New Approach for the Construction and Calibration of Gas-Tight Setups for Biohydrogen Production at the Small Laboratory Scale
Abstract
1. Introduction
2. Results
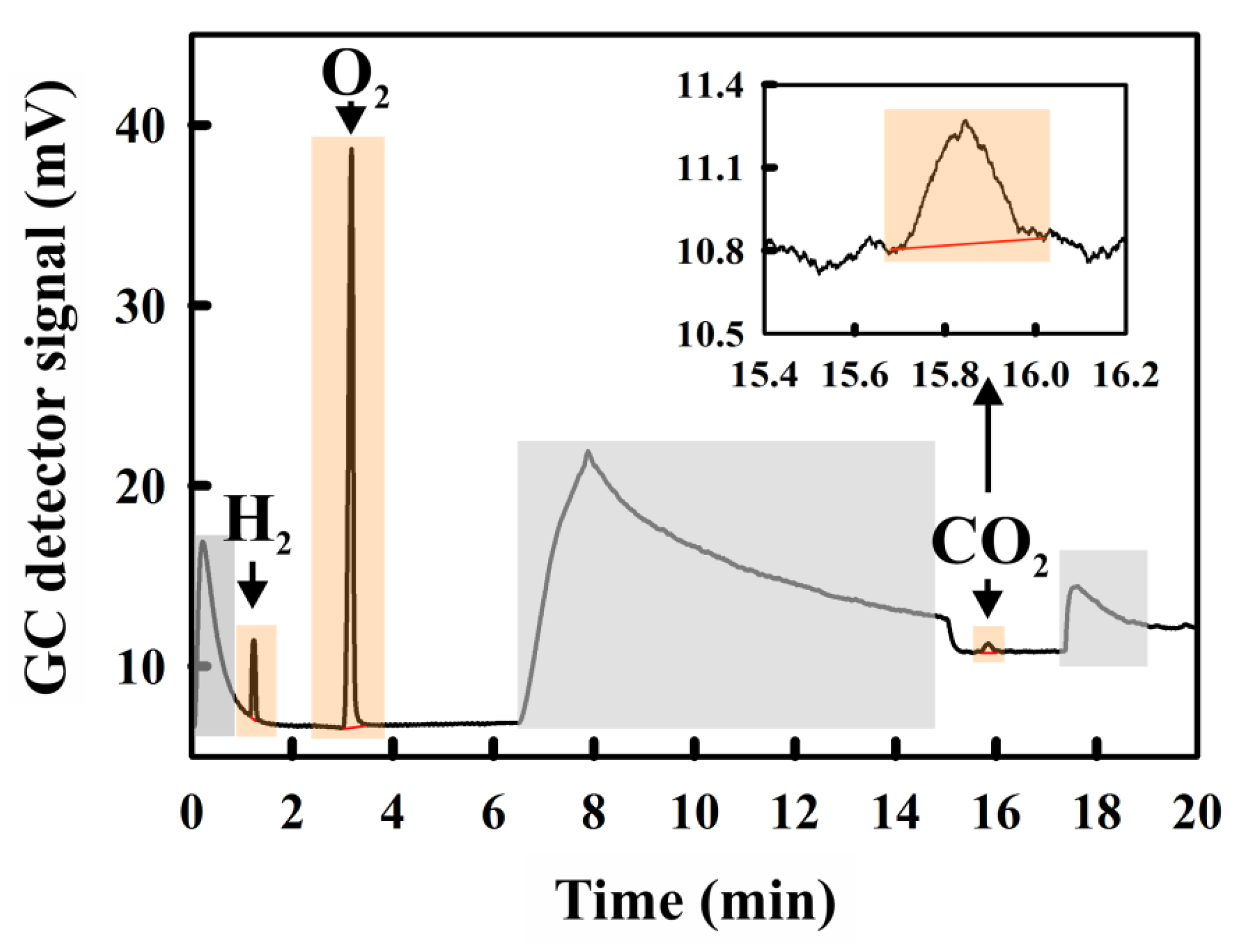
2.1. Calculation of the Conversion Coefficients for the Determination of the Concentrations of O2, H2 and CO2 Directly from the GC Peak Area
2.1.1. The Reference Value: The GC-Conversion Coefficient for O2

2.1.2. Determination of the GC-Conversion Coefficient for H2

2.1.3. Determination of the GC-Conversion Coefficient for CO2

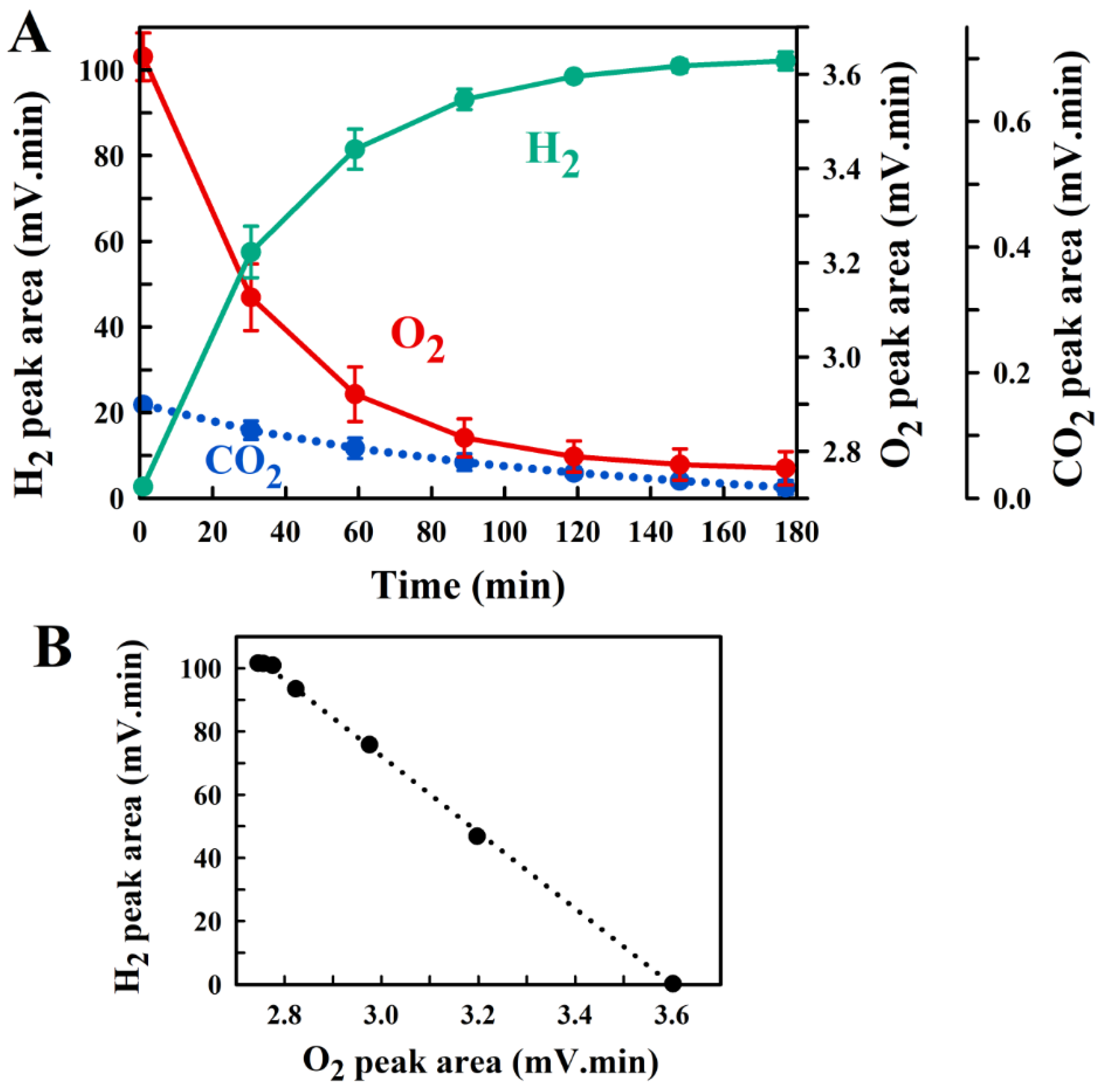
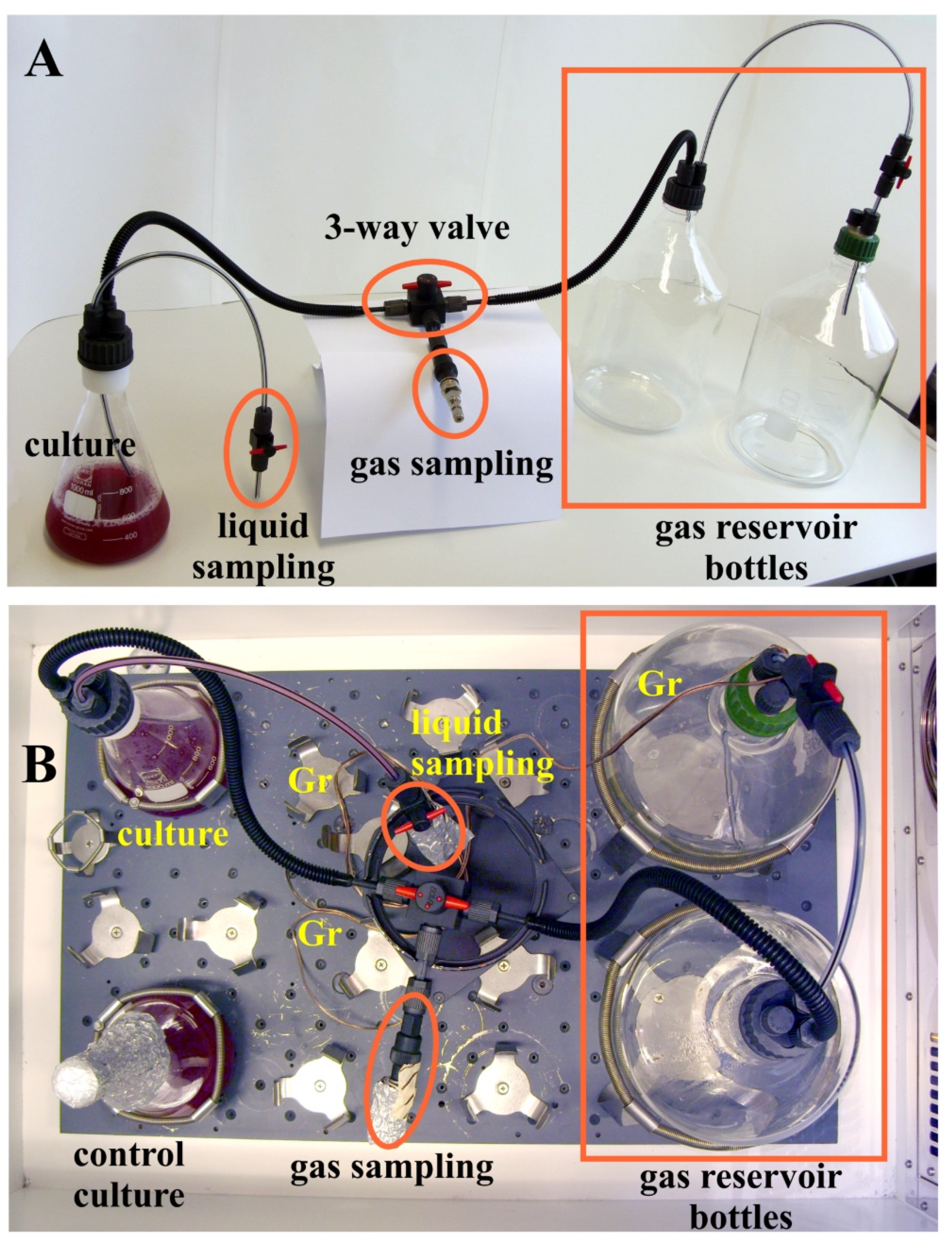
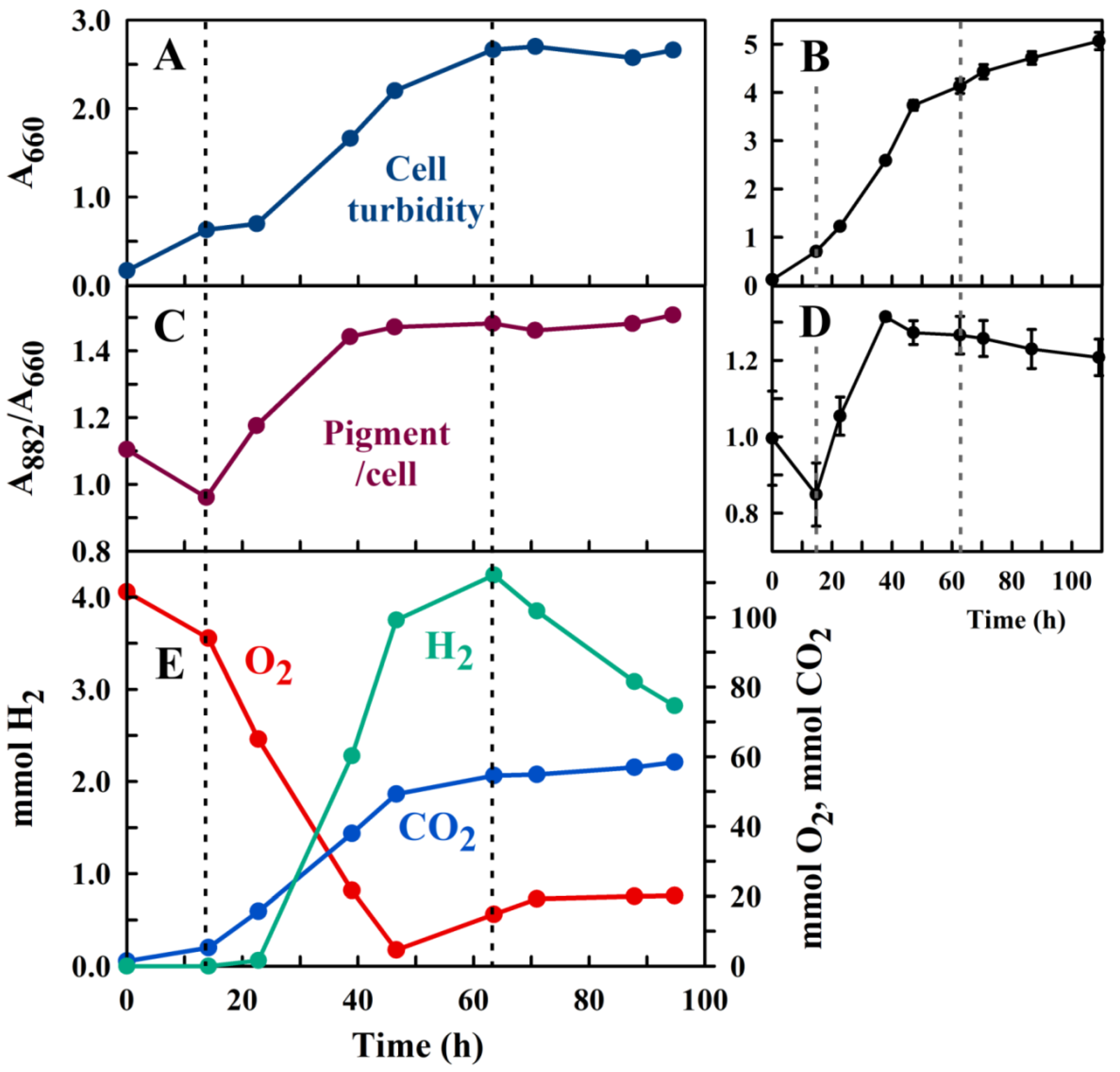
2.2. Experimental Setup for Semi-Aerobic Growth of a H2 Producing Photosynthetic Bacterium, R. Rubrum
3. Discussion
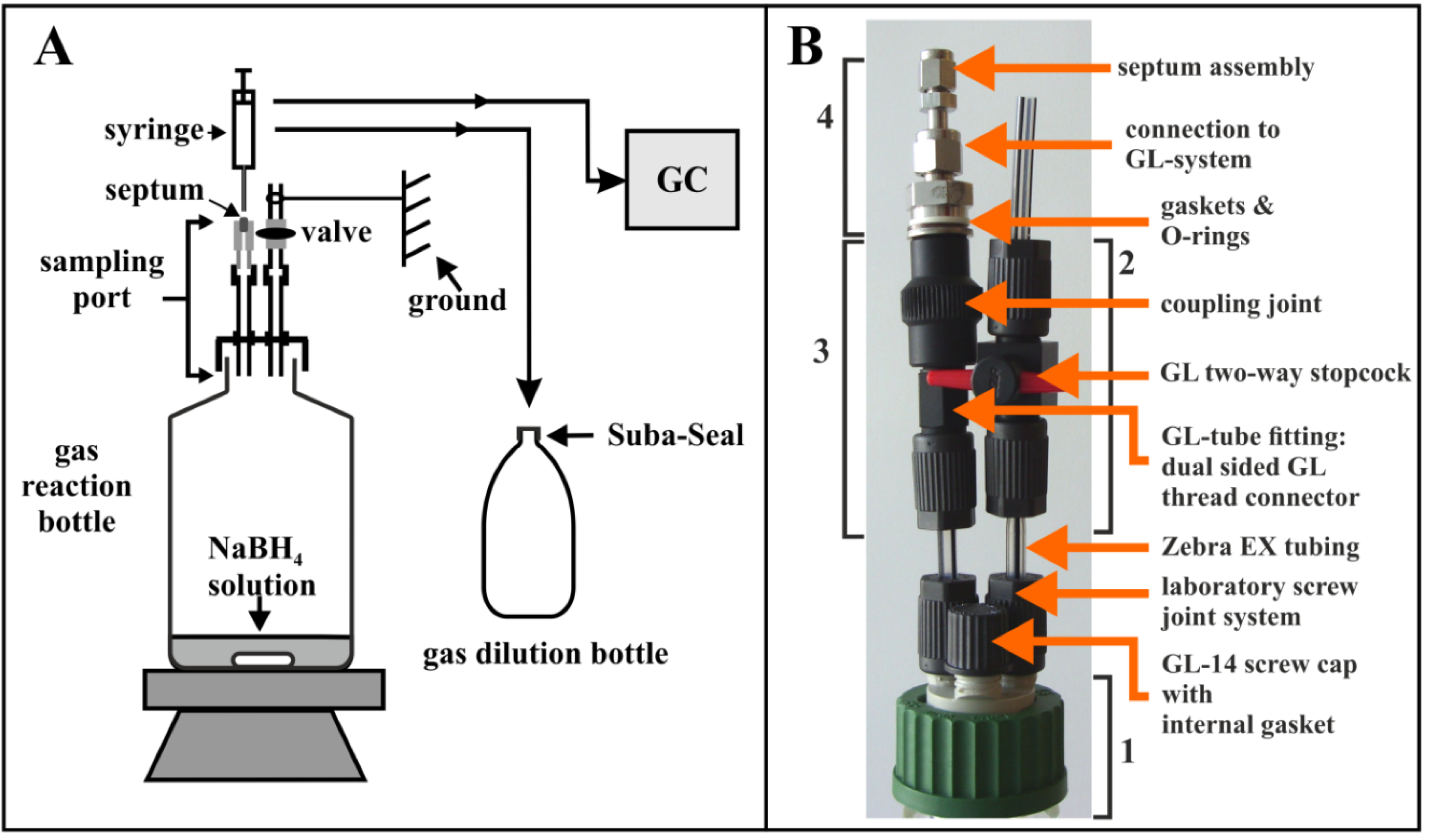
4. Materials and Methods
4.1. Chemicals and Materials
4.2. The Gas Chromatograph Protocol
Considerations for the Effect of Elevated Pressure in Gas Samples upon the GC Measurement of Gas Concentrations
4.3. BOLA and Swagelok Components and Connections used for Constructing Gas Sampling Assemblies
4.4. Calibration of the H2 GC-Signal
4.5. Bacterial Strain, Growth Conditions, and Spectroscopic and Biochemical Analysis of Growth Parameters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kruse, O.; Hankamer, B. Microalgal hydrogen production. Curr. Opin. Biotech. 2010, 21, 238–243. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Harwood, C.S. Photobiological production of hydrogen gas as a biofuel. Curr. Opin. Biotech. 2010, 21, 244–251. [Google Scholar] [CrossRef]
- Mahidhara, G.; Burrow, H.; Sasikala, C.; Ramana, C.V. Biological hydrogen production: Molecular and electrolytic perspectives. World J. Microb. Biotechnol. 2019, 35, 116. [Google Scholar] [CrossRef]
- Eroglu, E.; Melis, A. Microalgal hydrogen production. Int. J. Hydrog. Energy 2016, 41, 12772–12798. [Google Scholar] [CrossRef]
- Hassan, A.H.S.; Mietzel, T.; Brunstermann, R.; Schmuck, S.; Schoth, J.; Küppers, M.; Widmann, R. Fermentative hydrogen production from low-value substrates. World J. Microb. Biotechnol. 2018, 34, 176. [Google Scholar] [CrossRef] [PubMed]
- Voelskow, H.; Schön, G. H2 production of Rhodospirillum rubrum during adaptation to anaerobic dark conditions. Arch. Microbiol. 1980, 125, 245–249. [Google Scholar] [CrossRef]
- Zurrer, H.; Bachofen, R. Aspects of growth and hydrogen production of the photosynthetic bacterium Rhodospirillum rubrum in continuous cultures. Biomass 1982, 2, 165–174. [Google Scholar] [CrossRef]
- Ghirardi, M.L.; Togasaki, R.K.; Seibert, M. Oxygen sensitivity of algal H2- production. Appl. Biochem. Biotechnol. 1997, 63, 141–151. [Google Scholar] [CrossRef]
- Melis, A.; Zhang, L.P.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000, 122, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, M.K.; Kim, M.S.; Lee, J.K. Molecular hydrogen production by nitrogenase of Rhodobacter sphaeroides and by Fe-only hydrogenase of Rhodospirillum rubrum. Int. J. Hydrog. Energy 2008, 33, 1516–1521. [Google Scholar] [CrossRef]
- Hemschemeier, A.; Melis, A.; Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae of Chlamydomonas reinhardtii. Photosynth. Res. 2009, 102, 523–540. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Stöckel, J.; Min, H.; Sherman, L.A.; Pakrasi, H.B. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat. Commun. 2010, 1, 1–7. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Buitrón, G.; Moreno-Andrade, I.; Tapia-Rodríguez, A.C.; Palomo-Briones, R.; Razo-Flores, E.; Aguilar-Juárez, O.; Arreola-Vargas, J.; Bernet, N.; Braga, A.F.M.; et al. Standardized protocol for determination of biohydrogen potential. MethodsX 2020, 7, 100754. [Google Scholar] [CrossRef]
- Bosagh, F.; Rostami, K. A review of measurement methods of biological hydrogen. Int. J. Hydrog. Energy 2020, 45, 24424–24452. [Google Scholar] [CrossRef]
- Ghosh, R.; Hardmeyer, A.; Thoenen, I.; Bachofen, R. Optimization of the Sistrom culture medium for large-scale batch cultivation of Rhodospirillum rubrum under semi-aerobic conditions with maximal yield of photosynthetic membranes. Appl. Environ. Microbiol. 1994, 60, 1698–1700. [Google Scholar] [CrossRef]
- Bartkus, T.P.; T’ien, J.S.; Sung, C.-J. A semi-global reaction rate model based on experimental data for the self-hydrolysis kinetics of aqueous sodium borohydride. Int. J. Hydrog. Energy 2013, 38, 4024–4033. [Google Scholar] [CrossRef]
- Brack, P.; Dann, S.E.; Upul Wijayantha, K.G. Heterogeneous and homogeneous catalysts for hydrogen generation by hydrolysis of aqueous sodium borohydride (NaBH4) solutions. Energy Sci. Eng. 2015, 3, 174–188. Available online: https://www.scipedia.com/public/Brack_et_al_2015b (accessed on 28 September 2021). [CrossRef]
- Carius, L.; Hädicke, O.; Grammel, H. Stepwise reduction of the culture redox potential allows analysis of microaerobic metabolism and photosynthetic membrane synthesis in Rhodospirillum rubrum. Biotechnol. Bioeng. 2013, 110, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Alloul, A.; Muys, M.; Hertoghs, N.; Kerckhof, F.-M.; Vlaeminck, S.E. Cocultivating aerobic heterotrophs and purple bacteria for microbial protein in sequential photo- and chemotrophic reactors. Bioresour. Technol. 2021, 319, 124192. [Google Scholar] [CrossRef] [PubMed]
- Selao, T.T.; Edgren, T.; Wang, H.; Norén, A.; Nordlund, S. Effect of pyruvate on the metabolic regulation of nitrogenase activity in Rhodospirillum rubrum in darkness. Microbiology 2011, 157, 1834–1840. [Google Scholar] [CrossRef]
- Grammel, H.; Gilles, E.D.; Ghosh, R. Microaerophilic cooperation of reductive and oxidative pathways allows maximal photosynthetic membrane biosynthesis in Rhodospirillum rubrum. Appl. Environ. Microbiol. 2003, 69, 6577–6586. [Google Scholar] [CrossRef] [PubMed]
- Abo-Hashesh, M.; Hallenbeck, P.C. Microaerobic dark fermentative hydrogen production by the photosynthetic bacterium, Rhodobacter capsulatus JP91. Int. J. Low Carbon Technol. 2012, 7, 97–103. [Google Scholar] [CrossRef]
- Ormerod, J.G.; Gest, H. Symposium on metabolism of inorganic compounds IV—Hydrogen photosynthesis and alternative metabolic pathways in photosynthetic bacteria. Bacteriol. Rev. 1962, 26, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, R.H.; Ludden, P.W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J. Bacteriol. 1984, 158, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Schön, G.; Voelskow, H. Pyruvate fermentation in Rhodospirillum rubrum and after transfer from aerobic to anaerobic conditions in the dark. Arch. Microbiol. 1976, 107, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, T.E.; Uffen, R.L. Fermentative metabolism of pyruvate by Rhodospirillum rubrum after anaerobic growth in darkness. J. Bacteriol. 1977, 131, 533–543. [Google Scholar] [CrossRef]
- Adams, M.W.W.; Hall, D.O. Isolation of the membrane-bound hydrogenase from Rhodospirillum rubrum. Biochem. Biophys. Res. Commun. 1977, 77, 730–737. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tsapekos, P.; Alfaro, N.; Díaz, I.; Fdz-Polanco, M.; Rafiee, S.; Angelidaki, I. A review on prospects and challenges of biological H2S removal from biogas with focus on biotrickling filtration and microaerobic desulfurization. Biofuel Res. J. 2017, 16, 741–750. [Google Scholar] [CrossRef]
- Kosourov, S.N.; Batyrova, K.A.; Petushkova, E.P.; Tsygankov, A.A.; Ghirardi, M.L.; Seibert, M. Maximizing the hydrogen photoproduction yields in Chlamydomonas reinhardtii cultures: The effect of the H2 partial pressure. Int. J. Hydrogen Energy 2012, 37, 8850–8858. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J.; Keeler, J. Atkins’ Physical Chemistry, 11th ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Cohen-Bazire, G.; Sistrom, W.R.; Stanier, R.Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J. Cell Comp. Physiol. 1956, 49, 25–68. [Google Scholar] [CrossRef]
- Sistrom, W.R. A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. J. Gen. Microbiol. 1960, 22, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Lupo, D.; Ghosh, R. The reaction center H subunit is not required for high levels of light-harvesting complex 1 in Rhodospirillum rubrum mutants. J. Bacteriol. 2004, 186, 5585–5595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shaw, S.; Ghosh, R. A modified Kulka micromethod for the rapid and safe analysis of fructose and 1-deoxy-D-xylulose-5-phosphate. Metabolites 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Autenrieth, C.; Shaw, S.; Ghosh, R. New Approach for the Construction and Calibration of Gas-Tight Setups for Biohydrogen Production at the Small Laboratory Scale. Metabolites 2021, 11, 667. https://doi.org/10.3390/metabo11100667
Autenrieth C, Shaw S, Ghosh R. New Approach for the Construction and Calibration of Gas-Tight Setups for Biohydrogen Production at the Small Laboratory Scale. Metabolites. 2021; 11(10):667. https://doi.org/10.3390/metabo11100667
Chicago/Turabian StyleAutenrieth, Caroline, Shreya Shaw, and Robin Ghosh. 2021. "New Approach for the Construction and Calibration of Gas-Tight Setups for Biohydrogen Production at the Small Laboratory Scale" Metabolites 11, no. 10: 667. https://doi.org/10.3390/metabo11100667
APA StyleAutenrieth, C., Shaw, S., & Ghosh, R. (2021). New Approach for the Construction and Calibration of Gas-Tight Setups for Biohydrogen Production at the Small Laboratory Scale. Metabolites, 11(10), 667. https://doi.org/10.3390/metabo11100667






