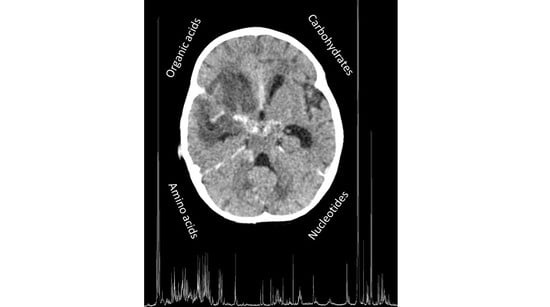
CSF Metabolomics of Tuberculous Meningitis: A Review
Abstract
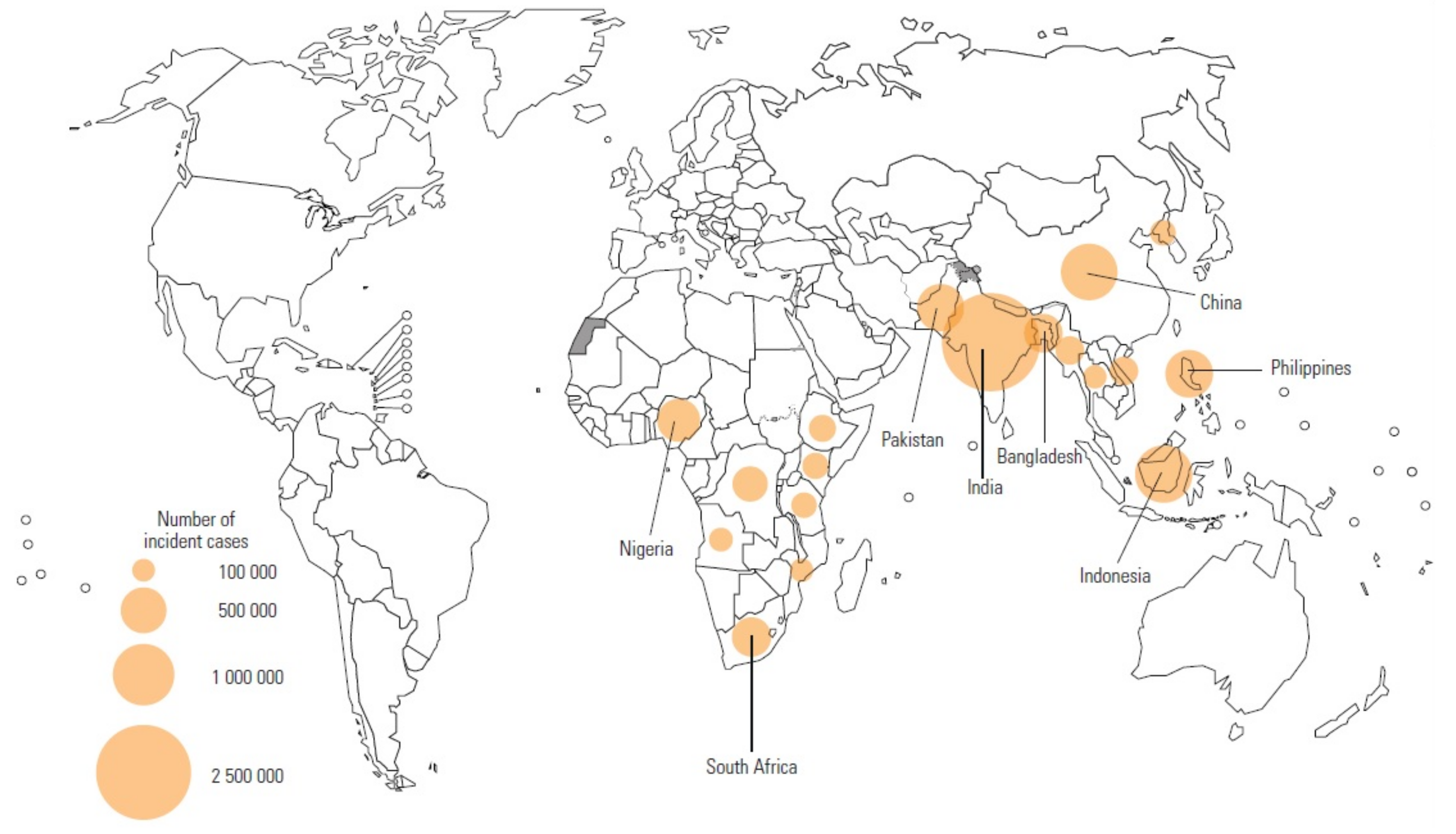
1. Introduction
2. CSF Metabolomics of TBM
2.1. Literature Search Parameters
2.2. Metabolomics—The Basics
2.3. History of CSF Metabolomics Studies of TBM in Human Subjects
3. Metabolic Markers of TBM
3.1. CSF Lactate
Diagnostic Value
3.2. CSF Glucose
3.3. Other Metabolic Markers of TBM
4. The Future of CSF Metabolomics in TBM Research
Author Contributions
Funding
Conflicts of Interest
References
- Hershkovitz, I.; Donoghue, H.D.; Minnikin, D.E.; May, H.; Lee, O.Y.C.; Feldman, M.; Galili, E.; Spigelman, M.; Rothschild, B.M.; Bar-Gal, G.K. Tuberculosis origin: The Neolithic scenario. Tuberculosis 2015, 95, S122–S126. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 16 July 2021).
- Seddon, J.A.; Tugume, L.; Solomons, R.; Prasad, K.; Bahr, N.C.; Tuberculous Meningitis International Research Consortium. The current global situation for tuberculous meningitis: Epidemiology, diagnostics, treatment and outcomes. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.A.; Hesseling, A.C.; Marais, B.J.; Jordaan, A.; Victor, T.; Schaaf, H.S. The evolving epidemic of drug-resistant tuberculosis among children in Cape Town, South Africa. Int. J. Tuberc. Lung Dis. 2012, 16, 928–933. [Google Scholar] [CrossRef]
- Bahr, N.C.; Marais, S.; Caws, M.; van Crevel, R.; Wilkinson, R.J.; Tyagi, J.S.; Thwaites, G.E.; Boulware, D.R.; Tuberculous Meningitis International Research Consortium. GeneXpert MTB/Rif to diagnose tuberculous meningitis: Perhaps the first test but not the last. Clin. Infect. Dis. 2016, 62, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Boyles, T.H.; Lynen, L.; Seddon, J.A.; Tuberculous Meningitis International Research Consortium. Decision-making in the diagnosis of tuberculous meningitis. Wellcome Open Res. 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, F.V.; Davis, A.G.; Sharma, K.; Roy, R.B.; Ganiem, A.R.; Kagimu, E.; Solomons, R.; Wilkinson, R.J.; Bahr, N.C.; Thuong, N.T.T.; et al. Recent developments in tuberculous meningitis pathogenesis and diagnostics. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Davis, A.G.; Nightingale, S.; Springer, P.E.; Solomons, R.; Arenivas, A.; Wilkinson, R.J.; Anderson, S.T.; Chow, F.C.; Tuberculous Meningitis International Research Consortium. Neurocognitive and functional impairment in adult and pediatric tuberculous meningitis. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.G.; Donovan, J.; Bremer, M.; van Toorn, R.; Schoeman, J.; Dadabhoy, A.; Lai, R.P.; Cresswell, F.V.; Boulware, D.R.; Wilkinson, R.J.; et al. Host directed therapies for tuberculous meningitis. Wellcome Open Res. 2020, 5. [Google Scholar] [CrossRef]
- Donovan, J.; Rohlwink, U.K.; Tucker, E.W.; Hiep, N.T.T.; Thwaites, G.E.; Figaji, A.A.; Tuberculous Meningitis International Research Consortium. Checklists to guide the supportive and critical care of tuberculous meningitis. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Donovan, J.; Cresswell, F.V.; Thuong, N.T.T.; Boulware, D.R.; Thwaites, G.E.; Bahr, N.C. Xpert MTB/RIF ultra for the diagnosis of tuberculous meningitis: A small step forward. Clin. Infect. Dis. 2020, 71, 2002–2005. [Google Scholar] [CrossRef]
- Imran, D.; Hill, P.C.; McKnight, J.; van Crevel, R.; Tuberculous Meningitis International Research Consortium. Establishing the cascade of care for patients with tuberculous meningitis. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Marais, B.J.; Heemskerk, A.D.; Marais, S.S.; van Crevel, R.; Rohlwink, U.; Caws, M.; Meintjes, G.; Misra, U.K.; Mai, N.T.; Ruslami, R.; et al. Standardized methods for enhanced quality and comparability of tuberculous meningitis studies. Clin. Infect. Dis. 2017, 64, 501–509. [Google Scholar] [CrossRef]
- Marais, S.; van Toorn, R.; Chow, F.C.; Manesh, A.; Siddiqi, O.K.; Figaji, A.; Schoeman, J.F.; Meintjes, G.; Tuberculous Meningitis International Research Consortium. Management of intracranial tuberculous mass lesions: How long should we treat for? Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J.; Tuberculous Meningitis International Research Consortium. Mechanism, spectrum, consequences and management of hyponatremia in tuberculous meningitis. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Rohlwink, U.K.; Chow, F.C.; Wasserman, S.; Dian, S.; Lai, R.P.; Chaidir, L.; Hamers, R.L.; Wilkinson, R.J.; Boulware, D.R.; Cresswell, F.V.; et al. Standardized approaches for clinical sampling and endpoint ascertainment in tuberculous meningitis studies. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Seddon, J.A.; Thwaites, G.E.; Tuberculous Meningitis International Research Consortium. Tuberculous meningitis: New tools and new approaches required. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Seddon, J.A.; Wilkinson, R.; van Crevel, R.; Figaji, A.; Thwaites, G.E.; Tuberculous Meningitis International Research Consortium. Knowledge gaps and research priorities in tuberculous meningitis. Wellcome Open Res. 2019, 4. [Google Scholar] [CrossRef]
- Dai, Y.N.; Huang, H.J.; Song, W.Y.; Tong, Y.X.; Yang, D.H.; Wang, M.S.; Huang, Y.C.; Chen, M.J.; Zhang, J.J.; Ren, Z.Z.; et al. Identification of potential metabolic biomarkers of cerebrospinal fluids that differentiate tuberculous meningitis from other types of meningitis by a metabolomics study. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Isaiah, S.; Loots, D.T.; Solomons, R.; van Der Kuip, M.; Tutu Van Furth, A.; Mason, S. Overview of brain-to-gut axis exposed to chronic CNS bacterial infection(s) and a predictive urinary metabolic profile of a brain infected by Mycobacterium tuberculosis. Front. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Du, B.; Li, J.; Zhang, J.; Zheng, X.; Jia, H.; Xing, A.; Sun, Q.; Liu, F.; Zhang, Z. Cerebrospinal fluid metabolomic profiling in tuberculous and viral meningitis: Screening potential markers for differential diagnosis. Clin. Chim. Acta 2017, 466, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; van Furth, A.M.; Mienie, L.J.; Engelke, U.F.; Wevers, R.A.; Solomons, R.; Reinecke, C.J. A hypothetical astrocyte–microglia lactate shuttle derived from a 1H NMR metabolomics analysis of cerebrospinal fluid from a cohort of South African children with tuberculous meningitis. Metabolomics 2015, 11, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Reinecke, C.J.; Kulik, W.; van Cruchten, A.; Solomons, R.; van Furth, A.M.T. Cerebrospinal fluid in tuberculous meningitis exhibits only the L-enantiomer of lactic acid. BMC Infect. Dis. 2016, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mason, S. Lactate shuttles in neuroenergetics—Homeostasis, allostasis and beyond. Front. Neurosci. 2017, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Reinecke, C.J.; Solomons, R. Cerebrospinal fluid amino acid profiling of pediatric cases with tuberculous meningitis. Front. Neurosci. 2017, 11, 534. [Google Scholar] [CrossRef]
- Mason, S. A novel, multi-faceted perception of lactate in neurology. Front. Neurosci. 2020, 14, 460. [Google Scholar] [CrossRef]
- Van Laarhoven, A.; Dian, S.; Aguirre-Gamboa, R.; Avila-Pacheco, J.; Ricaño-Ponce, I.; Ruesen, C.; Annisa, J.; Koeken, V.A.; Chaidir, L.; Li, Y.; et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: An observational cohort study. Lancet Infect. Dis. 2018, 18, 526–535. [Google Scholar] [CrossRef]
- Van Zyl, C.D.W.; Loots, D.T.; Solomons, R.; van Reenen, M.; Mason, S. Metabolic characterization of tuberculous meningitis in a South African pediatric population using 1H NMR metabolomics. J. Infect. 2020, 81, 743–752. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, W.; Lang, Y.; Qu, Y.; Chen, J.; Cui, L. 1H nuclear magnetic resonance-based metabolic profiling of cerebrospinal fluid to identify metabolic features and markers for tuberculosis meningitis. Infect. Genet. Evol. 2019, 68, 253–264. [Google Scholar] [CrossRef]
- Coen, M.; O’sullivan, M.; Bubb, W.A.; Kuchel, P.W.; Sorrell, T. Proton nuclear magnetic resonance—Based metabonomics for rapid diagnosis of meningitis and ventriculitis. Clin. Infect. Dis. 2005, 41, 1582–1590. [Google Scholar] [CrossRef]
- Chatterji, T.; Singh, S.; Sen, M.; Singh, A.K.; Maurya, P.K.; Husain, N.; Srivastava, J.K.; Mandal, S.K.; Roy, R. Comprehensive 1H NMR metabolic profiling of body fluids for differentiation of meningitis in adults. Metabolomics 2016, 12, 1–14. [Google Scholar] [CrossRef]
- Chatterji, T.; Singh, S.; Sen, M.; Singh, A.K.; Agarwal, G.R.; Singh, D.K.; Srivastava, J.K.; Singh, A.; Srivastava, R.N.; Roy, R. Proton NMR metabolic profiling of CSF reveals distinct differentiation of meningitis from negative controls. Clin. Chim. Acta 2017, 469, 42–52. [Google Scholar] [CrossRef]
- Carlini, S.M.; Beyers, N.; Schoeman, J.F.; Nel, E.D.; Truter, E.J.; Donald, P.R. Adenylate kinase activity in the cerebrospinal fluid of children with tuberculous meningitis and its relationship to neurological outcome. Scand. J. Infect. Dis. 1997, 29, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Arief, G.; Arifin, M.Z.; Nataprawira, H.M. Correlation of lactate concentration in peripheral plasma and cerebrospinal fluid with Glasgow outcome scale for patients with tuberculous meningitis complicated by acute hydrocephalus treated with fluid diversions. World Neurosurg. 2018, 111, e178–e182. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, A.; Camina, F.; Lojo, S.; Rodríguez-Segade, S.; Castro-Gago, M. Concentrations of nucleotides, nucleosides, purine bases and urate in cerebrospinal fluid of children with meningitis. Acta Paediatr. 1993, 82, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, A.; Cid, E.; Rodríguez-García, J.; Camiña, F.; Rodríguez-Segade, S.; Castro-Gago, M. Neuron-specific enolase, nucleotides, nucleosides, purine bases, oxypurines and uric acid concentrations in cerebrospinal fluid of children with meningitis. Brain Dev. 2003, 25, 102–106. [Google Scholar] [CrossRef]
- Donald, P.R.; Malan, C. Cerebrospinal fluid lactate and lactate dehydrogenase levels as diagnostic aids in tuberculous meningitis. S. Afr. Med. J. 1985, 67, 19–20. [Google Scholar] [PubMed]
- Tang, L.M. Serial lactate determinations in tuberculous meningitis. Scand. J. Infect. Dis. 1988, 20, 81–83. [Google Scholar] [CrossRef]
- Donald, P.R.; Schoeman, J.F.; van Zyl, L. Cerebrospinal fluid lactate and raised intracranial pressure in tuberculous meningitis. J. Trop. Pediatr. 1989, 35, 263–264. [Google Scholar] [CrossRef]
- Lu, C.H.; Chang, W.N.; Chang, H.W. The prognostic factors of adult tuberculous meningitis. Infection 2001, 29, 299–304. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Chau, T.T.H.; Stepniewska, K.; Phu, N.H.; Chuong, L.V.; Sinh, D.X.; White, N.J.; Parry, C.M.; Farrar, J.J. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 2002, 360, 1287–1292. [Google Scholar] [CrossRef]
- Thwaites, G.E.; Simmons, C.P.; Than Ha Quyen, N.; Thi Hong Chau, T.; Phuong Mai, P.; Thi Dung, N.; Hoan Phu, N.; White, N.P.; Tinh Hien, T.; Farrar, J.J. Pathophysiology and prognosis in Vietnamese adults with tuberculous meningitis. J. Infect. Dis. 2003, 188, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Solomons, R.S.; Visser, D.H.; Donald, P.R.; Marais, B.J.; Schoeman, J.F.; van Furth, A.M. The diagnostic value of cerebrospinal fluid chemistry results in childhood tuberculous meningitis. Childs Nerv. Syst. 2015, 31, 1335–1340. [Google Scholar] [CrossRef]
- Cresswell, F.V.; Tugume, L.; Bahr, N.C.; Kwizera, R.; Bangdiwala, A.S.; Musubire, A.K.; Rutakingirwa, M.; Kagimu, E.; Nuwagira, E.; Mpoza, E.; et al. Xpert MTB/RIF Ultra for the diagnosis of HIV-associated tuberculous meningitis: A prospective validation study. Lancet Infect. Dis. 2020, 20, 308–317. [Google Scholar] [CrossRef]
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998, 16, 373–378. [Google Scholar] [CrossRef]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genomics 2001, 2, 155–168. [Google Scholar] [CrossRef]
- Mason, S.; Reinecke, C.J.; Solomons, R.; van Furth, A. Tuberculous meningitis in infants and children: Insights from nuclear magnetic resonance metabolomics. S. Afr. J. Sci. 2016, 112, 1–8. [Google Scholar] [CrossRef][Green Version]
- Zhang, P.; Zhang, W.; Lang, Y.; Qu, Y.; Chu, F.; Chen, J.; Cui, L. Mass spectrometry-based metabolomics for tuberculosis meningitis. Clin. Chim. Acta 2018, 483, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Guo, A.C.; Chaudhary, K.K.; Liu, P.; Yallou, F.S.; Dong, E.; Aziat, F.; Wishart, D.S. Multi-platform characterization of the human cerebrospinal fluid metabolome: A comprehensive and quantitative update. Genome Med. 2012, 4, 1–11. [Google Scholar] [CrossRef]
- Leen, W.G.; Willemsen, M.A.; Wevers, R.A.; Verbeek, M.M. Cerebrospinal fluid glucose and lactate: Age-specific reference values and implications for clinical practice. PLoS ONE 2012, 7, e42745. [Google Scholar] [CrossRef]
- Qureshi, G.A.; Baig, S.M.; Bednar, I.; Halawa, A.; Parvez, S.H. The neurochemical markers in cerebrospinal fluid to differentiate between aseptic and tuberculous meningitis. Neurochem. Int. 1998, 32, 197–203. [Google Scholar] [CrossRef]
- Marais, S.; Thwaites, G.; Schoeman, J.F.; Török, M.E.; Misra, U.K.; Prasad, K.; Donald, P.R.; Wilkinson, R.J.; Marais, B.J. Tuberculous meningitis: A uniform case definition for use in clinical research. Lancet Infect. Dis. 2010, 10, 803–812. [Google Scholar] [CrossRef]
- Sakushima, K.; Hayashino, Y.; Kawaguchi, T.; Jackson, J.L.; Fukuhara, S. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: A meta-analysis. J. Infect. 2011, 62, 255–262. [Google Scholar] [CrossRef]
- Huy, N.T.; Thao, N.T.; Diep, D.T.; Kikuchi, M.; Zamora, J.; Hirayama, K. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: A systemic review and meta-analysis. Crit. Care 2010, 14. [Google Scholar] [CrossRef]
- Boss, E.A.; Moolenaar, S.H.; Massuger, L.F.; Boonstra, H.; Engelke, U.F.; de Jong, J.G.; Wevers, R.A. High-resolution proton nuclear magnetic resonance spectroscopy of ovarian cyst fluid. NMR Biomed. 2000, 13, 297–305. [Google Scholar] [CrossRef]
- Gowda, G.N.; Raftery, D. Can NMR solve some significant challenges in metabolomics? J. Magn. Reson. 2015, 260, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
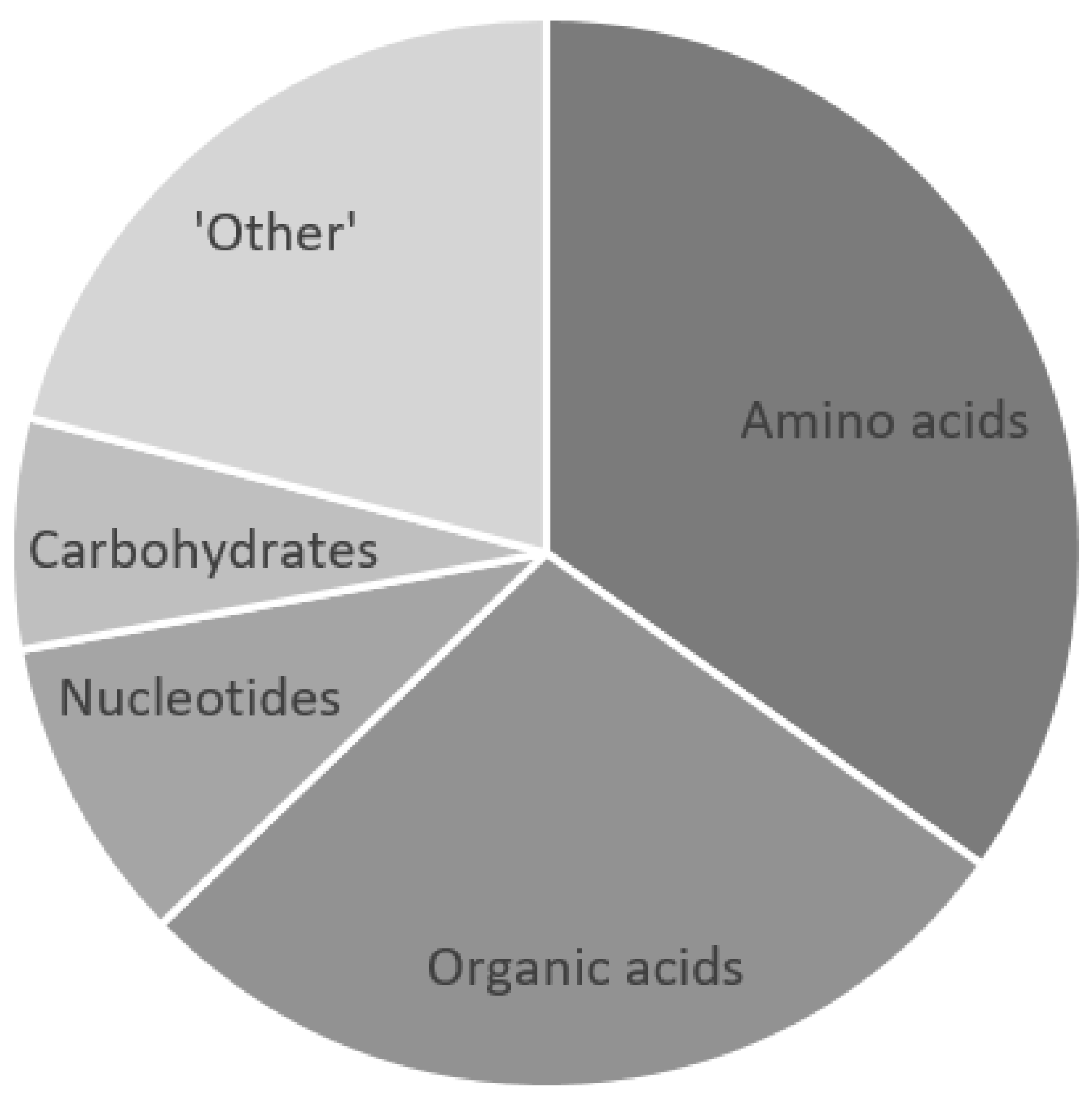
| Metabolites | Mason et al. 2015 [22] | Chatterji et al. 2016 [31] | Mason et al. 2017 [25] | Zhang et al. 2019 [29] | van Zyl et al. 2020 [28] | Rodríguez-Núñez et al. 2003 [36] | Reference Ranges | ||
|---|---|---|---|---|---|---|---|---|---|
| TBM cases (n) | 17 TBM | 26 TBM | 33 TBM | 25 TBM | 23 TBM | 9 TBM | |||
| Pediatric/Adult | Pediatric | Adult | Pediatric | Adult | Pediatric | Pediatric | Pediatric | Adult | |
| Amino acids | Alanine | 99.5 ± 71.9 | 77.3 ± 52 | 126.4 ± 82.2 | 154.7 ± 73.4 | 11–53.5 | 46 ± 27 | ||
| Asparagine | 19.9 ± 14 | 4–10 | 4 ± 2 | ||||||
| Glutamate | 54.4 ± 31.1 | 0–15 | 40 ± 52 | ||||||
| Glutamine | 697.1 ± 213.9 | 285–820 | 398 ± 150 | ||||||
| Glycine | 58.2 ± 31.7 | 6.3 ± 4.3 | 1–10 | 6.1 ± 1.4 | |||||
| Histidine | 18.69 ± 26.42 | 0–17 | 15 ± 8 | ||||||
| Isoleucine | 54 ± 27.6 | 97.58 ± 438.33 | 55 ± 40.9 | 25.5 ± 18.6 | 1.2–7.5 | 7 ± 5 | |||
| Leucine | 3.3–21.3 | 16 ± 9 | |||||||
| Lysine | 86.6 ± 40.7 | 36.5 ± 25.2 | 183.5 ± 69.2 | 6–37 | 29 ± 13 | ||||
| Methionine | 5 ± 1.7 | 0–5 | 5 ± 4 | ||||||
| Phenylalanine | 37.4 ± 18.4 | 72.04 ± 56.9 | 4–20 | 15 ± 8 | |||||
| Proline | 24.3 ± 25.8 | 105 ± 46.4 | 0–3 | 1.9 ± 1 | |||||
| Tyrosine | 31 ± 14.8 | 45.81 ± 32.56 | 3.9–18.5 | 12 ± 9 | |||||
| Valine | 69.5 ± 40.1 | 71.7 ±70 | 87.6 ± 56.6 | 71.5 ± 49.5 | 7.5–23 | 19 ± 13 | |||
| Carbohydrates | Glucose | 2689.1 ± 1217 | 2016.41 ± 792.65 | 2379.21 ± 1194.25 | 1900–4900 | 2600–4500 | |||
| α-Glucose | 1841.14 ± 1271.65 | N/A | N/A | ||||||
| β-Glucose | 3606.24 ± 3056.17 | N/A | N/A | ||||||
| myo-Inositol | 177.3 ± 95.6 | 206.5 ± 163.87 | 79.35 ± 31.02 | 293.08 ± 181.89 | N/A | 84 ± 40 | |||
| 2-Hydroxybutyrate | 82.6 ± 115.26 | 108.22 ± 53.46 | 37 ± 21 | 40 ± 24 | |||||
| Organic acids | Lactate | 7363.7 ± 2361.3 | 17019.32 ± 9554.84 | 7071.45 ± 3093.38 | 7593.41 ± 3592.63 | 1000–2200 | 1200–2700 | ||
| Pyruvate | 82 ± 35.6 | 92.53 ± 28.78 | 88.8 ± 40.35 | 27 ± 11 | 53 ± 42 | ||||
| Acetate | 183.38 ± 111.22 | 6.16 ± 5.66 | 43.07 ± 15.74 | 284 ± 126 | 58 ± 27 | ||||
| Citrate | 206.02 ± 29.47 | 262.23 ± 53.72 | N/A | 255 ± 96 | |||||
| Formate | 8.04 ± 9.99 | N/A | 32 ± 16 | ||||||
| 3-Hydroxybutyrate | 126.89 ±110.95 | N/A | 34 ± 31 | ||||||
| 3-Hydroxyisovalerate | 2.15 ± 2.05 | 11.87 ± 9.26 | N/A | 4 ± 2 | |||||
| 2-Hydroxyisovalerate | 15.78 ± 12.24 | N/A | 8 ± 6 | ||||||
| 2-Oxogluturate | 12.17 ± 5.56 | 2.1 ± 0.7 | 5 ± 4 | ||||||
| Isobutyrate | 0.92 ± 0.57 | 7.32 ± 5.68 | N/A | 0–3.6 | |||||
| Isovalerate | 5.75 ± 3.35 | N/A | 0–2.7 | ||||||
| Caprate | 12.86 ± 10.11 | N/A | N/A | ||||||
| Nucleotides | 1,3-Dimethylurate | 1.87 ± 0.98 | N/A | N/A | |||||
| Inosine | 1.14 ± 1.02 | 0.0–1.2 | 0.64 ± 0.35 | ||||||
| Xanthine | 6.14 ± 3.74 | 2.5 ± 0.7 | 13 ± 7 | ||||||
| Urate | 88.87 ± 69.89 | 10.2 ± 6.8 | 15 ± 10 | ||||||
| Other | Choline | 4.3 ± 3.4 | 1.51 ± 1.03 | 5.31 ± 2.92 | N/A | 7–13 | |||
| Creatinine | 47.7 ± 20.3 | 44.59 ± 19.81 | 78.55 ± 45.62 | N/A | 43 ± 12 | ||||
| Dimethyl sulfone | 4.9 ± 5.4 | N/A | 2 ± 1 | ||||||
| Acetamide | 2.32 ± 1.84 | N/A | N/A | ||||||
| Glycerophosphocholine | 0.78 ± 1.52 | N/A | 3.9 ± 1.2 | ||||||
| Creatine | 8.31 ± 3.97 | 153.04 ± 57.5 | N/A | 44 ± 13 | |||||
| Creatine phosphate | 32.48 ± 18.11 | N/A | N/A | ||||||
| Carnitine | 20.43 ± 8.55 | N/A | 1.9 ± 0.5 | ||||||
| Guanidinoacetate | 165.16 ± 74.32 | N/A | 2.3 ± 0.9 | ||||||
| Study | Pediatrics/ Adults | Population | TBM Cases (n) | Platform | Metabolites Characterizing TBM (n) |
|---|---|---|---|---|---|
| Coen et al. 2005 [30] | Adults | Australian | 2 | NMR | N/A |
| Mason et al. 2015 [22] | Pediatrics | South African | 33 | NMR | 17 |
| Chatterji et al. 2016 [31] | Adults | Indian | 30 | NMR | 15 |
| Li et al. 2017 [21] | Adults | Chinese | 18 | NMR | N/A |
| Mason et al. 2017 [25] | Pediatrics | South African | 33 | GC-MS | 5 |
| Dai et al. 2017 [19] | Adults | Chinese | 50 | LC-MS | N/A |
| van Laarhoven et al. 2018 [27] | Adults | Indonesian | 32 | LC-MS | 6 |
| Zhang et al. 2019 [29] | Adults | Chinese | 31 | NMR | 21 |
| van Zyl et al. 2020 [28] | Pediatrics | South African | 23 | NMR | 20 |
| Lactate | Glucose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Pediatrics/Adults | n | Mean | SD | Range | n | Mean | SD | Range |
| Donald and Malan 1985 [37] | Both | 26 | 4.86 | 38 | 1.94 | ||||
| Tang 1988 [38] | Adults | 21 | 7 | 2.3 | 3.9–11 | 21 | 1.7 | 0.8 | 0.6–3.2 |
| Donald et al. 1989 [39] | |||||||||
| Stage 2 | Pediatrics | 20 | 5.33 | 3.11–9.87 | 20 | 1.6 | 1–4 | ||
| Stage 3 | Pediatrics | 14 | 5.77 | 2.53–6.95 | 14 | 1.6 | 1–5.2 | ||
| Carlini et al. 1997 [33] | Pediatrics | 61 | 6.4 | 1.4–17.9 | 79 | 1.8 | 0.1–9.1 | ||
| Lu et al. 2001 [40] | |||||||||
| Poor outcome | Adults | 16 | 7.16 | 2.165 | 18 | 1.78 | 0.966 | ||
| Good outcome | Adults | 18 | 4.97 | 2.31 | 18 | 2.098 | 0.73 | ||
| Thwaites et al. 2002 [41] | Adults | 102 | 5.4 | 1.5–9.8 | |||||
| Thwaites et al. 2003 [42] | |||||||||
| Survived | Adults | 16 | 5.9 | 2.6 | |||||
| Died | Adults | 5 | 9.5 | 3.3 | |||||
| Mason et al. 2015 [22] | Pediatrics | 17 | 7.36 | 2.36 | 17 | 2.69 | 1.22 | ||
| Solomons et al. 2015 [43] | Pediatrics | 469 | 1.85 | 1.18 | |||||
| Chatterji et al. 2016 [31] | Adults | 26 | 17 | 9.55 | |||||
| Mason et al. 2016 [23] | Pediatrics | 20 | 5.2 | 2.6 | 1.5–10.5 | 16 | 1.96 | 1.35 | 0.2–5.1 |
| Faried et al. 2018 [34] | Both | 34 | 3.04 | 1.05 | 1.5–6.4 | ||||
| Zhang et al. 2019 [29] | Adults | 25 | 7.07 | 3.09 | 25 | 2.01 | 0.79 | ||
| Cresswell et al. 2020 [44] | Adults | 18 | 9.5 | 4.6–11 | |||||
| Van Zyl et al. 2020 [28] | Pediatrics | 23 | 7.59 | 3.59 | 23 | 2.4 | 1.19 | ||
| Weighted summary: | N = 462 | 6.59 | 2.89 | 3.04–17 | N = 758 | 1.88 | 0.18 | 1.6–2.69 | |
| Reference: | |||||||||
| Leen et al. 2012 [50] | Pediatrics | 1.0–2.2 | 1.9–4.9 | ||||||
| Leen et al. 2012 [50] | Adults | 1.2–2.7 | 2.6–4.5 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, S.; Solomons, R. CSF Metabolomics of Tuberculous Meningitis: A Review. Metabolites 2021, 11, 661. https://doi.org/10.3390/metabo11100661
Mason S, Solomons R. CSF Metabolomics of Tuberculous Meningitis: A Review. Metabolites. 2021; 11(10):661. https://doi.org/10.3390/metabo11100661
Chicago/Turabian StyleMason, Shayne, and Regan Solomons. 2021. "CSF Metabolomics of Tuberculous Meningitis: A Review" Metabolites 11, no. 10: 661. https://doi.org/10.3390/metabo11100661
APA StyleMason, S., & Solomons, R. (2021). CSF Metabolomics of Tuberculous Meningitis: A Review. Metabolites, 11(10), 661. https://doi.org/10.3390/metabo11100661