Application of Thin-Film Microextraction to Analyze Volatile Metabolites in A549 Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
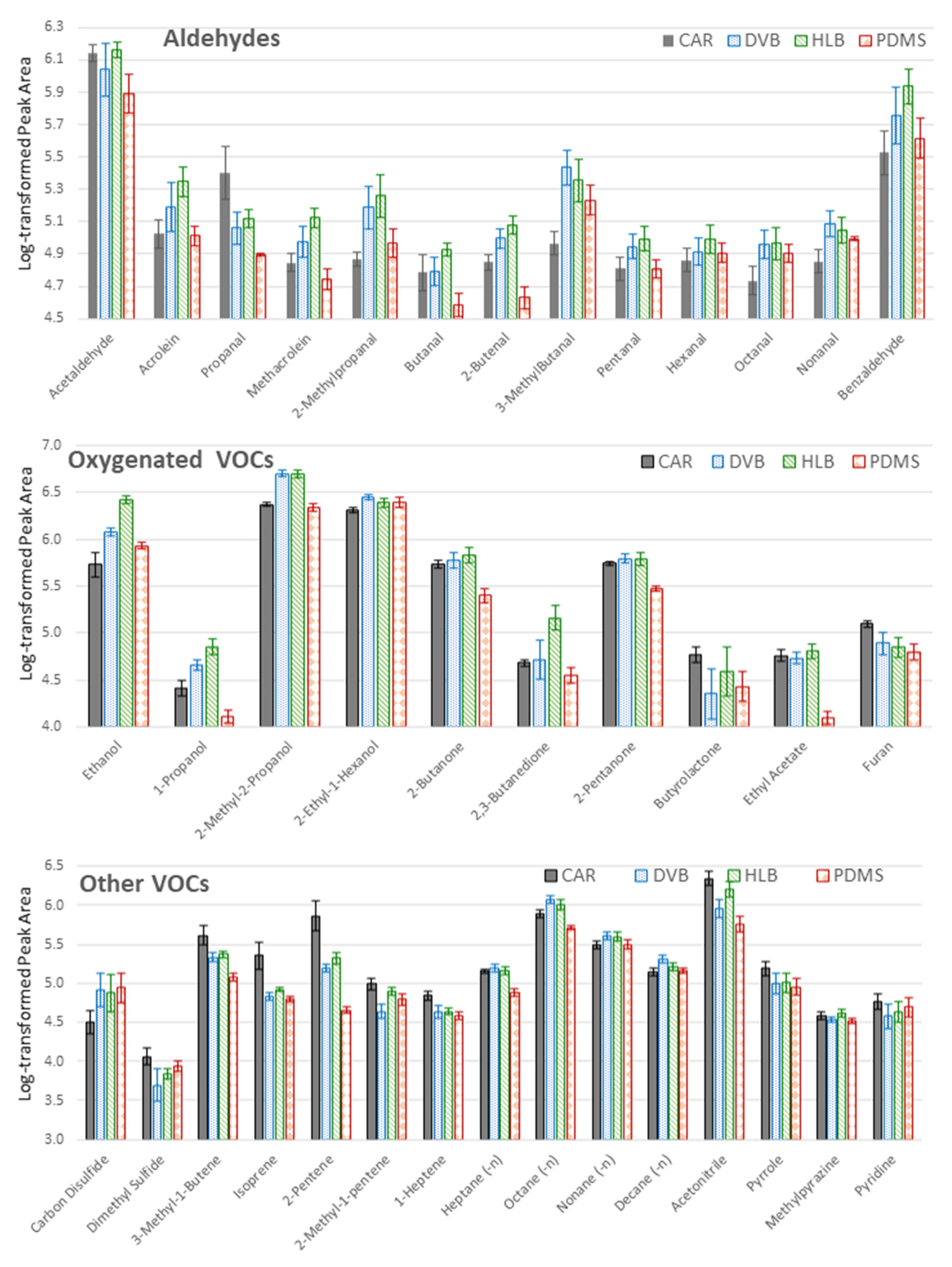
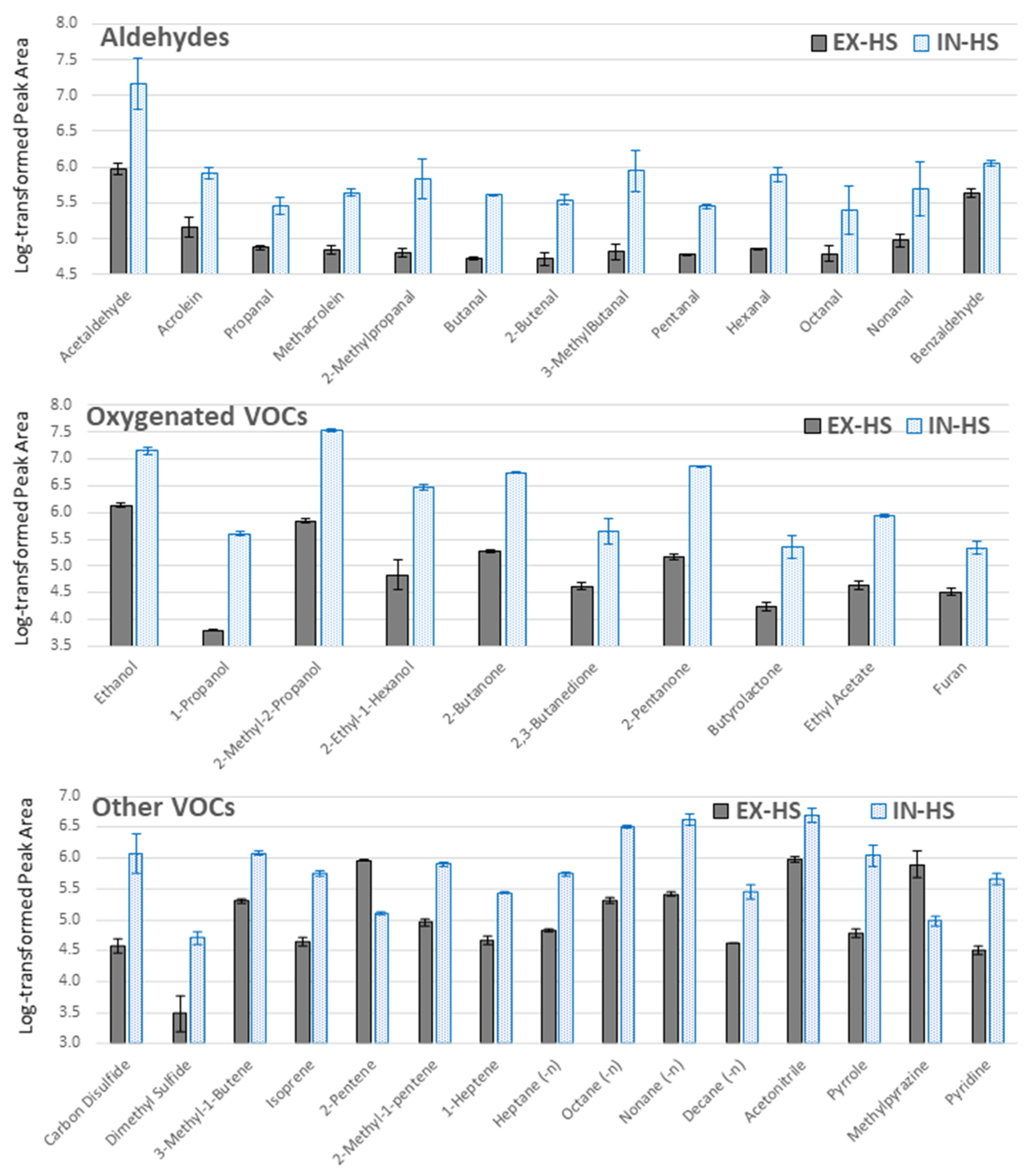
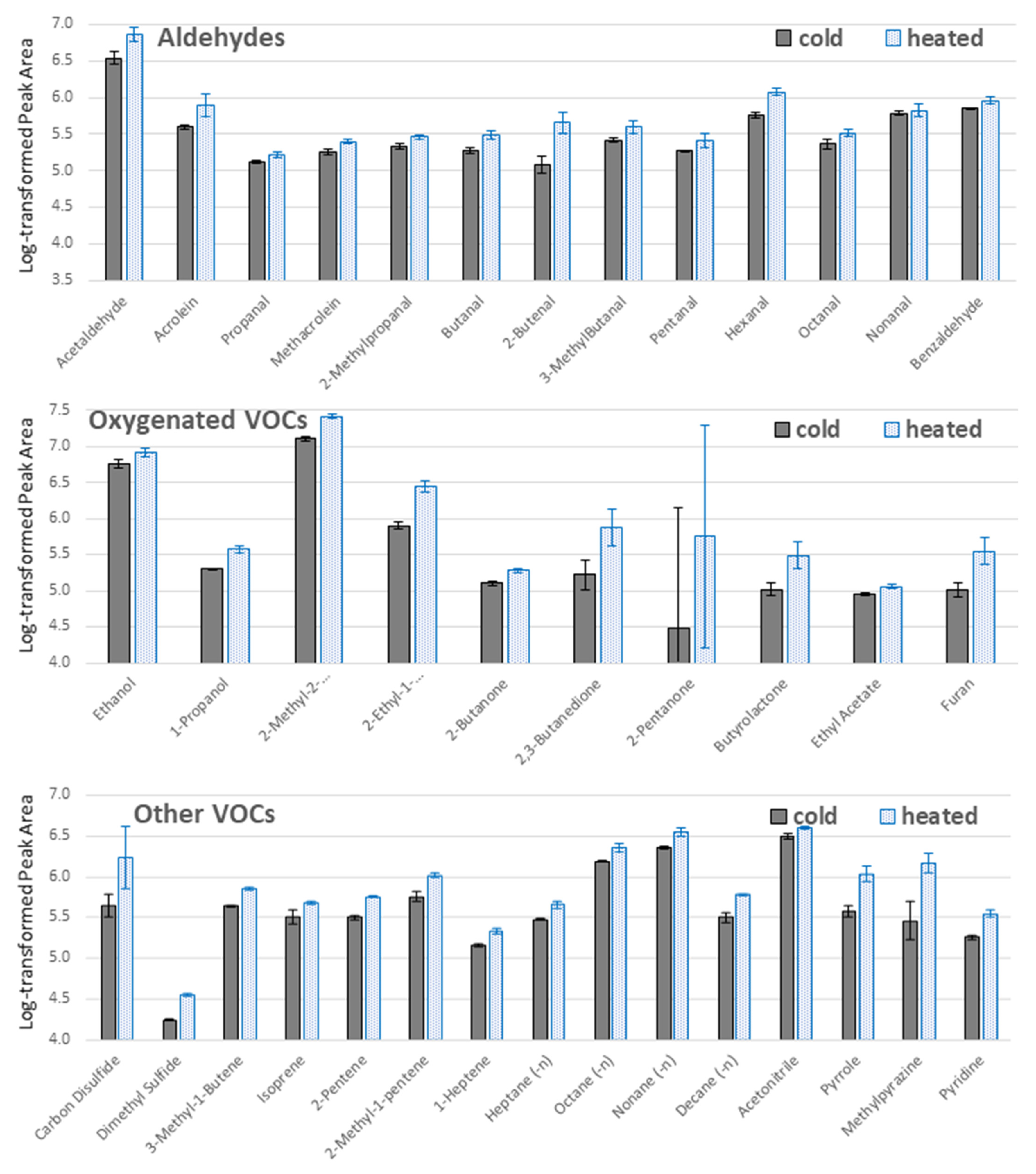
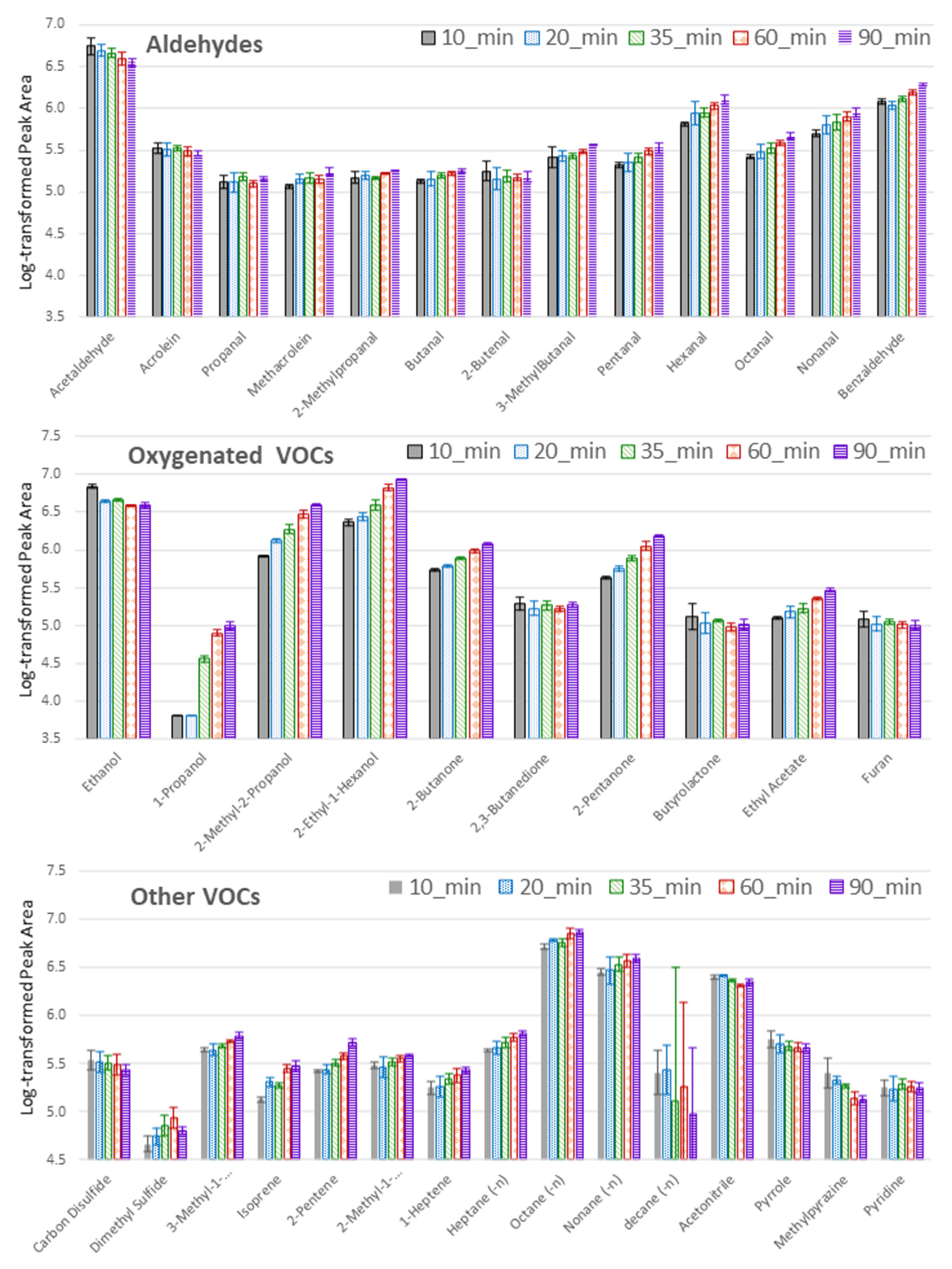
2.1. Optimization of the TFME Extraction Procedure
2.2. VOCs Released from A549 Cancer Cells
2.3. Study Limitations
3. Materials and Methods
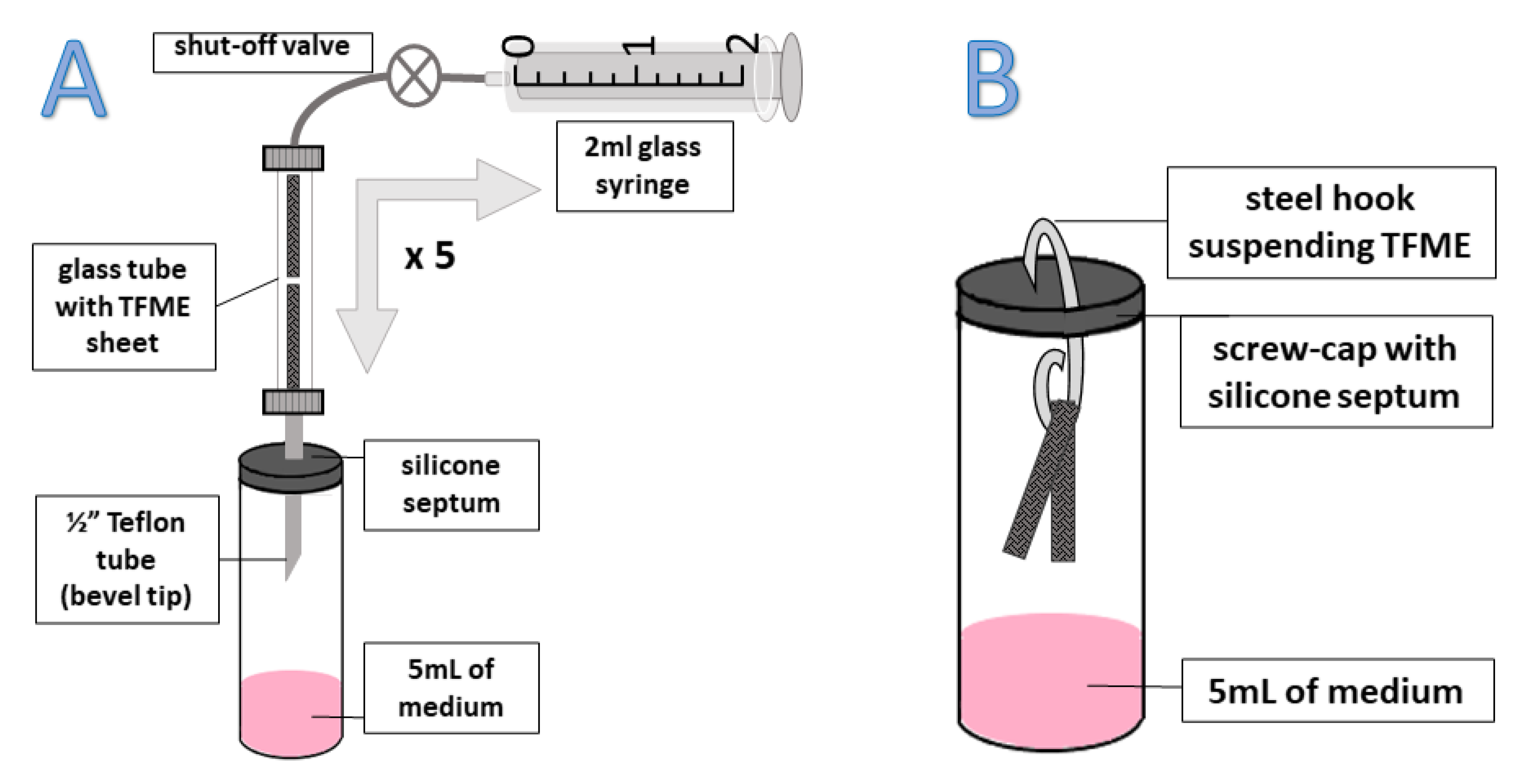
3.1. Thin-Film Microextraction Devices (TFME)
3.2. Thermal Desorption Gas Chromatography Mass Spectrometry (TD-GC-MS)
3.3. Cell Cultures
3.4. Sampling Optimization
3.4.1. Selection of Sorbent
3.4.2. Selection of Sampling Protocol
3.4.3. Extraction Temperature Optimization
3.4.4. Extraction Time Optimization
3.5. Calibration of Target VOCs
- σ—standard deviation of the Y-intercept of the regression line
- S—slope of the regression line
3.6. Data Processing and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Kocher, F.; Hilbe, W.; Seeber, A.; Pircher, A.; Schmid, T.; Greil, R.; Auberger, J.; Nevinny-Stickel, M.; Sterlacci, W.; Tzankov, A.; et al. Longitudinal analysis of 2293 NSCLC patients: A comprehensive study from the TYROL registry. Lung Cancer 2015, 87, 193–200. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid Volatolomics and Disease Detection. Angew. Chem.-Int. Ed. 2015, 54, 11036–11048. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid-phase microextraction with thermal-desorption using fused-silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Filipiak, W.; Bojko, B. SPME in clinical, pharmaceutical, and biotechnological research—How far are we from daily practice? Trac-Trends Anal. Chem. 2019, 115, 203–213. [Google Scholar] [CrossRef]
- Ligor, T.; Szeliga, J.; Jackowski, M.; Buszewski, B. Preliminary study of volatile organic compounds from breath and stomach tissue by means of solid phase microextraction and gas chromatography-mass spectrometry. J. Breath Res. 2007, 1, 016001. [Google Scholar] [CrossRef]
- Ma, H.Y.; Li, X.; Chen, J.M.; Wang, H.J.; Cheng, T.T.; Chen, K.; Xu, S.F. Analysis of human breath samples of lung cancer patients and healthy controls with solid-phase microextraction (SPME) and flow-modulated comprehensive two-dimensional gas chromatography (GC × GC). Anal. Methods 2014, 6, 6841–6849. [Google Scholar] [CrossRef]
- Bianchi, F.; Riboni, N.; Carbognani, P.; Gnetti, L.; Dalcanale, E.; Ampollini, L.; Careri, M. Solid-phase microextraction coupled to gas chromatography-mass spectrometry followed by multivariate data analysis for the identification of volatile organic compounds as possible biomarkers in lung cancer tissues. J. Pharm. Biomed. Anal. 2017, 146, 329–333. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Jung, C.; Rolff, J.; Fichtner, I.; Nehls, I. Investigation of cell culture volatilomes using solid phase micro extraction: Options and pitfalls exemplified with adenocarcinoma cell lines. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2015, 1006, 158–166. [Google Scholar] [CrossRef]
- Thriumani, R.; Zakaria, A.; Hashim, Y.; Jeffree, A.I.; Helmy, K.M.; Kamarudin, L.M.; Omar, M.I.; Shakaff, A.Y.M.; Adom, A.H.; Persaud, K.C. A study on volatile organic compounds emitted by in-vitro lung cancer cultured cells using gas sensor array and SPME-GCMS. BMC Cancer 2018, 18, 362. [Google Scholar] [CrossRef]
- Pyo, J.S.; Ju, H.K.; Park, J.H.; Kwon, S.W. Determination of volatile biomarkers for apoptosis and necrosis by solid-phase microextraction-gas chromatography/mass spectrometry: A pharmacometabolomic approach to cisplatin’s cytotoxicity to human lung cancer cell lines. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2008, 876, 170–174. [Google Scholar] [CrossRef]
- Alexovic, M.; Horstkotte, B.; Solich, P.; Sabo, J. Automation of static and dynamic non-dispersive liquid phase microextraction. Part 2: Approaches based on impregnated membranes and porous supports. Anal. Chim. Acta 2016, 907, 18–30. [Google Scholar] [CrossRef]
- Grandy, J.J.; Boyaci, E.; Pawliszyn, J. Development of a Carbon Mesh Supported Thin Film Microextraction Membrane As a Means to Lower the Detection Limits of Benchtop and Portable GC/MS Instrumentation. Anal. Chem. 2016, 88, 1760–1767. [Google Scholar] [CrossRef] [Green Version]
- Pawliszyn, J. (Ed.) Handbook of Solid Phase Microextraction; Chemical Industry Press of China: Beijing, China, 2012; pp. 1–478. [Google Scholar]
- Zanella, D.; Henket, M.; Schleich, F.; Dejong, T.; Louis, R.; Focant, J.-F.; Stefanuto, P.-H. Comparison of the effect of chemically and biologically induced inflammation on the volatile metabolite production of lung epithelial cells by GCxGC-TOFMS. Analyst 2020, 145, 5148–5157. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Zwaka, H.; Menzel, R.; Johnen, D.; Fischer-Tenhagen, C.; Rolff, J.; Nehls, I. In vitro cultured lung cancer cells are not suitable for animal-based breath biomarker detection. J. Breath Res. 2015, 9, 027103. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, T.; Ishii, R.; Onishi, H.; Ota, S. Elucidation of Biochemical Pathways Underlying VOCs Production in A549 Cells. Front. Mol. Biosci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Filipiak, W.; Sponring, A.; Filipiak, A.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. TD-GC-MS Analysis of Volatile Metabolites of Human Lung Cancer and Normal Cells In vitro. Cancer Epidemiol. Biomark. Prev. 2010, 19, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P.; et al. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014, 8, 027111. [Google Scholar] [CrossRef]
- Filipiak, W.; Mochalski, P.; Filipiak, A.; Ager, C.; Cumeras, R.; Davis, C.E.; Agapiou, A.; Unterkofler, K.; Troppmair, J. A Compendium of Volatile Organic Compounds (VOCs) Released By Human Cell Lines. Curr. Med. Chem. 2016, 23, 2112–2131. [Google Scholar] [CrossRef] [Green Version]
- Marcato, P.; Dean, C.A.; Giacomantonio, C.A.; Lee, P.W.K. Aldehyde dehydrogenase Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle 2011, 10, 1378–1384. [Google Scholar] [CrossRef]
- Watanabe, M. Polymorphic CYP genes and disease predisposition—What have the studies shown so far? Toxicol. Lett. 1998, 103, 167–171. [Google Scholar] [CrossRef]
- Mlakar, A.; Spiteller, G. Reinvestigation of Lipid-Peroxidation of Linolenic Acid. Biochim. Biophys. Acta-Lipids Lipid Metab. 1994, 1214, 209–220. [Google Scholar]
- Mitsui, T.; Kondo, T. Inadequacy of theoretical basis of breath methylated alkane contour for assessing oxidative stress. Clin. Chim. Acta 2003, 333, 91. [Google Scholar] [CrossRef]
- Grandy, J.J.; Singh, V.; Lashgari, M.; Gauthier, M.; Pawliszyn, J. Development of a Hydrophilic Lipophilic Balanced Thin Film Solid Phase Microextraction Device for Balanced Determination of Volatile Organic Compounds. Anal. Chem. 2018, 90, 14072–14080. [Google Scholar] [CrossRef]
- EMEA 2006: CPMP/ICH/381/95. ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 15 September 2021).
- MetaboAnalyst 5.0. Available online: https://www.metaboanalyst.ca (accessed on 18 June 2021).
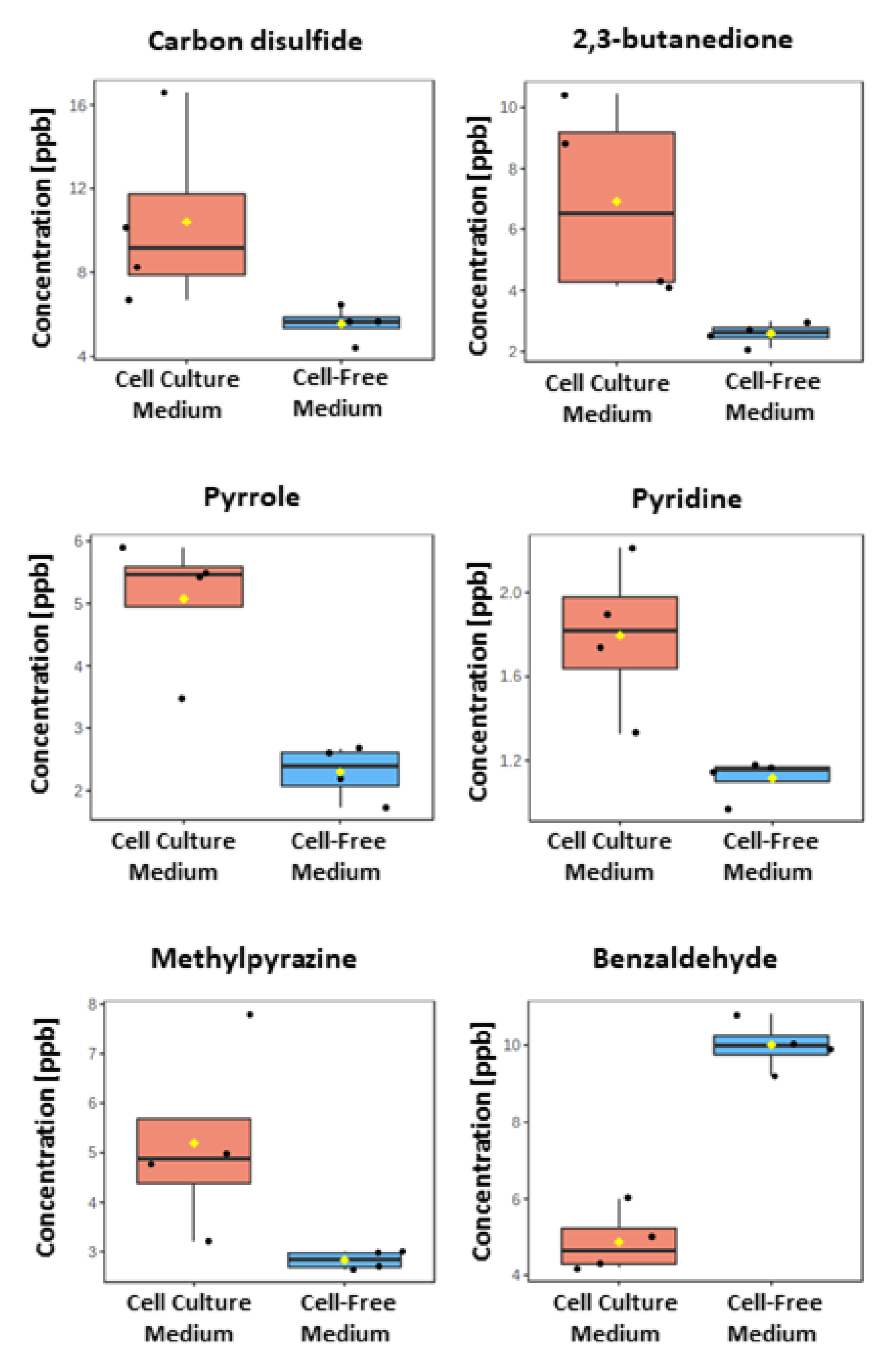
| Compound Name | CAS | R2 | LOD [ppb] | LOQ [ppb] | Cell Culture Medium | Cell-Free Medium | ||
|---|---|---|---|---|---|---|---|---|
| Mean Conc. [ppb] | Standard Deviation [ppb] | Mean Conc. [ppb] | Standard Deviation [ppb] | |||||
| Carbon Disulfide | 75-15-0 | 0.9957 | 1.360 | 4.121 | 10.423 | 4.343 | 5.552 | 0.851 |
| 2,3-Butanedione | 431-03-8 | 0.9880 | 2.280 | 6.909 | 6.918 | 3.184 | 2.581 * | 0.370 |
| Pyrrole | 109-97-7 | 0.9986 | 0.782 | 2.370 | 5.073 | 1.087 | 2.297 | 0.435 |
| Pyridine | 110-86-1 | 0.9986 | 0.763 | 2.313 | 1.796 * | 0.369 | 1.114 * | 0.097 |
| Methylpyrazine | 109-08-0 | 0.9948 | 1.496 | 4.533 | 5.191 | 1.916 | 2.827 * | 0.185 |
| Benzaldehyde | 100-52-7 | 0.9987 | 0.747 | 2.264 | 4.872 | 0.823 | 10.011 | 0.654 |
| Compound Name | CAS | Best TFME Type | Best Extraction Time [min] | Literature Finding | Literature Sample Prep. | Reference |
|---|---|---|---|---|---|---|
| 2,3-Butanedione | 431-03-8 | HLB | 10 | ex vivo human lung tissue | Tenax-CAR TD tubes | [19] |
| Benzaldehyde | 100-52-7 | HLB | 90 | A549 cells | CAR-DVB SPME fiber | [10] |
| ex vivo human lung tissue | CAR-DVB SPME fiber | [8] | ||||
| A549 cells | Graphite and C18 silica monolith monotrap | [17] | ||||
| ex vivo human lung tissue | Tenax-CAR TD tubes | [19] | ||||
| Carbon Disulfide | 75-15-0 | PDMS | 10 | |||
| Methylpyrazine | 109-08-0 | HLB | 10 | |||
| Pyridine | 110-86-1 | CAR | 35 | ex vivo human lung tissue | Tenax-CAR TD tubes | [18] |
| Pyrrole | 109-97-7 | CAR | 10 | A549 cells | CAR SPME fiber | [9] |
| A549 cells | Tenax-CAR TD tubes | [18] | ||||
| ex vivo human lung tissue | Tenax-CAR TD tubes | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipiak, W.; Jaroch, K.; Szeliska, P.; Żuchowska, K.; Bojko, B. Application of Thin-Film Microextraction to Analyze Volatile Metabolites in A549 Cancer Cells. Metabolites 2021, 11, 704. https://doi.org/10.3390/metabo11100704
Filipiak W, Jaroch K, Szeliska P, Żuchowska K, Bojko B. Application of Thin-Film Microextraction to Analyze Volatile Metabolites in A549 Cancer Cells. Metabolites. 2021; 11(10):704. https://doi.org/10.3390/metabo11100704
Chicago/Turabian StyleFilipiak, Wojciech, Karol Jaroch, Paulina Szeliska, Karolina Żuchowska, and Barbara Bojko. 2021. "Application of Thin-Film Microextraction to Analyze Volatile Metabolites in A549 Cancer Cells" Metabolites 11, no. 10: 704. https://doi.org/10.3390/metabo11100704
APA StyleFilipiak, W., Jaroch, K., Szeliska, P., Żuchowska, K., & Bojko, B. (2021). Application of Thin-Film Microextraction to Analyze Volatile Metabolites in A549 Cancer Cells. Metabolites, 11(10), 704. https://doi.org/10.3390/metabo11100704








