Clinical Predictors of Mortality and Critical Illness in Patients with COVID-19 Pneumonia
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics of the Surviving and Non-Surviving COVID-19 Patients
2.2. Association between Risk Factors and Mortality
2.3. Correlation between Blood Tests, Severity of Pneumonia and Mortality in COVID-19 Patients
2.4. Discriminant Analysis with Diagnostic Accuracy of the Correlations between Clinical Parameters and Survival
2.5. Correlation between the 4-C Score, Cytokines Storm Syndrome, Liver Injury, ARDS and Mortality in COVID-19 Patients
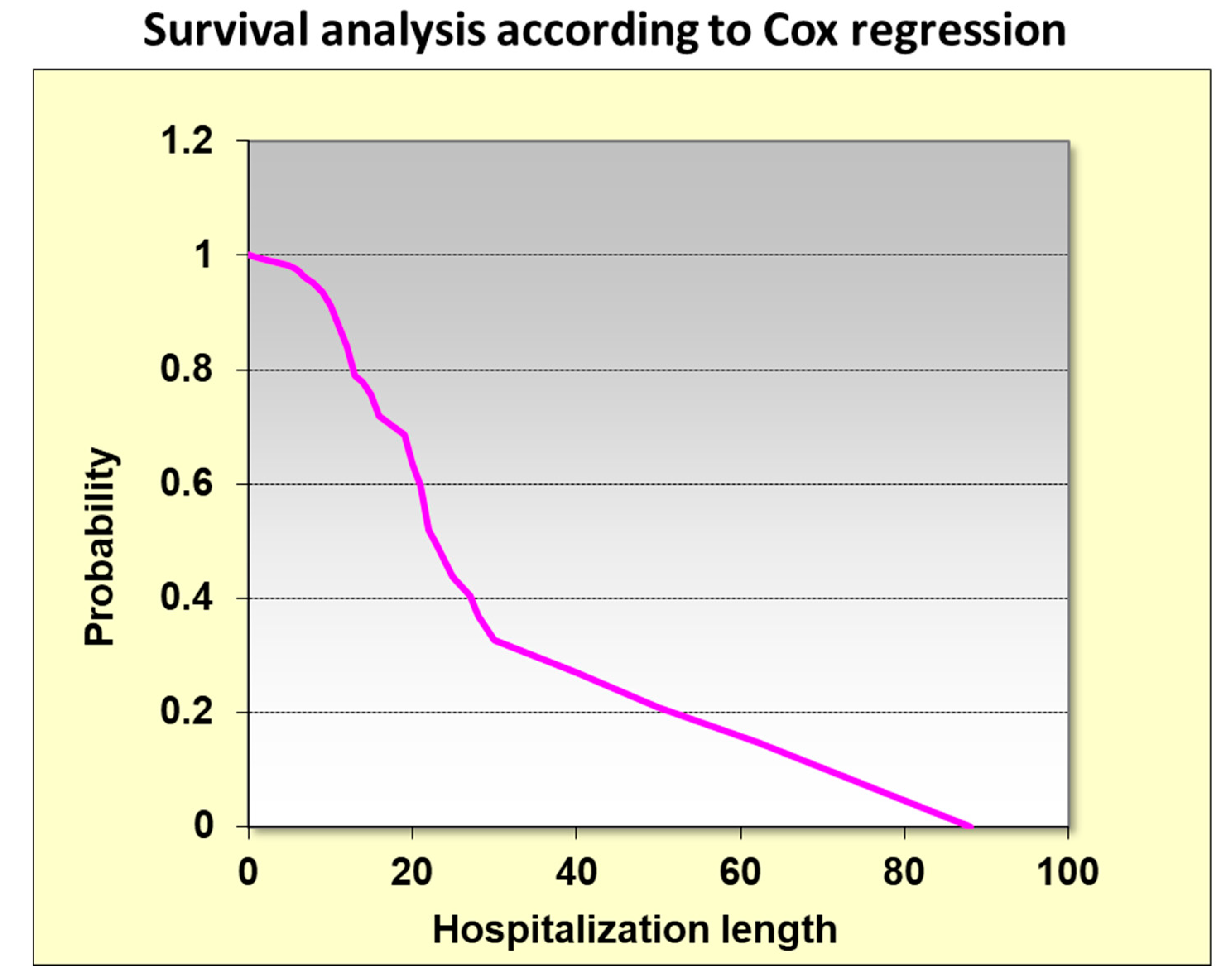
2.6. Kaplan Meir Survival Analysis According to HDL Classes
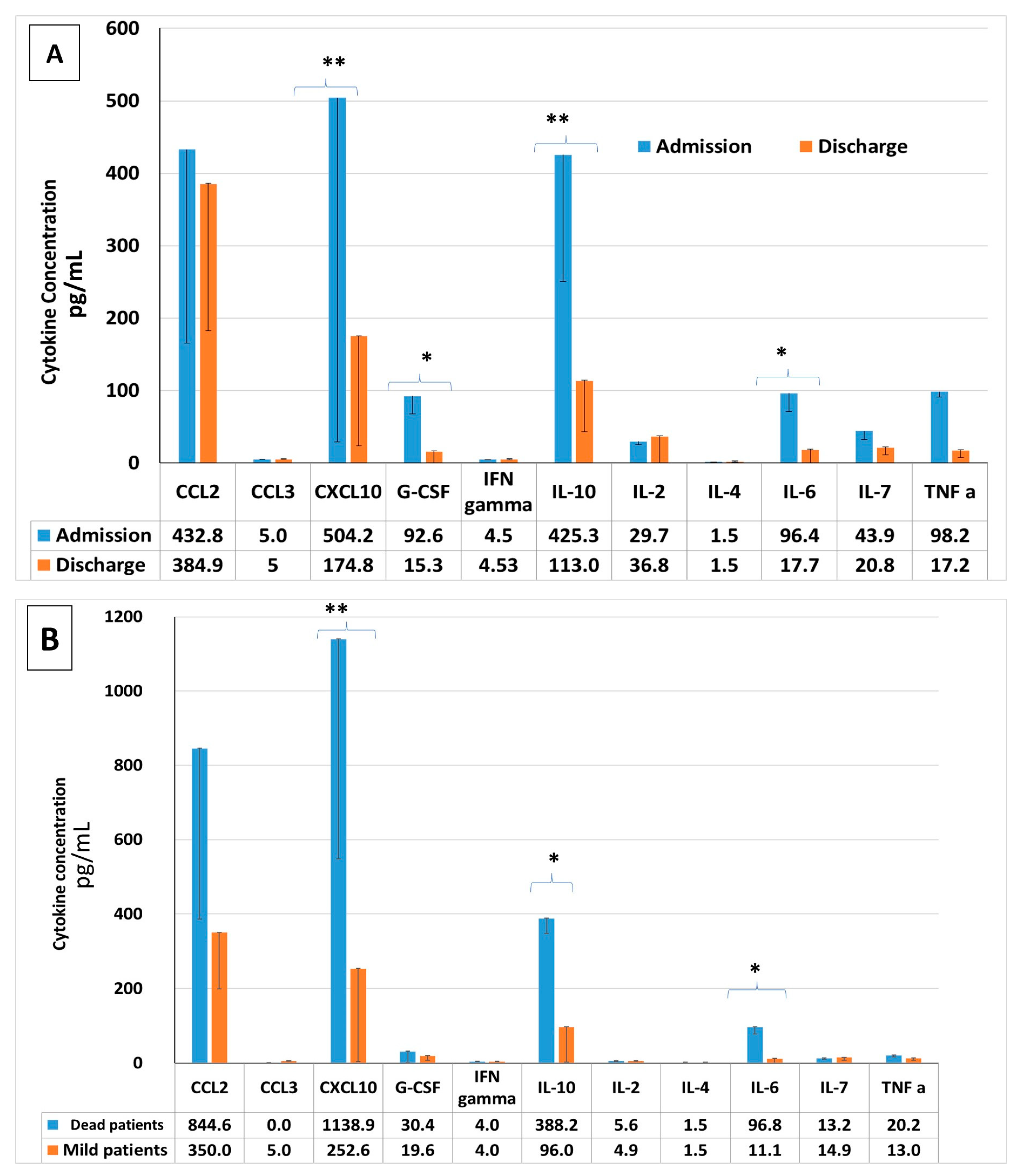
2.7. Pro-Inflammatory Cytokine Concentrations in COVID-19 Patients: Upon Admission vs. upon Discharge, Severe vs. Mild COVID-19 Patients, Survivors vs. Non-Survivors
3. Discussion
4. Methods
4.1. Study Population
4.2. Study Design
4.3. Luminex-Based Multiplex Assay for Serum Cytokine Concentration
4.4. Ethics
4.5. Outcomes
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities and outcomes among 5,700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Tan, B.K.; Mainbourg, S.; Friggeri, A.; Bertoletti, L.; Douplat, M.; Dargaud, Y.; Grange, C.; Lobbes, H.; Provencher, S.; Lega, J.-C. Arterial and venous thromboembolism in COVID-19: A study-level meta-analysis. Thorax 2021, 76, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Tan, B.K.; Mainbourg, S.; Potus, F.; Cucherat, M.; Lega, J.-C.; Provencher, S. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: A systematic review with meta-analysis. Vasc. Pharmacol. 2021, 139, 106882. [Google Scholar] [CrossRef]
- Miró, Ò.; Jiménez, S.; Mebazaa, A.; Freund, Y.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberechts, E.J.; Alquézar-Arbé, A.; Jacob, J.; et al. Pulmonary embolism in patients with COVID-19: Incidence, risk factors, clinical characteristics, and outcome. Eur. Heart J. 2021, 24, 1148. [Google Scholar]
- Liu, P.P.; Blet, A.; Smyth, D.; Li, H. The science underlying COVID-19: Implications for the cardiovascular system. Circulation 2020, 142, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Hendren, N.S.; Drazner, M.H.; Bozkurt, B.; Cooper, L.T., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation 2020, 141, 1903–1914. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Clinical Management: Living Guidance; 2021. 25 January 2021, COVID-19: Clinical Care. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed on 29 September 2021).
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2021. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf (accessed on 29 September 2021).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Wongvibulsin, S.; Garibaldi, B.T.; Antar, A.A.; Wen, J.; Wang, M.-C.; Gupta, A.; Bollinger, R.; Xu, Y.; Wang, K.; Betz, J.F.; et al. Development of severe COVID-19 adaptive risk predictor (SCARP), a calculator to predict severe disease or death in hospitalized patients with COVID-19. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Zou, X.; Li, S.; Fang, M.; Hu, M.; Bian, Y.; Ling, J.; Yu, S.; Jing, L.; Li, D.; Huang, J. Acute Physiology and Chronic Health Evaluation II Score as a Predictor of Hospital Mortality in Patients of Coronavirus Disease 2019. Crit. Care Med. 2020, 48, 657–665. [Google Scholar] [CrossRef]
- Iqbal, F.M.; Lam, K.; Sounderajah, V.; Clarke, J.M.; Ashrafian, H.; Darzi, A. Characteristics and predictors of acute and chronic post-COVID syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100899. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinnadurai, R.; Ogedengbe, O.; Agarwal, P.; Money-Coomes, S.; Abdurrahman, A.Z.; Mohammed, S.; Kalra, P.A.; Rothwell, N.; Pradhan, S. Older age and frailty are the chief predictors of mortality in COVID-19 patients admitted to an acute medical unit in a secondary care setting- a cohort study. BMC Geriatr. 2020, 20, 409. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Haghbin, H.; Lee-Smith, W.; Goyal, H.; Nawras, A.; Adler, D.G. Gastrointestinal predictors of severe COVID-19: Systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 615–630. [Google Scholar] [CrossRef]
- Knight, S.R.; Ho, A.; Pius, R.; Buchan, I.; Carson, G.; Drake, T.M.; Dunning, J.; Fairfield, C.J.; Gamble, C.; Green, C.A.; et al. Stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterization Protocol: Development and validation of the 4-C Mortality Score. BMJ 2020, 370, m3339. [Google Scholar] [CrossRef]
- Caricchio, R.; Gallucci, M.; Dass, C.; Zhang, X.; Gallucci, S.; Fleece, D.; Bromberg, M.; Criner, G.J.; Temple University COVID-19 Research Group. Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021, 80, 88–95. [Google Scholar] [CrossRef]
- Lim, S.; Shin, S.M.; Nam, G.E.; Jung, C.H.; Koo, B.K. Proper Management of People with Obesity during the COVID-19 Pandemic. J. Obes. Metab. Syndr. 2020, 29, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; De Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef] [Green Version]
- Sattar, N.; McInnes, I.B.; McMurray, J.J.V. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6. [Google Scholar] [CrossRef]
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed on 29 September 2021).
- Sun, Y.; Dong, Y.; Wang, L.; Xie, H.; Li, B.; Chang, C.; Wang, F.-S. Characteristics and prognostic factors of disease severity in patients with COVID-19: The Beijing experience. J. Autoimmun. 2020, 112, 102473. [Google Scholar] [CrossRef]
- Gotsman, I.; Zwas, D.; Planer, D.; Admon, D.; Lotan, C.; Keren, A. The significance of serum urea and renal function in patients with heart failure. Medicine 2010, 89, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Brisco, M.A.; Zile, M.; ter Maaten, J.M.; Hanberg, J.S.; Wilson, F.; Parikh, C.; Testani, J.M. The risk of death associated with proteinuria in heart failure is restricted to patients with an elevated blood urea nitrogen to creatinine ratio. Int. J. Cardiol. 2016, 215, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Murata, A.; Kasai, T.; Matsue, Y.; Matsumoto, H.; Yatsu, S.; Kato, T.; Suda, S.; Hiki, M.; Takagi, A.; Daida, H. Relationship between blood urea nitrogen-to-creatinine ratio at hospital admission and long-term mortality in patients with acute decompensated heart failure. Heart Vessel. 2018, 33, 877–885. [Google Scholar] [CrossRef]
- Chu, D.K.; Kim, L.H.; Young, P.J. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef]
- Mellado-Artigas, R.; Ferreyro, B.L.; Angriman, F.; Hernández-Sanz, M.; Arruti, E.; Torres, A.; Villar, J.; Brochard, L.; Ferrando, C. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit. Care 2021, 25, 58. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Huang, F.; Yang, Y.; Wang, F.; Yuan, J.; Zhang, Z.; Qin, Y.; Li, X.; Zhao, D.; et al. 2019-novel Coronavirus (2019-nCoV) Infections Trigger an Exaggerated Cytokine Response Aggravating Lung Injur. Sci. China Life Sci. 2020, 63, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020, 17, ciaa449. [Google Scholar]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.S.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef]
- Boyle, A.J.; Di Gangi, S.; Hamid, U.I.; Mottram, L.-J.; McNamee, L.; White, G.; Cross, L.M.; McNamee, J.J.; O’Kane, C.M.; McAuley, D.F. Aspirin therapy in patients with acute respiratory distress syndrome (ARDS) is associated with reduced intensive care unit mortality: A prospective analysis. Crit. Care 2015, 19, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Janz, D.R.; Bastarache, J.A.; May, A.K.; O’Neal, H.R., Jr.; Bernard, G.R.; Ware, L.B. Prehospital aspirin use is associated with reduced risk of acute respiratory distress syndrome in critically ill patients: A propensity-adjusted analysis. Crit. Care Med. 2015, 43, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Erlich, J.M.; Talmor, D.S.; Cartin-Ceba, R.; Gajic, O.; Kor, D.J. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: A population-based cohort study. Chest 2011, 139, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kor, D.J.; Carter, R.E.; Park, P.K.; Festic, E.; Banner-Goodspeed, V.M.; Hinds, R.; Talmor, D.; Gajic, O.; Ware, L.B.; Gong, M.N.; et al. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: The LIPS-A randomized clinical trial. JAMA 2016, 315, 2406–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kor, D.J.; Erlich, J.; Gong, M.N.; Malinchoc, M.; Carter, R.E.; Gajic, O.; Talmor, D.S. Association of prehospitalization aspirin therapy and acute lung injury: Results of a multicenter international observational study of at-risk patient. Crit. Care Med. 2011, 39, 2393–2400. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Survivors | Non-Survivors | p-Value |
|---|---|---|---|
| Total | N = 318 | N = 74 | |
| Age | 58 ± 18 | 78 ± 12 | 0.001 |
| Male (%) | 47% | 60% | 600 |
| BMI | 29 ± 6 | 32 ± 11 | 0.008 |
| Comorbidities % | |||
| Diabetes (%) | 30 | 57 | 0.001 |
| Hypertension (%) | 48 | 84 | 0.001 |
| Lung disease (%) | 9 | 18 | 0.03 |
| Hemodialysis (%) | 5 | 15 | 0.003 |
| Aspirin use (%) | 30 | 61 | 0.001 |
| Symptom’s duration before admission to hospitals (days) | 6 ± 5 | 9 ± 12 | 0.19 |
| Symptoms before admission (% of total) | |||
| Fever % | 57 | 56 | 0.77 |
| Diarrhea % | 6 | 2 | 0.26 |
| Dyspnea % | 59 | 59 | 0.74 |
| Clinical severity on admission % | 30 | 54 | 0.001 |
| Lab Findings upon admission | |||
| Hemoglobin (mg/dL) | 13 ± 3 | 12 ± 1.2 | 0.06 |
| Absolute neutrophil count (×103/µL) | 13 ± 9 | 13 ± 6 | 0.9 |
| Absolute lymphocyte count (×103/µL) | 2.06 ± 11 | 1.4 ± 4 | 0.001 |
| Neutrophil to lymphocyte ratio (NLR) | 7.01 ± 3 | 9.1 ± 0.8 | 0.001 |
| Platelet (×103/µL) | 220 ± 86 | 220 ± 90 | 0.79 |
| BUN (mg/dL) | 19 ± 14 | 40 ± 27 | 0.001 |
| Creatinine (mg/dL) | 2 ± 8 | 2.2 ± 2 | 0.7 |
| Triglycerides (mg/dL) | 145 ± 148 | 157 ± 48 | 0.08 |
| HDL (mg/dL) | 33 ± 12 | 30 ± 13 | 0.09 |
| Insulin Resistance (TG/HDL) | 5.4 ± 5.8 | 6.1 ± 5.0 | 0.02 |
| C-reactive protein (CRP) (mg/dL) | 68 ± 78 | 109 ± 97 | 0.001 |
| Ferritin | 632 ± 987 | 1041 ± 3594 | 0.57 |
| D-dimer | 1664 ± 3513 | 2539 ± 3374 | 0.001 |
| Fibrinogen | 641 ± 183 | 660 ± 180 | 0.33 |
| IL-6 | 34 ± 52 | 264 ± 111 | 0.001 |
| ALT | 23 ± 21 | 37 ± 51 | 0.001 |
| Cytokine storm (% of total) | 25 | 51 | 0.001 |
| 4-C score | 8 ± 20 | 12 ± 3 | 0.001 |
| SOFA score | 1.3 ± 1.4 | 2.7 ± 2.2 | 0.001 |
| O2 supplement on admission % | 55 | 100 | 0.001 |
| High flow use (% of total) | 14 | 82 | 0.001 |
| Mechanical ventilation (% of total) | 2 | 46 | 0.001 |
| ARDS (% of total) | 17 | 34 | 0.006 |
| O2 supply at day 7 (% of total) | 18 | 99 | 0.001 |
| Hospitalization length | 9.5 ± 10 | 17.2 ± 13 | 0.001 |
| Time to negative PCR | 17 ± 8 | 20 ± 8 | 0.16 |
| Treatment in hospitalization | |||
| Steroid therapy (% of total) Methylprednisolone Dexamethasone | 93 86 14 | 97 90 10 | 0.26 0.75 0.75 |
| LMWH (% of total) | 96 | 99 | 0.34 |
| Remdesivir (% of total) | 12 | 19 | 0.21 |
| Vitamin D (% of total) | 97 | 97 | 0.9 |
| A | |||||
| Coefficient | 95%Conf. (±) | Std. Error | T | p-Value | |
| Constant | |||||
| Age | 0.01 | 0.002 | 0.001 | 5.4 | 0.00001 |
| Gender | −0.04 | 0.07 | 0.036 | −1.36 | 0.17 |
| Ethnicity | −0.04 | 0.07 | 0.03 | −1.23 | 0.21 |
| BMI | 0.001 | 0.005 | 0.002 | 2.23 | 0.02 |
| DM | 0.04 | 0.088 | 0.044 | 1.05 | 0.2 |
| HTN | −0.02 | 0.048 | 0.048 | 0.5 | 0.6 |
| Hemodialysis | 0.1 | 0.07 | 0.07 | 2.2 | 0.02 |
| B | |||||
| Coefficient | 95%Conf. (±) | Std. Error | T | p-Value | |
| Constant | |||||
| Age | 0.01 | 0.001 | 0.001 | 7.7 | 0.00001 |
| BMI | 0.01 | 0.005 | 0.0025 | 2.3 | 0.00001 |
| Hemodialysis | 0.176 | 0.13 | 0.069 | 2.5 | 0.00001 |
| C | |||||
| Coefficient | 95%Conf. (±) | Std. Error | T | p-Value | |
| Constant | |||||
| Past Aspirin use | 0.14 | 0.08 | 0.04 | 3.6 | 0.0003 |
| O2 supplement before admission | 0.25 | 0.08 | 0.04 | 6.2 | 0.00001 |
| A | |||||
| Coefficient | 95%Conf. (±) | Std. Error | T | p-Value | |
| Constant | |||||
| Age | 0.01 | 0.01 | 0.008 | 1.35 | 0.18 |
| BMI | 0.008 | 0.024 | 0.01 | 0.89 | 0.38 |
| SO2 upon admission | −0.004 | 0.044 | 0.02 | −0.26 | 0.79 |
| % Pneumonia | 0.0003 | 0.007 | 0.003 | 0.104 | 0.9 |
| PAO2/FIO2 | 0.0002 | 0.001 | 0.0004 | 0.42 | 0.67 |
| ARDS class | 0.187 | 0.37 | 0.18 | 1.043 | 0.3 |
| D-Dimer | −0.0001 | 8.6 | 0.001 | −0.50 | 0.62 |
| Fibrinogen | −0.000 | 0.0007 | 0.001 | −1.26 | 0.21 |
| NLR | 0.00125 | 0.0014 | 0.001 | 1.84 | 0.076 |
| Ferritin | 0.001 | 0.0001 | 0.001 | 0.75 | 0.45 |
| IL-6 | 0.0007 | 0.002 | 0.001 | 0.54 | 0.58 |
| ALT | −0.001 | 0.007 | 0.001 | −0.51 | 0.61 |
| BUN | 0.004 | 0.014 | 0.007 | 0.56 | 0.56 |
| BUN Class | 0.096 | 0.36 | 0.175 | 0.557 | 0.58 |
| Cytokine storme | −0.068 | 0.33 | 0.16 | −0.427 | 0.67 |
| CRP | 0.0001 | 0.002 | 0.001 | 0.1 | 0.92 |
| HDL | −0.006 | 0.016 | 0.008 | −0.81 | 0.42 |
| HDL Class | 0.003 | 0.34 | 0.169 | 0.01 | 0.98 |
| TG | −0.0004 | 0.002 | 0.0014 | −0.29 | 0.76 |
| TG/HDL | −0.0004 | 0.055 | 0.026 | −0.017 | 0.98 |
| 4C-score | −0.012 | 0.07 | 0.03 | −0.36 | 0.71 |
| High flow use | 0.18 | 0.38 | 0.18 | 0.96 | 0.34 |
| B | |||||
| Coefficient | 95%Conf. (±) | Std. Error | T | p-Value | |
| Constant | |||||
| Age | 0.009 | 0.005 | 0.002 | 3.1 | 0.003 |
| NLR | 0.001 | 0.001 | 0.0005 | 2.3 | 0.022 |
| BUN | 0.006 | 0.004 | 0.002 | 2.9 | 0.004 |
| High flow use | 0.258 | 0.183 | 0.091 | 2.8 | 0.006 |
| A | ||
| Actual count | 0 | 1 |
| 218 | 189 | 29 |
| 47 | 4 | 43 |
| B | ||
| Specificity | 59.7 | |
| Sensitivity | 97.9 | |
| Positive predictive value | 86.7 | |
| Negative predictive value | 91.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basheer, M.; Saad, E.; Hagai, R.; Assy, N. Clinical Predictors of Mortality and Critical Illness in Patients with COVID-19 Pneumonia. Metabolites 2021, 11, 679. https://doi.org/10.3390/metabo11100679
Basheer M, Saad E, Hagai R, Assy N. Clinical Predictors of Mortality and Critical Illness in Patients with COVID-19 Pneumonia. Metabolites. 2021; 11(10):679. https://doi.org/10.3390/metabo11100679
Chicago/Turabian StyleBasheer, Maamoun, Elias Saad, Rechnitzer Hagai, and Nimer Assy. 2021. "Clinical Predictors of Mortality and Critical Illness in Patients with COVID-19 Pneumonia" Metabolites 11, no. 10: 679. https://doi.org/10.3390/metabo11100679
APA StyleBasheer, M., Saad, E., Hagai, R., & Assy, N. (2021). Clinical Predictors of Mortality and Critical Illness in Patients with COVID-19 Pneumonia. Metabolites, 11(10), 679. https://doi.org/10.3390/metabo11100679







