The Liability Threshold Model for Predicting the Risk of Cardiovascular Disease in Patients with Type 2 Diabetes: A Multi-Cohort Study of Korean Adults
Abstract
1. Introduction
2. Results
2.1. Nongenetic Risk Factors for DCVD
2.2. Genetic Risk Factors for DCVD
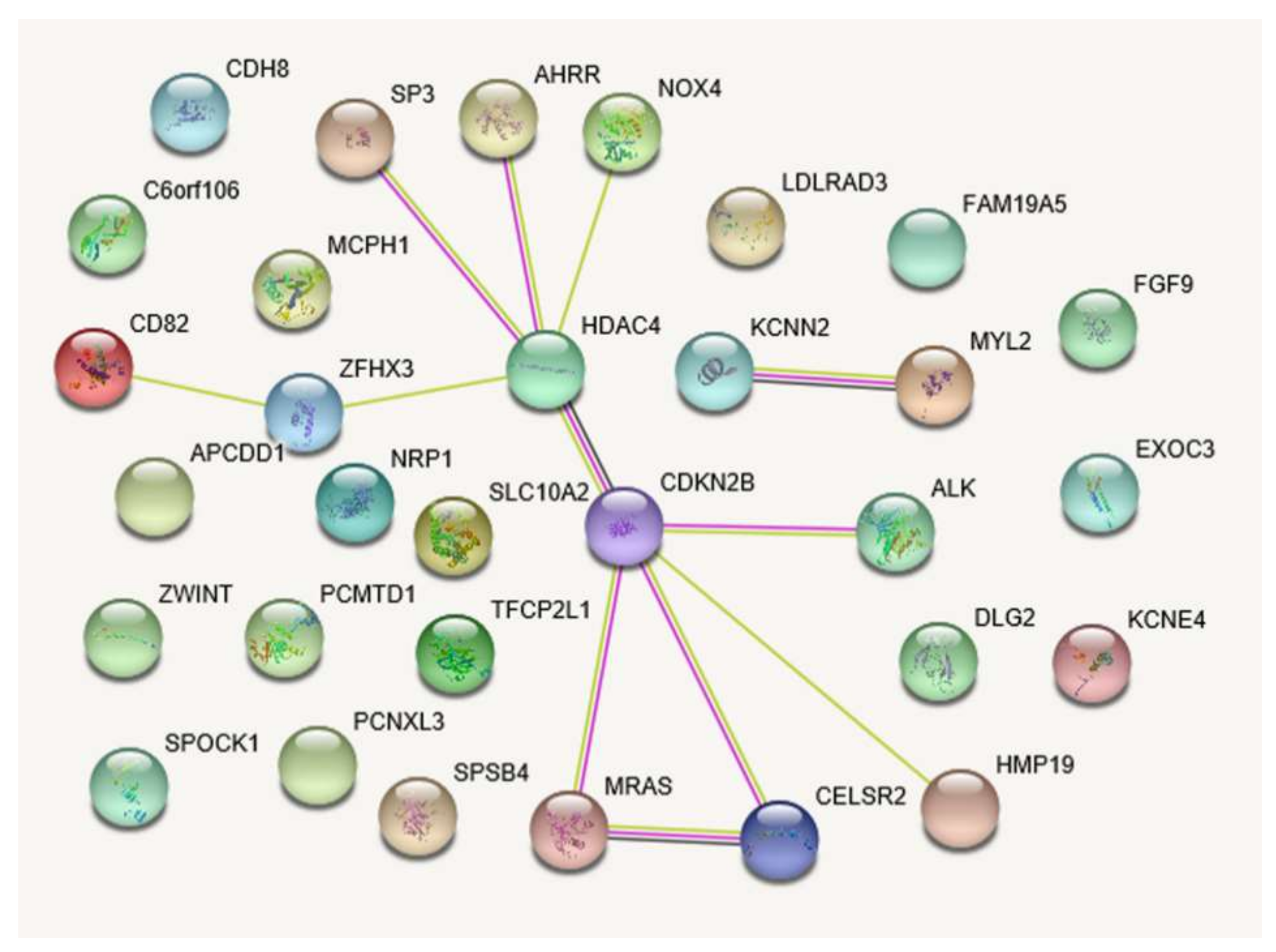
2.3. Gene Function Prediction
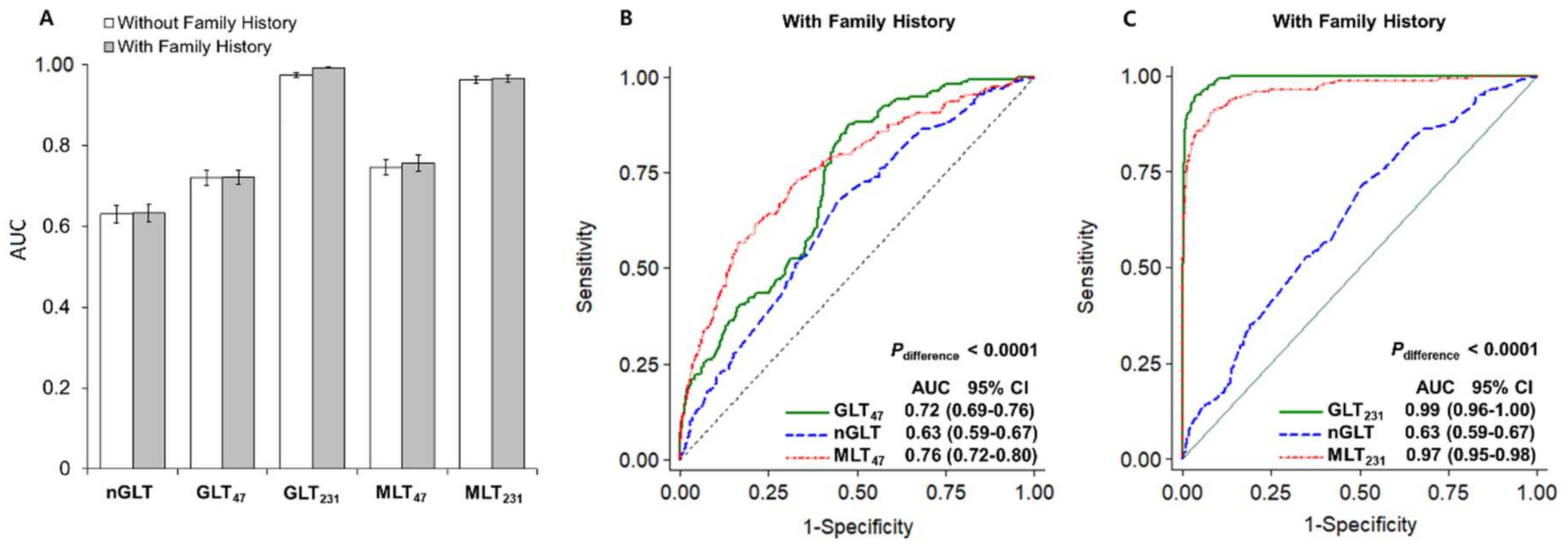
2.4. DCVD Risk Prediction
2.4.1. Genetic Risk Prediction
2.4.2. Multifactorial Risk Prediction
3. Discussion
4. Materials and Methods
4.1. Study Populations
4.2. Genotyping and Quality Controls
4.3. Statistical Analysis
4.3.1. Association of Conventional Risk Factors with the Development of DCVD
4.3.2. Genetic Association Analysis of DCVD Based on Generalized Linear Mixed Model
4.3.3. Gene Functional Enrichment, Pathway, and Network Analyses
4.3.4. Risk Prediction of DCVD in T2D Patients Using
Incidence-Based Liability Threshold Models
Polygenic Risk Scores
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States; Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. Available online: https://www.cdc.gov/diabetes/pubs (accessed on 23 December 2017).
- Kim, B.Y.; Won, J.C.; Lee, J.H.; Kim, H.S.; Park, J.W.; Ha, K.H.; Won, K.C.; Kim, D.J.; Park, K.S. Diabetes Fact Sheets in Korea, 2018: An Appraisal of Current Status. Diabetes Metab. J. 2019, 43, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Lee, C.H.; Yang, B.R.; Hwang, S.S.; Choi, N.K. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: A National Health Insurance Database Study. PLoS ONE 2014, 9, e110650. [Google Scholar] [CrossRef] [PubMed]
- Flannick, J.; Florez, J.C. Type 2 diabetes: Genetic data sharing to advance complex disease research. Nat. Rev. Genet. 2016, 17, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C. Genetics of cardiovascular and renal complications in diabetes. J. Diabetes Investig. 2016, 7, 139–154. [Google Scholar] [CrossRef]
- Qi, L.; Qi, Q.; Prudente, S.; Mendonca, C.; Andreozzi, F.; di Pietro, N.; Sturma, M.; Novelli, V.; Mannino, G.C.; Formoso, G.; et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA 2013, 310, 821–828. [Google Scholar] [CrossRef]
- Yang, J.; Zaitlen, N.A.; Goddard, M.E.; Visscher, P.M.; Price, A.L. Advantages and pitfalls in the application of mixed-model association methods. Nat. Genet. 2014, 46, 100–106. [Google Scholar] [CrossRef]
- Chatterjee, N.; Shi, J.; Garcia-Closas, M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat. Rev. Genet. 2016, 17, 392–406. [Google Scholar] [CrossRef]
- Murea, M.; Ma, L.; Freedman, B.I. Genetic and environmental factors associated with type 2 diabetes and diabetic vascular complications. Rev. Diabet. Stud. 2012, 9, 6–22. [Google Scholar] [CrossRef]
- Lee, S.H.; Wray, N.R.; Goddard, M.E.; Visscher, P.M. Estimating missing heritability for disease from genome-wide association studies. Am. J. Hum. Genet. 2011, 88, 294–305. [Google Scholar] [CrossRef]
- Flannick, J. The Contribution of Low-Frequency and Rare Coding Variation to Susceptibility to Type 2 Diabetes. Curr. Diab. Rep. 2019, 19, 25. [Google Scholar] [CrossRef]
- Martin-Timon, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Canizo-Gomez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef] [PubMed]
- Gheith, O.; Farouk, N.; Nampoory, N.; Halim, M.A.; Al-Otaibi, T. Diabetic kidney disease: World wide difference of prevalence and risk factors. J. Nephropharmacol. 2015, 5, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes Hypertension;and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Mo, E.Y.; Shin, S.J.; Moon, S.D.; Han, J.H.; Kim, E.S. Serum gamma-glutamyltransferase is not associated with subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.; Leite, N.C.; Salles, G.F. Prognostic Importance of C-Reactive Protein in High Cardiovascular Risk Patients With Type 2 Diabetes Mellitus: The Rio de Janeiro Type 2 Diabetes Cohort Study. J. Am. Heart Assoc. 2016, 5, e004554. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Smyth, A.; Rangarajan, S.; Ramasundarahettige, C.; Bangdiwala, S.I.; AlHabib, K.F.; Avezum, A.; Bengtsson Boström, K.; Chifamba, J.; Gulec, S.; et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income; middle-income; and high-income countries: The Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob. Health 2019, 7, e748–e760. [Google Scholar] [CrossRef]
- Doria, A. Leveraging Genetics to Improve Cardiovascular Health in Diabetes: The 2018 Edwin Bierman Award Lecture. Diabetes 2019, 68, 479–489. [Google Scholar] [CrossRef]
- Qi, L.; Parast, L.; Cai, T.; Powers, C.; Gervino, E.V.; Hauser, T.H.; Hu, F.B.; Doria, A. Genetic susceptibility to coronary heart disease in type 2 diabetes: 3 independent studies. J. Am. Coll. Cardiol. 2011, 58, 2675–2682. [Google Scholar] [CrossRef]
- Angelakopoulou, A.; Shah, T.; Sofat, R.; Shah, S.; Berry, D.J.; Cooper, J.; Palmen, J.; Tzoulaki, I.; Wong, A.; Jefferis, B.J.; et al. Comparative analysis of genome-wide association studies signals for lipids; diabetes; and coronary heart disease: Cardiovascular Biomarker Genetics Collaboration. Eur. Heart J. 2012, 33, 393–407. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef]
- Parmar, P.G.; Taal, H.R.; Timpson, N.J.; Thiering, E.; Lehtimaki, T.; Marinelli, M.; Lind, P.A.; Howe, L.D.; Verwoert, G.; Aalto, V.; et al. International Genome-Wide Association Study Consortium Identifies Novel Loci Associated With Blood Pressure in Children and Adolescents. Circ. Cardiovasc. Genet. 2016, 9, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Thorolfsdottir, R.B.; Fritsche, L.G.; Zhou, W.; Skov, M.W.; Graham, S.E.; Herron, T.J.; McCarthy, S.; Schmidt, E.M.; Sveinbjornsson, G.; et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018, 50, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, O.Y.; Li, Y.; Gorlov, I.; Ying, J.; Chen, W.V.; Assassi, S.; Reveille, J.D.; Arnett, F.C.; Zhou, X.; Bossini-Castillo, L.; et al. Gene-level association analysis of systemic sclerosis: A comparison of African-Americans and White populations. PLoS ONE 2018, 13, e0189498. [Google Scholar] [CrossRef] [PubMed]
- Divers, J.; Palmer, N.D.; Langefeld, C.D.; Brown, W.M.; Lu, L.; Hicks, P.J.; Smith, S.C.; Xu, J.; Terry, J.G.; Register, T.C.; et al. Genome-wide association study of coronary artery calcified atherosclerotic plaque in African Americans with type 2 diabetes. BMC Genet. 2017, 18, 105. [Google Scholar] [CrossRef]
- Parsa, A.; Chang, Y.P.; Kelly, R.J.; Corretti, M.C.; Ryan, K.A.; Robinson, S.W.; Gottlieb, S.S.; Kardia, S.L.R.; Shuldiner, A.R.; Liggett, S.B. Hypertrophy-associated polymorphisms ascertained in a founder cohort applied to heart failure risk and mortality. Clin. Transl. Sci. 2011, 4, 17–23. [Google Scholar] [CrossRef]
- Wray, N.R.; Yang, J.; Hayes, B.J.; Price, A.L.; Goddard, M.E.; Visscher, P.M. Pitfalls of predicting complex traits from SNPs. Nat. Rev. Genet. 2013, 14, 507–515. [Google Scholar] [CrossRef]
- Torkamani, A.; Wineinger, N.E.; Topol, E.J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 2018, 19, 581–590. [Google Scholar] [CrossRef]
- Morieri, M.L.; Gao, H.; Pigeyre, M.; Shah, H.S.; Sjaarda, J.; Mendonca, C.; Hastings, T.; Buranasupkajorn, P.; Motsinger-Reif, A.A.; Rotroff, D.M.; et al. Genetic Tools for Coronary Risk Assessment in Type 2 Diabetes: A Cohort Study From the ACCORD Clinical Trial. Diabetes Care. 2018, 41, 2404–2413. [Google Scholar] [CrossRef]
- Martin, A.R.; Kanai, M.; Kamatani, Y.; Okada, Y.; Neale, B.M.; Daly, M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019, 51, 584–591. [Google Scholar] [CrossRef]
- Duncan, L.; Shen, H.; Gelaye, B.; Meijsen, J.; Ressler, K.; Feldman, M.; Peterson, R.; Domingue, B. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 2019, 10, 3328. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B.; Pencina, K.M.; Janssens, A.C.J.W.; Greenland, P. Interpreting incremental value of markers added to risk prediction models. Am. J. Epidemiol. 2012, 176, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.S.; Janse, H.; Li, C.I. Net risk reclassification p values: Valid or misleading? J. Natl. Cancer Inst. 2014, 106, dju041. [Google Scholar] [CrossRef] [PubMed]
- Moorthie, S.; de Villiers, C.B.; Brigden, T.; Gaynor, L.; Hall, A.; Johnson, E.; Sanderson, S.; Kroese, M. Polygenic Scores Risk and Cardiovascular Disease; PHG Foundation: Cambridge, UK, 2019; Available online: https://www.phgfoundation.org/documents/prs-report-final-web.pdf (accessed on 19 October 2019).
- Cho, Y.S.; Go, M.J.; Kim, Y.J.; Heo, J.Y.; Oh, J.H.; Ban, H.J.; Yoon, D.; Lee, M.H.; Kim, D.J.; Park, M.; et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat. Genet. 2009, 41, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Go, M.J.; Hu, C.; Hong, C.B.; Kim, Y.K.; Lee, J.Y.; Hwang, J.Y.; Oh, J.H.; Kim, D.J.; Kim, N.H.; et al. Large-scale genome-wide association studies in East Asians identify new genetic loci influencing metabolic traits. Nat. Genet. 2011, 43, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Kim, S.; Kim, S.H.; Park, J.; Lim, H.A.; Park, H.J.; Choi, H.; Ng, D.; Lee, M.K.; Nam, M. Combined genome-wide linkage and association analyses of fasting glucose level in healthy twins and families of Korea. J. Korean. Med. Sci. 2013, 28, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Kato, N.; Hwang, J.Y.; Guo, X.; Tabara, Y.; Li, H.; Dorajoo, R.; Yang, X.; Tsai, F.J.; Li, S.; et al. Genome-wide association studies in East Asians identify new loci for waist-hip ratio and waist circumference. Sci. Rep. 2016, 6, 17958. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Howie, B.N.; Donnelly, P.; Marchini, J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Goddard, M.E.; Visscher, P.M. GCTA: A tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011, 88, 76–82. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H. STRING v11: Protein-protein association networks with increased coverage; supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- So, H.C.; Kwan, J.S.; Cherny, S.S.; Sham, P.C. Risk prediction of complex diseases from family history and known susceptibility loci; with applications for cancer screening. Am. J. Hum. Genet. 2011, 88, 548–565. [Google Scholar] [CrossRef] [PubMed]
- Barakat, K.; Hitman, G.A. Genetic susceptibility to macrovascular complications of type 2 diabetes mellitus. Best Pract. Res. Clin. Endocrinol. Metab. 2001, 15, 359–370. [Google Scholar] [CrossRef] [PubMed]
- So, H.C.; Sham, P.C. Exploring the predictive power of polygenic scores derived from genome-wide association studies: A study of 10 complex traits. Bioinformatics 2017, 33, 886–892. [Google Scholar] [CrossRef]
- Suarez-Alvarez, M.M.; Pham, D.T.; Prostov, M.Y.; Prostov, Y.I. Statistical approach to normalization of feature vectors and clustering of mixed datasets. Proc. R. Soc. A 2012, 468, 2630–2651. [Google Scholar] [CrossRef]
- Longton, G.; Pepe, M. Current Methods for Evaluating Prediction Performance of Biomarkers and Tests. Available online: http://research.fhcrc.org/content/dam/stripe/diagnostic-biomarkers-statistical-center/files/incrisk.pdf. (accessed on 1 February 2020).
| DCVD | T2D Only | Logistic Regression † | ||
|---|---|---|---|---|
| Characteristics * | (N = 168) | (N = 2210) | OR (95% CI) | p |
| Men, N (%) | 91 (54.2) | 1159 (52.4) | 1.07 (0.78–1.47) | 0.666 |
| Age, years (%) | 61.1 ± 0.5 | 56.9 ± 0.2 | 1.07 (1.05–1.09) | 3.2 × 10−9 ‡ |
| Income, N (%) | ||||
| <1 million won | 82 (48.8) | 908 (41.1) | Reference | |
| 1 million won ≤ | 69 (41.1) | 1110 (50.2) | 0.69 (0.49–0.96) | 0.027 |
| Education, N (%) | ||||
| <High school | 108 (64.3) | 1444 (65.3) | Reference | |
| High school ≤ | 58 (34.5) | 752 (34.0) | 1.03 (0.74–1.44) | 0.856 |
| Smoking status, N (%) | ||||
| Nonsmoker | 93 (55.4) | 1255 (56.8) | Reference | |
| Current smoker | 30 (17.9) | 510 (23.1) | 0.79 (0.52–1.21) | 0.286 |
| Ex-smoker | 45 (26.8) | 426 (19.3) | 1.43 (0.98–2.07) | 0.062 |
| Drinking status, N (%) | ||||
| Nondrinker | 75 (44.6) | 1042 (47.2) | Reference | |
| Current drinker | 65 (38.7) | 973 (44.0) | 0.93 (0.66–1.31) | 0.670 |
| Ex-drinker | 26 (15.5) | 184 (8.3) | 1.96 (1.22–3.15) | 0.005 |
| Family history, N (%) | ||||
| T2D, yes | 112 (66.7) | 1367 (61.9) | 0.96 (0.66–1.39) | 0.824 |
| DCVD, yes | 13 (11.7) | 82 (5.5) | 1.22 (0.66–2.25) | 0.530 |
| BMI, kg/m2 | 25.9 ± 0.3 | 25.2 ± 0.1 | 1.06 (1.01–1.12) | 0.012 ‡ |
| SBP, mm Hg | 131.1 ± 1.5 | 127.9 ± 0.4 | 1.01 (1.00–1.02) | 0.032 |
| DBP, mm Hg | 80.8 ± 0.9 | 79.8 ± 0.2 | 1.01 (0.99–1.02) | 0.256 |
| TC, mg/dL | 192.9 ± 3.3 | 201.1 ± 0.9 | 0.995 (0.992–0.999) | 0.018 |
| TG, mg/dL | 176.0 ± 7.5 | 199.7 ± 3.2 | 0.998 (0.997–0.999) | 0.042 |
| GGT, IU/L | 38.4 ± 2.5 | 57.8 ± 2.7 | 0.996 (0.992–1.000) | 0.027 |
| AST, IU/L | 29.8 ± 1.3 | 32.2 ± 0.6 | 0.995 (0.985–1.004) | 0.246 |
| ALT, IU/L | 30.9 ± 1.5 | 33.7 ± 0.8 | 0.996 (0.988–1.003) | 0.263 |
| Creatinine, mg/dL | 1.01 ± 0.03 | 0.91 ± 0.01 | 2.62 (1.64–4.19) | 5.3 × 10−5 ‡ |
| CRP, mg/L | 2.73 ± 0.49 | 2.57 ± 0.10 | 1.01 (0.98–1.04) | 0.689 |
| FPG, mg/dL | 124.7 ± 3.3 | 137.0 ± 1.2 | 0.99 (0.99–1.00) | 0.004 |
| 2 h PG, mg/dL | 200.3 ± 10.7 | 240.9 ± 3.0 | 0.99 (0.99–1.00) | 0.001 |
| Hemoglobin A1c | 7.09 ± 1.49 | 7.44 ± 1.74 | 0.87 (0.74–1.03) | 0.101 |
| Gene | Chr | SNP | Function | N/R | LMM † | ||
|---|---|---|---|---|---|---|---|
| RAF (Ca/Co) | OR | p | |||||
| 15 previously reported SNPs (p < 0.05)‡ | |||||||
| LOC107986441 (KCNN2) * | 5q22.2 | rs4621553 | intron | A/G | 0.09/0.05 | 1.05 | 0.002 |
| MRAS * | 3q22.3 | rs9818870 | 3’ UTR | C/T | 0.03/0.01 | 1.08 | 0.011 |
| CELSR2, PSRC1 * | 1p13.3 | rs599839 | 500bp~3′ UTR | A/G | 0.09/0.06 | 1.04 | 0.015 |
| IBTK * | 6q14.1 | rs16893526 | intergenic | G/A | 0.15/0.11 | 1.03 | 0.017 |
| ZFHX3 * | 16q22.3 | rs879324 | intron | A/G | 0.67/0.62 | 1.02 | 0.022 |
| CDKN2B * | 9p21.3 | rs1333042 | intron | A/G | 0.71/0.65 | 1.02 | 0.025 |
| MREGP1 | 12p11.21 | rs11610422 | intergenic | A/G | 0.07/0.05 | 1.04 | 0.027 |
| LOC100288146 | 4q24 | rs17035270 | intron | C/T | 0.99/0.04 | 1.04 | 0.028 |
| SPSB4 * | 3q23 | rs16851055 | intron (ncRNA) | G/A | 0.23/0.18 | 1.02 | 0.036 |
| ILRUN (C6orf106) * | 6p21.31 | rs2814993 | intron | G/A | 0.03/0.01 | 1.07 | 0.037 |
| MTAP * | 9p21.3 | rs7865618 | intron | G/A | 0.90/0.86 | 1.02 | 0.037 |
| PCNXL3 | 11q13.1 | rs12801636 | intron | A/G | 0.56/0.49 | 1.02 | 0.038 |
| HDAC4 * | 2q37.3 | rs6706785 | intergenic | G/T | 0.32/0.27 | 1.02 | 0.040 |
| TFCP2L1 * | 2q14.2 | rs17006292 | intron | C/A | 0.04/0.03 | 1.05 | 0.043 |
| MYL2* | 12q24.11 | rs3782889 | intron | G/A | 0.88/0.83 | 1.02 | 0.046 |
| 32 SNPs associated with DCVD (p < 10−4) | |||||||
| MCPH1 * | 8p23.2 | rs4538911 | intergenic | C/G | 0.13/0.06 | 1.08 | 5.0 × 10−7 |
| LOC100505973 | 21q21.1 | rs9982069 | intergenic | G/A | 0.49/0.38 | 1.04 | 9.1 × 10−7 |
| CDH11 * | 16q21 | rs17465734 | intergenic | T/A | 0.05/0.01 | 1.14 | 8.0 × 10−6 |
| CD82 * | 11p11.2 | rs7946015 | intergenic | A/T | 0.26/0.17 | 1.04 | 8.2 × 10−6 |
| FAM19A5 (TAFA5) * | 22q13.31 | rs5768165 | intergenic | G/T | 0.11/0.05 | 1.07 | 1.3 × 10−5 |
| rs2338258 | intergenic | T/C | 0.13/0.07 | 1.06 | 3.6 × 10−5 | ||
| rs5768143 | intergenic | C/T | 0.13/0.07 | 1.05 | 9.1 × 10−5 | ||
| MGC45800 | 4q34.3 | rs17072597 | intron | C/T | 0.22/0.14 | 1.05 | 1.5 × 10−5 |
| KCNE4 * | 2q36.1 | rs16864293 | intergenic | T/A | 0.09/0.04 | 1.08 | 1.6 × 10−5 |
| SLC9A3 * | 5p15.33 | rs1053226 | intron | C/T | 0.05/0.02 | 1.11 | 1.8 × 10−5 |
| SP3 * | 2q31.1 | rs41326844 | intergenic | T/C | 0.47/0.36 | 1.03 | 2.5 × 10−5 |
| AHRR * | 5p15.33 | rs6555242 | intron | T/G | 0.07/0.03 | 1.09 | 3.1 × 10−5 |
| VAPA * | 18p11.22 | rs16956185 | intergenic | G/A | 0.15/0.08 | 1.06 | 3.2 × 10−5 |
| ZWINT *, MIR3924 | 10q21.1 | rs1503908 | intergenic | A/G | 0.19/0.12 | 1.05 | 3.9 × 10−5 |
| NOX4 * | 11q14.3 | rs319025 | intron | T/C | 0.67/0.56 | 1.03 | 4.1 × 10−5 |
| SPOCK1 * | 5q31.2 | rs6893667 | intergenic | C/T | 0.06/0.02 | 1.10 | 4.2 × 10−5 |
| C14orf64 (LINCO1550) | 14q32.2 | rs877455 | intergenic | G/A | 0.10/0.05 | 1.07 | 4.8 × 10−5 |
| LDLRAD3 * | 11p13 | rs1001715 | intron | G/A | 0.44/0.33 | 1.03 | 4.9 × 10−5 |
| rs12276510 | intron | G/A | 0.43/0.33 | 1.03 | 5.5 × 10−5 | ||
| ST18 * | 8q11.23 | rs2450153 | intergenic | G/A | 0.63/0.52 | 1.03 | 5.3 × 10−5 |
| rs3843918 | intergenic | T/C | 0.46/0.44 | 1.03 | 7.0 × 10−5 | ||
| CYP2B6 * | 19q13.2 | rs1872125 | intron | T/C | 0.24/0.16 | 1.04 | 5.7 × 10−5 |
| FGF9 * | 13q12.11 | rs9506827 | intergenic | T/C | 0.29/0.20 | 1.04 | 5.9 × 10−5 |
| MIRN656 | 14q32.31 | rs8016145 | intergenic | G/A | 0.09/0.04 | 1.08 | 6.4 × 10−5 |
| DLG2 * | 11q14.1 | rs349083 | intron | G/A | 0.47/0.36 | 1.03 | 6.5 × 10−5 |
| LOC646700 | 9p21.1 | rs10968749 | intergenic | A/G | 0.19/0.12 | 1.04 | 7.4 × 10−5 |
| METTL21EP, SLC10A2 * | 13q33.1 | rs9586032 | intergenic | G/A | 0.23/0.15 | 1.04 | 7.4 × 10−5 |
| PPIAL3 | 21q21.1 | rs2825256 | intergenic | T/A | 0.67/0.55 | 1.03 | 7.4 × 10−5 |
| HMP19 * | 5q35.2 | rs2913472 | intergenic | A/C | 0.05/0.02 | 1.11 | 7.9 × 10−5 |
| ALK * | 2p23.2 | rs4575680 | intron | G/C | 0.08/0.04 | 1.07 | 9.0 × 10−5 |
| MIR1261 | 11q14.3 | rs10501726 | intergenic | A/T | 0.08/0.04 | 1.08 | 9.5 × 10−5 |
| NRP1 * | 10p11.22 | rs767164 | intergenic | T/A | 0.30/0.21 | 1.04 | 9.8 × 10−5 |
| Biological Function * | Gene, N | p† | FDR, % † | Gene Set |
|---|---|---|---|---|
| GO:0014911~positive regulation of smooth muscle cell migration | 3 | 0.0012 | 2.0 | NOX4, HDAC4, NRP1 |
| GO:0048731~system development | 16 | 0.0014 | 2.3 | NOX4, NRP1, MYL2, FGF9, MRAS, TFCP2L1, SPOCK1, CELSR2, ALK, APCDD1, HDAC4, CDKN2B, SP3, MCPH1, ZFHX3, DLG2 |
| GO:0061061~muscle structure development | 6 | 0.0026 | 4.2 | NOX4, HDAC4, MYL2, FGF9, MRAS, ZFHX3 |
| GO:0048513~animal organ development | 13 | 0.0027 | 4.3 | NOX4, NRP1, MYL2, FGF9, MRAS, TFCP2L1, CELSR2, APCDD1, HDAC4, CDKN2B, SP3, MCPH1, ZFHX3 |
| GO:0007517~muscle organ development | 5 | 0.0030 | 4.8 | HDAC4, MYL2, FGF9, MRAS, ZFHX3 |
| GO:0014910~regulation of smooth muscle cell migration | 3 | 0.0035 | 5.6 | NOX4, HDAC4, NRP1 |
| GO:0014909~smooth muscle cell migration | 3 | 0.0040 | 6.4 | NOX4, HDAC4, NRP1 |
| GO:0048523~negative regulation of cellular process | 15 | 0.0048 | 7.5 | NOX4, NRP1, MYL2, FGF9, TFCP2L1, SPOCK1, APCDD1, HDAC4, AHRR, CDKN2B, SP3, ZWINT, MCPH1, ZFHX3, DLG2 |
| GO:0007275~multicellular organism development | 16 | 0.0055 | 8.7 | NOX4, NRP1, MYL2, FGF9, MRAS, TFCP2L1, SPOCK1, CELSR2, ALK, APCDD1, HDAC4, CDKN2B, SP3, MCPH1, ZFHX3, DLG2 |
| GO:0014812~muscle cell migration | 3 | 0.0055 | 8.7 | NOX4, HDAC4, NRP1 |
| Model | Ca/Co, N * | Ca/Co, Mean (Range) † | OR (95% CI) ‡ | p-Value | AUC |
|---|---|---|---|---|---|
| Nongenetic | |||||
| nGLT | 167/2195 | 0.24 (0.06–0.40)/0.20 (0.06–0.40) | 1.23 (1.15–1.32) | 4.8 × 10−9 | 0.63 (0.59–0.67) |
| nGRS | 167/2195 | 2.32 (0.00–4.25)/1.78 (0.00–4.41) | 1.21 (1.13–1.29) | 8.9 × 10−9 | 0.64 (0.60–0.68) |
| Genetic | |||||
| GLT15 | 164/2172 | 0.15 (0.11–0.23)/0.15 (0.10–0.26) | 1.05 (0.99–1.10) | 0.089 | 0.54 (0.49–0.58) |
| GLT47 | 163/2076 | 0.25 (0.14–0.42)/0.20 (0.12–0.39) | 1.54 (1.41–1.68) | 7.3 × 10−22 | 0.73 (0.70- 0.77) |
| GLT231 | 114/1558 | 0.38 (0.21–0.66)/0.16 (0.06–0.45) | 14.13 (9.08–21.97) | 7.4 × 10−32 | 0.99 (0.99–0.99) |
| L: < 0.21 | 0 (0.0)/1911 (86.5) | Reference | NA | 0.93 (0.93–0.94) | |
| H: 0.21 ≤ | 168 (100.0)/299 (13.5) | NA | NA | 100/86.8/87.7 § | |
| PRS15 | 164/2172 | 0.27 (0.16–0.42)/0.26 (0.12–0.48) | 1.16 (1.02–1.30) | 0.019 | 0.55 (0.50–0.60) |
| PRS47 | 163/2076 | 0.93 (0.51–1.50)/0.69 (0.29–1.31) | 2.72 (2.38–3.10) | 3.0 × 10−49 | 0.84 (0.81–0.87) |
| PRS231 | 114/1558 | 5.40 (4.57–6.82)/4.19 (3.28–5.81) | 18.41 (11.17–30.35) | 3.2 × 10−30 | 0.99 (0.99–0.99) |
| L: < 4.57 | 0 (0.0)/ 1369 (62.0) | Reference | NA | 0.81 (0.80–0.82) | |
| H: 4.57 ≤ | 168 (100.0)/ 841 (38.1) | NA | NA | 100/61.9/64.6 § | |
| Multifactorial | |||||
| MLT47 | 162/2062 | 0.23 (0.06–0.38)/0.17 (0.04–0.38) | 1.84 (1.65–2.04) | 1.9 × 10−29 | 0.76 (0.72–0.80) |
| MLT231 | 113/1552 | 0.41 (0.08–0.72)/0.15 (0.02–0.51) | 7.79 (5.67–10.68) | 4.9 × 10−37 | 0.97 (0.95–0.99) |
| MRS47 | 162/2062 | 3.26 (0.68–5.43)/2.47 (0.37–5.40) | 1.39 (1.28–1.51) | 2.5 × 10−15 | 0.71 (0.67–0.76) |
| MRS231 | 113/1552 | 7.71 (4.57–9.99)/5.92 (3.48–8.74) | 2.98 (2.48–3.58) | 3.0 × 10−31 | 0.86 (0.82–0.89) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, E.P.; Heo, S.G.; Park, J.W. The Liability Threshold Model for Predicting the Risk of Cardiovascular Disease in Patients with Type 2 Diabetes: A Multi-Cohort Study of Korean Adults. Metabolites 2021, 11, 6. https://doi.org/10.3390/metabo11010006
Hong EP, Heo SG, Park JW. The Liability Threshold Model for Predicting the Risk of Cardiovascular Disease in Patients with Type 2 Diabetes: A Multi-Cohort Study of Korean Adults. Metabolites. 2021; 11(1):6. https://doi.org/10.3390/metabo11010006
Chicago/Turabian StyleHong, Eun Pyo, Seong Gu Heo, and Ji Wan Park. 2021. "The Liability Threshold Model for Predicting the Risk of Cardiovascular Disease in Patients with Type 2 Diabetes: A Multi-Cohort Study of Korean Adults" Metabolites 11, no. 1: 6. https://doi.org/10.3390/metabo11010006
APA StyleHong, E. P., Heo, S. G., & Park, J. W. (2021). The Liability Threshold Model for Predicting the Risk of Cardiovascular Disease in Patients with Type 2 Diabetes: A Multi-Cohort Study of Korean Adults. Metabolites, 11(1), 6. https://doi.org/10.3390/metabo11010006







