Food Chem. 2004, 52, 4740–4750. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yang, Z.; Nishizawa, T.; Mori, T.; Saito, K. Top-down targeted metabolomics reveals a sulfur-containing metabolite with inhibitory activity against angiotensin-converting enzyme in asparagus officinalis. J. Nat. Prod. 2015, 78, 1179–1183. [Google Scholar] [CrossRef]
- Jaramillo-Carmona, S.; Rodriguez-Arcos, R.; Jiménez-Araujo, A.; López, S.; Gil, J.; Moreno, R.; Guillén-Bejarano, R. Saponin Profile of Wild Asparagus Species. J. Food Sci. 2017, 82, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Dawid, C.; Hofmann, T. Identification of sensory-active phytochemicals in asparagus (Asparagus officinalis L.). J. Agric. Food Chem. 2012, 60, 11877–11888. [Google Scholar] [CrossRef]
- van Geem, M.; Gols, R.; van Dam, N.M.; van der Putten, W.H.; Fortuna, T.; Harvey, J.A. The importance of aboveground-belowground interactions on the evolution and maintenance of variation in plant defense traits. Front. Plant Sci. 2013, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Herppich, W.B.; Huyskens-Keil, S.; Herppick, W.; Huyskens-Keil, S. Cell wall biochemistry and biomechanics of harvested white asparagus shoots as affected by temperature. Ann. Appl. Biol. 2008, 152, 377–388. [Google Scholar] [CrossRef]
- Hassan, M.N.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Bumke-Vogt, C. Flavonoid Glycosides in Brassica Species Respond to UV-B Depending on Exposure Time and Adaptation Time. Molecules 2021, 26, 494. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Farahani-Kofoet, R.D.; Zrenner, R.; Henze, A.; Witzel, K. Tissue-specific signatures of metabolites and proteins in asparagus roots and exudates. Hortic. Res. 2021, 8. [Google Scholar] [CrossRef]
- Hayes, P.Y.; Jahidin, A.H.; Lehmann, R.; Penman, K.; Kitching, W.; de Voss, J.J. Steroidal saponins from the roots of Asparagus racemosus. Phytochemistry 2008, 69, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Dawid, C.; Hofmann, T. Quantitation and bitter taste contribution of saponins in fresh and cooked white asparagus (Asparagus officinalis L.). Food Chem. 2014, 145, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; de Souza, L.P.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028. [Google Scholar] [CrossRef]
- Yi, T.G.; Yeoung, Y.R.; Choi, I.-Y.; Park, N.-I. Transcriptome analysis of Asparagus officinalis reveals genes involved in the biosynthesis of rutin and protodioscin. PLoS ONE 2019, 14, e0219973. [Google Scholar] [CrossRef] [Green Version]
- Fuentes-Alventosa, J.M.; Jaramillo, S.; Rodriguez-Gutierrez, G.; Cermeño, P.; Espejo, J.A.; Jiménez-Araujo, A.; Guillén-Bejarano, R. Flavonoid profile of green asparagus genotypes. J. Agric. Food Chem. 2008, 56, 6977–6984. [Google Scholar] [CrossRef]
- Wang, M.; Tadmor, Y.; Wu, Q.L.; Chin, C.K.; Garrison, S.A.; Simon, J.E. Quantification of protodioscin and rutin in asparagus shoots by LC/MS and HPLC methods. J. Agric. Food Chem. 2003, 51, 6132–6136. [Google Scholar] [CrossRef]
- Chambers, E., IV; Koppel, K. Associations of volatile compounds with sensory aroma and flavor: The complex nature of flavor. Molecules 2013, 18, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Creydt, M.; Hudzik, D.; Rurik, M.; Kohlbacher, O.; Fischer, M. Food Authentication: Small-Molecule Profiling as a Tool for the Geographic Discrimination of German White Asparagus. J. Agric. Food Chem. 2018, 66, 13328–13339. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Antoniou, C.; Rouphael, Y.; Kyratzis, A.C.; Kyriacou, M.C. Changes in the primary and secondary metabolome of male green asparagus (Asparagus officinalis L.) as modulated by sequential harvesting. Food Chem. 2021, 358. [Google Scholar] [CrossRef] [PubMed]
- Creydt, M.; Fischer, M. Mass-Spectrometry-Based Food Metabolomics in Routine Applications: A Basic Standardization Approach Using Housekeeping Metabolites for the Authentication of Asparagus. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Hashimoto, K.; Mori, T.; Toyooka, K.; Sudo, H.; Saito, K. Spatial metabolomics using imaging mass spectrometry to identify the localization of asparaptine in Asparagus officinalis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Magazù, S.; Migliardo, F.; Telling, M.T.F. The effect of partial replacement of maltodextrin with vegetable fibres in spray-dried white asparagus powder on its physical and aroma properties. Food Chem. 2021, 356, 129567. [Google Scholar] [CrossRef]
- Tikunov, Y.; Lommen, A.; de Vos, C.H.R.; Verhoeven, H.A.; Bino, R.J.; Hall, R.D.; Bovy, A.G. A novel approach for nontargeted data analysis for metabolomics. Large-scale profiling of tomato fruit volatiles. Plant Physiol. 2005, 139, 1125–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Simon, C.; Ammerlaan, B.; van den Berg, M.; van Duynhoven, J.; Jacobs, D.; Mumm, R.; Hall, R.D. Comparison of volatile trapping techniques for the comprehensive analysis of food flavourings by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2020, 1624, 461191. [Google Scholar] [CrossRef]
- de Vos, R.C.H.; Moco, S.; Lommen, A.; Keurentjes, J.J.B.; Bino, R.J.; Hall, R.D. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef]
- van Treuren, R.; van Eekelen, H.D.L.M.; Wehrens, R.; de Vos, R.C.H. Metabolite variation in the lettuce gene pool: Towards healthier crop varieties and food. Metabolomics 2018, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lommen, A. MetAlign: Interface-Driven, Versatile Metabolomics Tool for Hyphenated Full-Scan Mass Spectrometry Data Preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Tikunov, Y.M.; Laptenok, S.; Hall, R.D.; Bovy, A.; de Vos, R.C.H. MSClust: A tool for unsupervised mass spectra extraction of chromatography-mass spectrometry ion-wise aligned data. Metabolomics 2012, 8, 714–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehrens, R.; Hageman, J.A.; van Eeuwijk, F.; Kooke, R.; Flood, P.J.; Wijnker, E.; de Vos, R.C. Improved batch correction in untargeted MS-based metabolomics. Metabolomics 2016, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Duchesnay, E. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. Available online: http://jmlr.org/papers/v12/pedregosa11a.html (accessed on 6 April 2021).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Ournal R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]


| (a) Volatiles | |||||||
|---|---|---|---|---|---|---|---|
| Metabolite Name | CAS | RI | LOI | Metabolite Name | CAS | RI | LOI |
| Significantly More Abundant in Green | Significantly More Abundant in White | ||||||
| Methanethiol | 74-93-1 | 548 | 1 | Hexanal | 66-25-1 | 794 | 1 |
| Pentane | 109-66-0 | 567 | 1 | 1,3-Dimethylbenzene | 108-38-3 | 872 | 2 |
| 2-Methyl-3-buten-2-ol | 115-18-4 | 623 | 1 | 2-Heptanone | 110-43-0 | 890 | 2 |
| 2-Methylfuran | 534-22-5 | 623 | 1 | Hexanoic acid | 142-62-1 | 964 | 3 |
| 1-Penten-3-ol | 616-25-1 | 678 | 1 | 2-Pentylfuran | 3777-69-3 | 993 | 1 |
| 3-Pentanone | 96-22-0 | 689 | 2 | unidentified | - | 1050 | 4 |
| Pentanal | 110-62-3 | 692 | 1 | 1,3-Diethylbenzene | 108-38-3 | 1056 | 1 |
| 1-Pentanol | 71-41-0 | 758 | 1 | (E)-2-Octenal | 2548-87-0 | 1062 | 1 |
| 3-Methylbutanoic acid | 503-74-2 | 824 | 3 | 1,2-Dimethoxybenzene | 91-16-7 | 1146 | 1 |
| (E)-2-Hexenal | 6728-26-3 | 850 | 1 | (E,E)-2,4-Nonadienal | 5910-87-2 | 1223 | 2 |
| 3-Methylhexanal | 19269-28-4 | 864 | 2 | (E,E)-2,4-Decadienal | 25152-84-5 | 1325 | 2 |
| Heptanal | 111-71-7 | 900 | 1 | 2,6-Di-tert-butylbenzoquinone | 719-22-2 | 1477 | 3 |
| Octanal | 124-13-0 | 968 | 1 | ||||
| 1-Octen-3-one | 4312-99-6 | 977 | 2 | ||||
| 6-Methyl-5-hepten-2-one | 110-93-0 | 984 | 1 | ||||
| (b) Non-Volatiles | |||||||
| Metabolite Name | CAS | M | LOI | Metabolite Name | CAS | M | LOI |
| Detected only in green | Detected only in white | ||||||
| Unidentified | 356.1116 | 4 | Furostane-3,22,26-triol | 1052.541 | 2 | ||
| 3-O-Feruloylquinic acid | 1899-29-2 | 368.1113 | 2 | Unidentified | 1050.525 | 4 | |
| Nicotiflorin | 31921-42-3 | 594.1594 | 2 | Spirostane-3,17-diol | 756.4305 | 2 | |
| Kaempferol-sinapoyl-trihexose | 978.2655 | 3 | Shatavarin VI OR V | 886.4996 | 3 | ||
| Blumenol C glucoside | 62512-23-6 | 372.2211 | 3 | Spirost-5-ene-3,21-diol | 884.4779 | 3 | |
| Ononin | 486-62-4 | 430.1271 | 3 | ||||
| Significantly ore abundant in green | Significantly more abundant in white | ||||||
| Chlorogenic acid | 906-33-2 | 354.0957 | 2 | Unidentified | 682.2542 | 4 | |
| 3-p-Coumaroylquinic acid | 87099-71-6 | 338.1008 | 2 | Unidentified | 265.9923 | 4 | |
| Asparagusic acid ester isomer II | 312.0399 | 3 | Unidentified | 674.3497 | 4 | ||
| Asparagusic acid ester isomer IV | 312.0399 | 3 | Protodioscin | 55056-80-9 | 1048.547 | 2 | |
| Unidentified | 432.2002 | 4 | Shatavarin IX | 902.4943 | 2 | ||
| Unidentified | 430.1903 | 4 | Desglucomusennin | 868.4888 | 3 | ||
| Rutin | 153-18-4 | 610.1543 | 2 | ||||
| Isoquercetin | 482-35-9 | 464.0964 | 2 | ||||
| Unidentified | 414.1321 | 4 | |||||
| Unidentified | 444.1428 | 4 | |||||
| Unidentified | 676.3738 | 4 | |||||
| Quercetin-3-O-glucosylrutinoside | 772.2074 | 2 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pegiou, E.; Zhu, Q.; Pegios, P.; De Vos, R.C.H.; Mumm, R.; Hall, R.D. Metabolomics Reveals Heterogeneity in the Chemical Composition of Green and White Spears of Asparagus (A. officinalis). Metabolites 2021, 11, 708. https://doi.org/10.3390/metabo11100708
Pegiou E, Zhu Q, Pegios P, De Vos RCH, Mumm R, Hall RD. Metabolomics Reveals Heterogeneity in the Chemical Composition of Green and White Spears of Asparagus (A. officinalis). Metabolites. 2021; 11(10):708. https://doi.org/10.3390/metabo11100708
Chicago/Turabian StylePegiou, Eirini, Qingrui Zhu, Paraskevas Pegios, Ric C. H. De Vos, Roland Mumm, and Robert D. Hall. 2021. "Metabolomics Reveals Heterogeneity in the Chemical Composition of Green and White Spears of Asparagus (A. officinalis)" Metabolites 11, no. 10: 708. https://doi.org/10.3390/metabo11100708
APA StylePegiou, E., Zhu, Q., Pegios, P., De Vos, R. C. H., Mumm, R., & Hall, R. D. (2021). Metabolomics Reveals Heterogeneity in the Chemical Composition of Green and White Spears of Asparagus (A. officinalis). Metabolites, 11(10), 708. https://doi.org/10.3390/metabo11100708