Abstract
Several differential panels of metabolites have been associated with the presence of metabolic syndrome and its related conditions, namely non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). This study aimed to perform a systematic review to summarize the most recent finding in terms of circulating biomarkers following NAFLD/NASH syndromes. Hence, the research was focused on NAFLD/NASH studies analysed by metabolomics approaches. Following Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines, a systematic search was conducted on the PubMed database. The inclusion criteria were (i) publication date between 2010 and 2021, (ii) presence of the combination of terms: metabolomics and NAFLD/NASH, and (iii) published in a scholarly peer-reviewed journal. Studies were excluded from the review if they were (i) single-case studies, (ii) unpublished thesis and dissertation studies, and (iii) not published in a peer-reviewed journal. Following these procedures, 10 eligible studies among 93 were taken into consideration. The metabolisms of amino acids, fatty acid, and vitamins were significantly different in patients affected by NAFLD and NASH compared to healthy controls. These findings suggest that low weight metabolites are an important indicator for NAFLD/NASH syndrome and there is a strong overlap between NAFLD/NASH and the metabolic syndrome. These findings may lead to new perspectives in early diagnosis, identification of novel biomarkers, and providing novel targets for pharmacological interventions.
1. Introduction
1.1. MetS General Characteristics
Metabolic syndrome (MetS) is a global epidemic that is leading to an increased risk of developing chronic diseases such as type 2 diabetes and cardiovascular diseases. The exact cause of MetS is not known, however the pathogenesis seems to be due to the presence of multiple factors including genetic predisposition, life-style habits, epigenetic modifications, and environmental expositions. Recent studies suggest that among risk factors, central obesity, hypertension, glucose intolerance, hypertriglyceridemia, and low serum high-density lipoprotein (HDL) have been considered important for the development and progression of this syndrome [1]. Awareness about the existence of the metabolic syndrome is relatively recent, since it was officially recognized by the World Health Organization (WHO) twenty-tree years ago (1998), when Alberti et al. [2] described this condition identifying as a mandatory criterion the presence of insulin resistance. The occurrence of at least two additional risk factors among obesity, dyslipidaemia, hypertension, and microalbuminuria [3] was also needed to attest the presence of MetS. In 2005, a new definition of MetS was shaped by the National Cholesterol Education Program (NCEP), the Adult Treatment Panel III (ATP III), the American Heart Association, and the National Heart Lung and Blood Institute [4]. MetS is present if three or more among the following five criteria are met: waist circumference over 101 cm (men) or 90 cm (women), blood pressure over 130/85 mmHg, fasting triglyceride (TG) level over 150 mg/dL, fasting high-density lipoprotein (HDL) cholesterol level less than 40 mg/dL (men) or 50 mg/dL (women), and fasting blood sugar over 100 mg/dL [5].
1.2. NAFLD and NASH
Accumulating evidence supports a metabolic association between MetS and non-alcoholic fatty liver disease (NAFLD) [6,7]. Their pathogenesis seems to have common pathophysiological mechanisms which involve cellular metabolism and has insulin resistance as a key factor. NAFLD represents a group of disorders that have in common the presence of fatty liver disease in individuals who do not consume alcohol or who consume very small quantities (less than 20 g of ethanol per week). NAFLD is considered the hepatic expression of MetS, with which it shares aetiology, prognosis, and treatment. It is characterized by an excessive accumulation of toxic lipids in the liver, including triglycerides, FFA, ceramides, and free cholesterol. NAFLD has become the most common cause of chronic liver disease in the United States, affecting over 3–5% of the population [8]. NAFLD may progress to an advanced stage named NASH (about 20–30% of individuals with NAFLD), in which the presence of inflammation, fibrosis, and cell damage can lead to cirrhosis and hepatocellular carcinoma (about 15% and 4–27% of individuals with NASH, respectively). However, it should be noted that NASH could progress to hepatocellular carcinoma in the absence of signs of cirrhosis. Currently, diagnosis is performed measuring the activity of hepatic enzymes, by examining the liver with ultrasound, computed tomography, and nuclear magnetic resonance spectroscopy. However, a clear correlation between the enzymatic alterations and the extent of steatosis do not exist, not even when is detected on liver biopsy. Liver biopsy is still considered the most informative examination, but, as it is not usually performed in subjects without alterations of the hepatic enzymes, the true prevalence and distribution of NAFLD is underestimated. Over the last few years, omics technologies have allowed us to obtain an integrated view of the NAFLD/NASH phenotype. The application of genome-wide associated study allowed the identification of several SNPs associated with MetS and NAFLD. Among these, the nonsynonymous rs738409 C/G variant in PNPLA3 (patatin-like phospholipase domain containing 3), which encodes the amino acid substitution I148M, was found as the major genetic component of NAFLD and NASH. The rs738409 was significantly associated with the accumulation of fat in the liver and with the histological disease severity and progression of NAFLD [9]. For this reason, it was considered a promising therapeutic target. However, its translational value from the bench to the bedside has been limited, as the reproducibility of these SNPs differed among groups of patients [10]. A step forward over the genomic era may be represented by the analysis of small metabolites and lipids contained in biofluids (plasma, serum, urine, and saliva). This approach is named metabolomics, and it may be particularly useful for the study of chronic metabolic diseases in which the phenotype is complex and dynamic, resulting from the occurrence of multiple interactions among genetic and environmental factors. For these reasons, metabolomics is expected to provide many more additional insights and clues to the mechanisms of biological processes and functions, and thus may increase our knowledge of the development and progression of the disease such as NAFLD and NASH. Therefore, this work aimed to conduct a systematic review of human studies on metabolite markers of MetS and to provide a list of shared metabolic pathways.
2. Methods
This systematic review follows the PRISMA guidelines and is reported in accordance with the PRISMA statement (http://www.prisma-statement.org/ accessed on 16 February 2021).
2.1. Search Strategy
A systematic search was conducted on PubMed for all publications with relation to metabolomics biomarkers of Metabolic syndrome reported from 2010 to July 2021, using the following combinations of terms: “Metabolomics or Metabonomics” and “Metabolic Syndrome” and “Human” and “Liver not Review” and “NAFLD/NASH”. Initially, titles and abstracts of all identified studies were screened and reviewed based on the established selection criteria.
2.2. Selection Criteria
English articles were selected based on their titles and abstracts for full-text review according to their relevance to the issue of interest. The following inclusion criteria were applied with no restriction to the bio-specimen used: identification of specific metabolites in NAFLD/NASH or Metabolic syndrome; identification of potential biomarkers of NAFLD/NASH or Metabolic syndrome diagnosis, or with a diagnosis of one or more traits of the disease; and level of standardization of the analytical platforms used and their limitations. Only metabolomics studies were included. Other “omics” results were excluded. In addition, reviews and studies made on animal models of Metabolic syndrome or on cell model systems were excluded. Systematic reviews and meta-analyses were excluded from the research.
2.3. Data Extraction
The selected studies were thoroughly examined, and the following information was extracted from each article: name of first author, year of publication, sample size, analytical platform used, use case, relevant biomarkers candidates, validation of biomarkers, statistical details, and relevant comments about the study. Data were independently extracted by three different reviewers (C.P, A.N., and L.I.) and disagreements regarding the selected information were solved by further review and discussion among them.
3. Results
A total of 94 studies were evaluated following the literature search (Figure 1).

Figure 1.
Flowchart of study selection. These articles were published between 2010 and 2021, the diagram shows the method by which relevant studies were retrieved from the databases, assessed, and selected, or excluded.
The full text was revised for 24 articles, and 13 were excluded from analysis after reading the full text (Table 1). The remaining 11 were considered relevant for inclusion in the systematic review. Therefore, we provided a narrative synthesis of the main results, organized by specific biochemical metabolism. As a second level, we organized results by the type of molecule identified.

Table 1.
Studies excluded after full text review.
3.1. NAFLD/NASH Biomarkers: Results from Case/Control Studies
Most of the studies were performed in populations aged between 7 and 80 years. Generally, NAFLD/NASH cases were compared with healthy controls (see Table 2). Biomarkers were mostly identified using targeted MS metabolomics or lipidomics on blood, urine, and saliva. About 100 different metabolites were identified and are presented in Table 2 with associated references and classified by families and direction of variation, as well as analytical methods for metabolomics/lipidomics and used statistical parameters/cofactors. The main classes were amino acids and derivatives, carbohydrates and derivatives, glycolysis related metabolites, glycerophospholipids, glycerolipids, sphingolipids, fatty acids, cholesterol and oxysterols, steroids, and peptides.

Table 2.
Selected Characteristics of Reviewed Studies on the Metabolomics of NAFLD and NASH syndromes.
3.2. Metabolism of Amino Acids and Derivatives
Three studies showed an increase in the concentration of alanine. Two of these studies, performed by Männisto et al. [11], and by Stechemesser et al. [12], showed an increase in alanine concentration in patients with MetS and NASH compared to healthy controls and with subjects with NAFLD. In particular, Stechemesser et al. [12] showed that the concentration of alanine was very different between the healthy control group and the MetS group, but did not discriminate when compared to subjects with high and normal iron parameters. The third study by Sookoian et al. [13] found an increased ratio of alanine/pyruvate in NAFLD patients compared to healthy controls. In particular, it was shown that hepatic transcriptional activity of aminotransferases (AST and ALT) was significantly upregulated in NAFLD patients and alanine was correlated with hepatic mRNA expression of GPT (gene encoding the cytosolic isoform ALT1), GPT2 (gene encoding the mitochondrial isoform ALT2), and GOT1 (gene encoding the cytoplasmic GOT). Furthermore, the L-alanine:pyruvate ratio was significantly correlated with BMI (R = 0.37, p = 0.01) even after adjustment for HOMA-IR and aminotransferase concentrations (β ± SE: 0.42 ± 0.18; p = 0.02). Six studies by Bhupathiraju et al. [14], Feldman et al. [15], Männisto et al. [11], Stechemesser et al. [12], Troisi et al. [16], and Masarone et al. [17] showed an increased concentration of branched-chain amino acids (BCAAs) in obese and NASH patients. Bhupathiraju et al. [14], while investigating the metabolomic profiles associated with distinct dietary patterns among a sample of Asian Indians living in the United States, found that western/nonvegetarian dietary pattern was positively associated with a high concentration of BCAAs.
Apparently, in contrast with the previous author, Feldam et al. [15] found an inverse correlation between obese subjects and BCAAs profile. However, the authors pointed out that obese patients had healthy livers, thus suggesting that obese patients with healthy liver have low blood concentrations of BCAAs. In agreement with Bhupathiraju et al. [14], the study performed by Mannisto et al. [11] discovered that the levels of serum BCAA were higher in NASH patients compared to patients with simple steatosis. The same variation in the concentration of BCAAs was observed by Stechemesser et al. [12] in MetS patients compared to healthy subjects. A significant increase in the BCAA isoleucine was also observed by Troisi et al. [16] in the saliva collected from paediatric obese patients compared to normal-weight subjects. Masarone et al. [17], in a recent study from 2021, showed how the BCAAs increased with the progress of the disease severity from steatosis disease to NASH, and NASH-cirrhosis. As for BCAA, the amino acid glutamate was found significantly increased in NAFLD patients by Lovric et al. [18] and Stechemesser et al. [12], whereas Sookoian et al. [13] were the only ones to identify an increased concentration of the amino acid kynurenine associated with serum concentrations of aminotransaminases and with BMI and HOMA-IR.
3.3. Metabolism of Fatty Acids and Derivatives
Lovric et al. [17] identified an increase in the concentration of sphingomyelin (SM) at the level of the ectopic adipose tissue. From the SM pool, diSM (18:0) stranded out with the highest positive correlation towards ectopic fat depots. A group of unsaturated fatty acids correlated with the metabolic status of obese individuals was found by Ni et al. [19]. The metabolites dihomo-gamma-linolenic acid (DGLA) and palmitoleic acid resulted significantly elevated in overweight/obese subjects with diabetes compared to their healthy counterparts. According to the authors, these metabolites were also able to predict the future development of MetS. In contrast to the previous authors, both Zhou et al. [9] and Feldman et al. [15] found a decreased concentration of lipids in NASH/NAFLD patients. In particular, the study of Zhou et al. 2016 [20] reported a reduction in the concentration of lysophosphocholine (16:0) in subjects with NASH compared to subjects without NASH, while Feldman et al. [15] found that low concentrations of some PCs (acyl–alkyl PCs) and sphingolipids were characteristic of obese NAFLD patients compared to healthy obese or lean subject.
3.4. Metabolism of Vitamin and Ketone Bodies
The alteration of retinoids was revealed by Zhong et al. [21]. The authors highlighted a reduction in the concentration of all-trans-retinyl palmitate-d4 (RP), all-trans-retinoic acid (atRA), 4-oxo- atRA, and 13-cisRA in subjects with NASH compared to controls. The NASH group showed also significant reduction in the ketone bodies β-hydroxybutyrate (β-OHB) (p-value = 0.004) and acetoacetate (p-value = 0.018) as demonstrated by Mannisto et al. [11]. In particular, lower levels of β-OHB were associated with the NASH predicting score p = 0.001.
4. Discussion
Cellular metabolism plays a crucial role in both health and disease, mirroring interactions between the host genome and the environment. Environmental and lifestyle factors have the potential to alter the individual metabolic phenotype both directly, by inducing perturbations in various metabolic pathways, and indirectly, by promoting epigenetic changes, which in turn lead to changes in gene expression, transcripts, and ultimately in the metabolic profile of a given cell, tissue, or biological fluid [22]. In the present systematic review, 10 studies matching with certain inclusive criteria were analysed, aiming to highlight common potential metabolites that characterized the onset or progression of the MetS, with a particular focus on metabolic abnormalities in the liver such as NAFLD and NASH syndromes. The discovery of new biomarkers in the study of the NAFLD/NASH could offer new investigative tools allowing an early diagnosis of the disease and consequently improving the prognosis. Furthermore, they could better clarify the mechanisms underlying the progression of the disease and eventually allow the identification of new possible therapeutic approaches. The metabolic profile identified by the considered studies was divided into subgroups to better evaluate the similarities among the class of metabolites.
4.1. BCAAs and Kynurenine
Half of the studies taken into consideration in this systematic review have demonstrated a positive correlation between circulating BCAAs (isoleucine, leucine, and valine) and NAFLD/NASH syndromes. The analysis of the adipose tissue demonstrated that obesity and insulin resistance can induce a decrease in the BCAAs intracellularly and an increased concentration in blood of the same aminoacids [11,12,14,15,16]. Several previous studies have suggested that BCAAs are predictors of insulin resistance and cardio-metabolic risk and are directly correlated with the accumulation of lipids in the liver [23,24,25]. In large-scale cohorts, BCAAs have been inversely associated with insulin sensitivity [25] and directly associated with insulin resistance, fasting blood glucose levels, and TG concentrations [24]. Animal studies have shown that a diet rich in BCAAs can cause significant liver damage, oxidative stress, and hepatocyte apoptosis [26]. The mechanisms determining liver damage are not entirely clear. However, Zhang et al. hypothesized that in adipocytes, BCAA activates AMPKα2 (Adenosine monophosphate (AMP)-activated protein kinase) and stimulates lipolysis, increasing plasma free fatty acids (FFA), and therefore the accumulation in the liver. In the liver, BCAA activates mTOR, which inhibits autophagy and the FFA to triglycerides conversion, blocking the hepatic outflow pathway of FFAs and thus intensifying the lipotoxicity of FFAs. Furthermore, the blockade of autophagy increased cell death by apoptosis [26]. An increase in BCAAs metabolism can cause the accumulation of catabolic intermediates and incomplete oxidation of fatty acids and glucose leading to mitochondrial dysfunction of pancreatic B-cells. Acylcarnitine C3 and C5, generated by the catabolism of BCAAs in the liver and skeletal muscle, have been associated with the direct onset of obesity and insulin resistance [14]. In conclusion, it seems that a high concentration of circulating BCAAs may be responsible for the switch from the lean to NAFLD/NASH phenotype [26]. Another metabolite, kynurenine, was found to be increased in subjects with Metabolic Syndrome. An alteration of the metabolic pathway of tryptophan/kynurenine [12,13], with a consequent increase in plasma levels of kynurenine, seems to be associated with apoptosis, pro-oxidant effects, and an increase in inflammation mediated by the arachidonic acid via [27].
4.2. Carbohydrates
The carbohydrate metabolism was largely impaired in obese individuals, as demonstrated by the study performed by Lovric et al. [18]. From a biological point of view, the increase in glucose concentration in the blood of obese patients can be determined by the overall adipose tissue, by the increase in IR, and the reduced ability of the adipose tissue to store glucose [28,29]. The product of glycolysis, pyruvate, was also found to increase in obese individuals. A possible explanation involves the conversion of pyruvate into lactate, which is the main precursor of gluconeogenesis during anaerobic glycolysis, which is over-regulated among obese individuals. Despite the interesting findings and assumptions of the study, glucose was the only metabolite that had significant associations with all ectopic fat stores.
4.3. Fatty Acids and Derivatives
Reduced levels of phosphatidylcholine (PC) and sphingolipids have been found in NAFLD patients [15]. A study on human adipocyte cultures from healthy obese patients and sick obese patients showed similar results [30]. Phospholipids are substrates necessary for the synthesis of triglycerides in the liver. A study based on an animal model of metabolic syndrome has demonstrated that reduced PC concentrations may be caused by an increase in adipocyte turnover [31]. In fact, it is hypothesized that unique mutations in the Ccna2 promoter of obese mice influence the expression of the Ccna2 gene in adipose tissue, resulting in increased mitotic activity of adipocytes [31]. In obese patients, a high turnover of adipocytes has been highlighted, as well as an association between adipocyte hypertrophy and metabolic complications [32]. This could explain the reduction in PC, and sphingolipid levels in NAFLD patients. The study by Zhou et al. 2016 [20] showed a reduction in SM levels in patients with NASH. According to the study, this alteration appeared to promote cellular stress, mitochondrial dysfunction, and alteration of the insulin-signalling pathway [33], which is closely associated with the etiopathogenesis of the MetS. The obese status was positively correlated with two unsaturated fatty acids (UFA) DGLA and PA as showed by Ni et al. [19]. Both UFAs appeared to be good inflammation markers useful in predicting the risk of developing metabolic syndrome and monitoring the metabolic status of overweight/obese individuals. In particular, a high concentration of PA is related to the augmented activity of stearoyl-CoA desaturase and hepatic de novo lipogenesis [34] which, in turn, leads to an increased synthesis of diacylglycerol (DAG), which contributes to the inflammatory mechanism through the release of arachidonic acid (AA). DGLA represents a pro-inflammatory agent that stimulates the increase in prostaglandins and leukotrienes that is observed in obesity and MetS. In agreement with Ni et al. [19], the study performed by Kurotani K et al. [35] showed that a high percentage of DGLA was associated with high concentrations of C reactive protein, a sensitive marker of inflammation associated with insulin resistance and T2D. Furthermore, the longitudinal study by Ni et al. showed that fasting UFA concentrations were elevated up to 10 years before the onset of the metabolic syndrome [19]. Surgical or dietary interventions in obese patients showed a significant reduction in UFA. UFAs, compared to saturated fatty acids (SFAs), decreased significantly after weight loss interventions, while they increased more significantly in obese subjects with MetS. These results suggest a more important role of UFAs than SFAs in determining the metabolic state of the individual. However, there is contradictory evidence regarding the effects of high SFA intake on the risk of developing type 2 diabetes mellitus [36].
4.4. Other Metabolites
The study performed by Männisto et al. [11] showed a reduction in ketone body levels (β-OHB and acetoacetate) in individuals with NASH compared to individuals with NAFLD. This could be justified by the fact that low levels of FFA, characteristic of the patient with NASH, would lead to a reduction in beta-oxidation and consequently to a reduction in circulating ketone bodies. However, a reduction in FFA has not been fully demonstrated. In several studies, the FFA concentration in individuals with NASH was increased compared to healthy controls and there were no differences between NAFLD and NASH [37]. In addition, genetic alterations of the genes ACAT1 and BDH1 that are involved in the regulation of ketolysis were found to be over-expressed in the liver of individuals with NASH. On the one hand, these results may suggest that the reduction in ketone bodies in individuals with NASH is determined by an increased ketolysis necessary to meet the increased need for acetyl-CoA to feed the Krebs cycle [38]. This hypothesis was also supported by the discovery that the levels of citrate, formed in the Krebs cycle from acetyl-CoA [39], were reduced in individuals with NASH. The reduction in the levels of ketone bodies and citrate in patients with NASH associated with an alteration in the hepatic expression of genes involved in ketolysis suggest a mitochondrial dysfunction typical of the patient with NASH compared to the patient with NAFLD. On the other hand, a study in which the patients were treated with a medium-chain fatty acids rich diet, showed an increase in the urinary concentration of metabolites of the Krebs cycle after twelve weeks [40]. These metabolites, and in particular citrate, seemed to be involved in the onset of obesity and related metabolic complications by increasing the synthesis of de novo fatty acids [41].
Youm et al. [41] highlighted a potential protective role of β-hydroxybutyrate in inhibiting the NLRP3 inflammasome and reducing the production of IL1β and IL-18 in human monocytes [42]. Several other studies have also shown an alteration in the concentration of other metabolites derived from the Krebs cycle. The study by Sookoian et al. 2016 [13] showed a reduction in citraconic acid and fumaric acid levels in subjects with NAFLD compared to healthy controls. The study by Troisi et al. 2018 [16] showed a reduction in citraconic acid levels in obese subjects compared to healthy controls. Overall, the data indicate an alteration of the Krebs cycle in metabolically ill patients, and this alteration seems to be more marked in subjects characterized by an advanced stage of the disease. In Figure 2, the different pathways and metabolites potentially involved in NAFLD/NASH have been summarized. Furthermore, a brief summary of principal metabolites and their potential role is reported in Table 3.
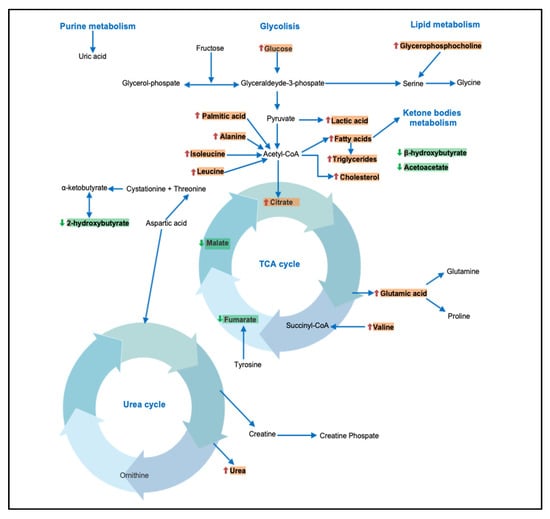
Figure 2.
A summary of different pathways potentially involved in NAFLD/NASH. Red arrow: increase in metabolite concentration. Green arrow: decrease in metabolite concentration.

Table 3.
Brief tabular summary of principal metabolites and their potential role.
5. Conclusions
Early diagnosis of NAFLD, NASH, and MetS is a relevant topic. This systematic review indicates that the metabolomics approach can identify new biomarkers of these conditions and suggest which are the principal altered metabolic pathways. For the future, there is a need to validate how sensitive these biomarkers are. Further investigations in large and well-characterized cohorts are recommended to gain new insights into the pathomechanisms of these conditions. This will also be useful to improve targeted therapies. Overall, a better understanding of the pathogenesis and the identification of new early biomarkers of MetS will be of value for this highly diffuse condition worldwide, often undiagnosed or diagnosed late.
Author Contributions
Conceptualization, C.P. and A.N.; investigation, C.P., A.N., and L.I.; writing—original draft preparation, C.P., A.N., and M.D.; writing—review and editing, V.P.L., V.F., and S.M.; supervision, C.P., A.N., and L.A.; and project administration, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data is available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lent-Schochet, D.; McLaughlin, M.; Ramakrishnan, N.; Jialal, I. Exploratory metabolomics of metabolic syndrome: A status report. World J. Diabetes 2019, 10, 23–36. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications, part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Cornier, M.A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Grund, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Cleeman, J.I.; Grundy, S.M.; Becker, D.; Clark, L. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar]
- Hamaguchi, M.; Kojima, T.; Takeda, N.; Nakagawa, T.; Taniguchi, H.; Fujii, K.; Omatsu, T.; Nakajima, T.; Sarui, H.; Shimazaki, M.; et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2005, 143, 722–728. [Google Scholar] [CrossRef]
- Hanley, A.J.; Williams, K.; Festa, A.; Wagenknecht, L.E.; D’Agostino, R.B.; Haffner, S.M. Liver markers and development of the metabolic syndrome: The insulin resistance atherosclerosis study. Diabetes 2005, 54, 3140–3147. [Google Scholar] [CrossRef] [Green Version]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Sookoian, S.; Pirola, C.J. Genetic predisposition in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2017, 23, 1–12. [Google Scholar] [CrossRef]
- Lind, L. Genome-Wide Association Study of the Metabolic Syndrome in UK Biobank. Metab. Syndr. Relat. Disord. 2019, 17, 505–511. [Google Scholar] [CrossRef]
- Männistö, V.T.; Simonen, M.; Hyysalo, J.; Soininen, P.; Kangas, A.J.; Kaminska, D.; Matte, A.K.; Venesmaa, S.; Käkelä, P.; Kärjä, V.; et al. Ketone body production is differentially altered in steatosis and non-alcoholic steatohepatitis in obese humans. Liver Int. 2015, 35, 1853–1861. [Google Scholar] [CrossRef]
- Stechemesser, L.; Eder, S.K.; Wagner, A.; Patsch, W.; Feldman, A.; Strasser, M.; Auer, S.; Niederseer, D.; Huber-Schönauer, U.; Paulweber, B.; et al. Metabolomic profiling identifies potential pathways involved in the interaction of iron homeostasis with glucose metabolism. Mol. Metab. 2016, 6, 38–47. [Google Scholar] [CrossRef]
- Sookoian, S.; Castaño, G.O.; Scian, R.; Fernández Gianotti, T.; Dopazo, H.; Rohr, C.; Gaj, G.; San Martino, J.; Sevic, I.; Flichman, D.; et al. Serum aminotransferases in nonalcoholic fatty liver disease are a signature of liver metabolic perturbations at the amino acid and Krebs cycle level. Am. J. Clin. Nutr. 2016, 103, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Bhupathiraju, S.N.; Guasch-Ferré, M.; Gadgil, M.D.; Newgard, C.B.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; Scholtens, D.M.; Hu, F.B.; Kanaya, A.M.; et al. Dietary Patterns among Asian Indians Living in the United States Have Distinct Metabolomic Profiles That Are Associated with Cardiometabolic Risk. J. Nutr. 2018, 148, 1150–1159. [Google Scholar] [CrossRef]
- Feldman, A.; Eder, S.K.; Felder, T.K.; Paulweber, B.; Zandanell, S.; Stechemesser, L.; Schranz, M.; Strebinger, G.; Huber-Schönauer, U.; Niederseer, D.; et al. Clinical and metabolic characterization of obese subjects without non-alcoholic fatty liver: A targeted metabolomics approach. Diabetes Metab. 2019, 45, 132–139. [Google Scholar] [CrossRef]
- Troisi, J.; Belmonte, F.; Bisogno, A.; Pierri, L.; Colucci, A.; Scala, G.; Cavallo, P.; Mandato, C.; Di Nuzzi, A.; Di Michele, L.; et al. Metabolomic Salivary Signature of Pediatric Obesity Related Liver Disease and Metabolic Syndrome. Nutrients 2019, 26, 274. [Google Scholar] [CrossRef] [Green Version]
- Masarone, M.; Troisi, J.; Aglitti., A.; Torre, P.; Colucci, A.; Dallio, M.; Federico, A.; Balsano, C.; Persico, M. Untargeted metabolomics as a diagnostic tool in NAFLD: Discrimination of statosis, steatohepatitis and cirrhosis. Metabolomics 2021, 17, 1–13. [Google Scholar] [CrossRef]
- Lovric, A.; Granér, M.; Bjornson, E.; Arif, M.; Benfeitas, R.; Nyman, K.; Ståhlman, M.; Pentikäinen, M.O.; Lundbom, J.; Hakkarainen, A.; et al. Characterization of different fat depots in NAFLD using inflammation-associated proteome, lipidome and metabolome. Sci. Rep. 2018, 8, 14200. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Zhao, L.; Yu, H.; Ma, X.; Bao, Y.; Rajani, C.; Loo, L.W.; Shvetsov, Y.B.; Yu, H.; Chen, T.; et al. Circulating Unsaturated Fatty Acids Delineate the Metabolic Status of Obese Individuals. EBioMedicine 2015, 2, 1513–1522. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Orešič, M.; Leivonen, M.; Gopalacharyulu, P.; Hyysalo, J.; Arola, J.; Verrijken, A.; Francque, S.; Van Gaal, L.; Hyötyläinen, T.; et al. Noninvasive Detection of Nonalcoholic Steatohepatitis Using Clinical Markers and Circulating Levels of Lipids and Metabolites. Clin. Gastroenterol. Hepatol. 2016, 14, 1463–1472. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Kirkwood, J.; Won, K.J.; Tjota, N.; Jeong, H.; Isoherranen, N. Characterization of Vitamin A Metabolome in Human Livers with and Without Nonalcoholic Fatty Liver Disease. J. Pharm. Exp. Ther. 2019, 370, 92–103. [Google Scholar] [CrossRef]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathé, E.A. Metabolomics and Multi-Omics Integration: A Survey of Computational Methods and Resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef]
- Haufe, S.; Witt, H.; Engeli, S.; Kaminski, J.; Utz, W.; Fuhrmann, J.C.; Rein, D.; Schulz-Menger, J.; Luft, F.C.; Boschmann, M.; et al. Branched-chain and aromatic amino acids, insulin resistance and liver specific ectopic fat storage in overweight to obese subjects. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 637–642. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’yasova, D.; Chen, Y.D.; Haffner, S.M.; Hanley, A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Oxenkrug, G.F. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann. N. Y. Acad. Sci. 2010, 1199, 1–14. [Google Scholar] [CrossRef]
- Park, S.; Sadanala, K.C.; Kim, E.K.A. Metabolomic Approach to Understanding the Metabolic Link between Obesity and Diabetes. Mol. Cells 2015, 38, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, R.; Gravenstein, K.S.; Metter, E.J.; Egan, J.M.; Ferrucci, L.; Chia, C.W. Selective contribution of regional adiposity, skeletal muscle, and adipokines to glucose disposal in older adults. J. Am. Geriatr. Soc. 2012, 60, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Böhm, A.; Halama, A.; Meile, T.; Zdichavsky, M.; Lehmann, R.; Weigert, C.; Fritsche, A.; Stefan, N.; Königsrainer, A.; Häring, H.U.; et al. Metabolic signatures of cultured human adipocytes from metabolically healthy versus unhealthy obese individuals. PLoS ONE 2014, 9, e93148. [Google Scholar] [CrossRef]
- Schäfer, N.; Yu, Z.; Wagener, A.; Millrose, M.K.; Reissmann, M.; Bortfeldt, R.; Dieterich, C.; Adamski, J.; Wang-Sattler, R.; Illig, T.; et al. Changes in metabolite profiles caused by genetically determined obesity in mice. Metabolomics 2014, 10, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Hanamatsu, H.; Ohnishi, S.; Sakai, S.; Yuyama, K.; Mitsutake, S.; Takeda, H.; Hashino, S.; Igarashi, Y. Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr. Diabetes 2014, 4, e141. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Campos, H.; McGarvey, S.; Wu, Z.; Goldberg, R.; Baylin, A. Adipose tissue palmitoleic acid and obesity in humans: Does it behave as a lipokine? Am. J. Clin. Nutr. 2011, 93, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Kurotani, K.; Sato, M.; Ejima, Y.; Nanri, A.; Yi, S.; Pham, N.M.; Akter, S.; Poudel-Tandukar, K.; Kimura, Y.; Imaizumi, K.; et al. High levels of stearic acid, palmitoleic acid, and dihomo-γ-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr. Res. 2012, 32, 669–675. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Bechmann, L.P.; Kocabayoglu, P.; Sowa, J.P.; Sydor, S.; Best, J.; Schlattjan, M.; Beilfuss, A.; Schmitt, J.; Hannivoort, R.A.; Kilicarslan, A.; et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology 2013, 57, 1394–1406. [Google Scholar] [CrossRef]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Mulholland, A.J.; Richards, W.G. Acetyl-CoA enolization in citrate synthase: A quantum mechanical/molecular mechanical (QM/MM) study. Proteins 1997, 27, 9–25. [Google Scholar] [CrossRef]
- Amer, B.; Clausen, M.R.; Bertram, H.C.; Bohl, M.; Nebel, C.; Zheng, H.; Skov, T.; Larsen, M.K.; Gregersen, S.; Hermansen, K.; et al. Consumption of Whey in Combination with Dairy Medium-Chain Fatty Acids (MCFAs) may Reduce Lipid Storage due to Urinary Loss of Tricarboxylic Acid Cycle Intermediates and Increased Rates of MCFAs Oxidation. Mol. Nutr. Food Res. 2017, 61, 1601048. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.B.; Vagelos, P.R. The mechanism of tricarboxylic acid cycle regulation of fatty acid synthesis. J. Biol. Chem. 1962, 237, 1787–1792. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).