Continuous Glucose and Heart Rate Monitoring in Young People with Type 1 Diabetes: An Exploratory Study about Perspectives in Nocturnal Hypoglycemia Detection
Abstract
1. Introduction
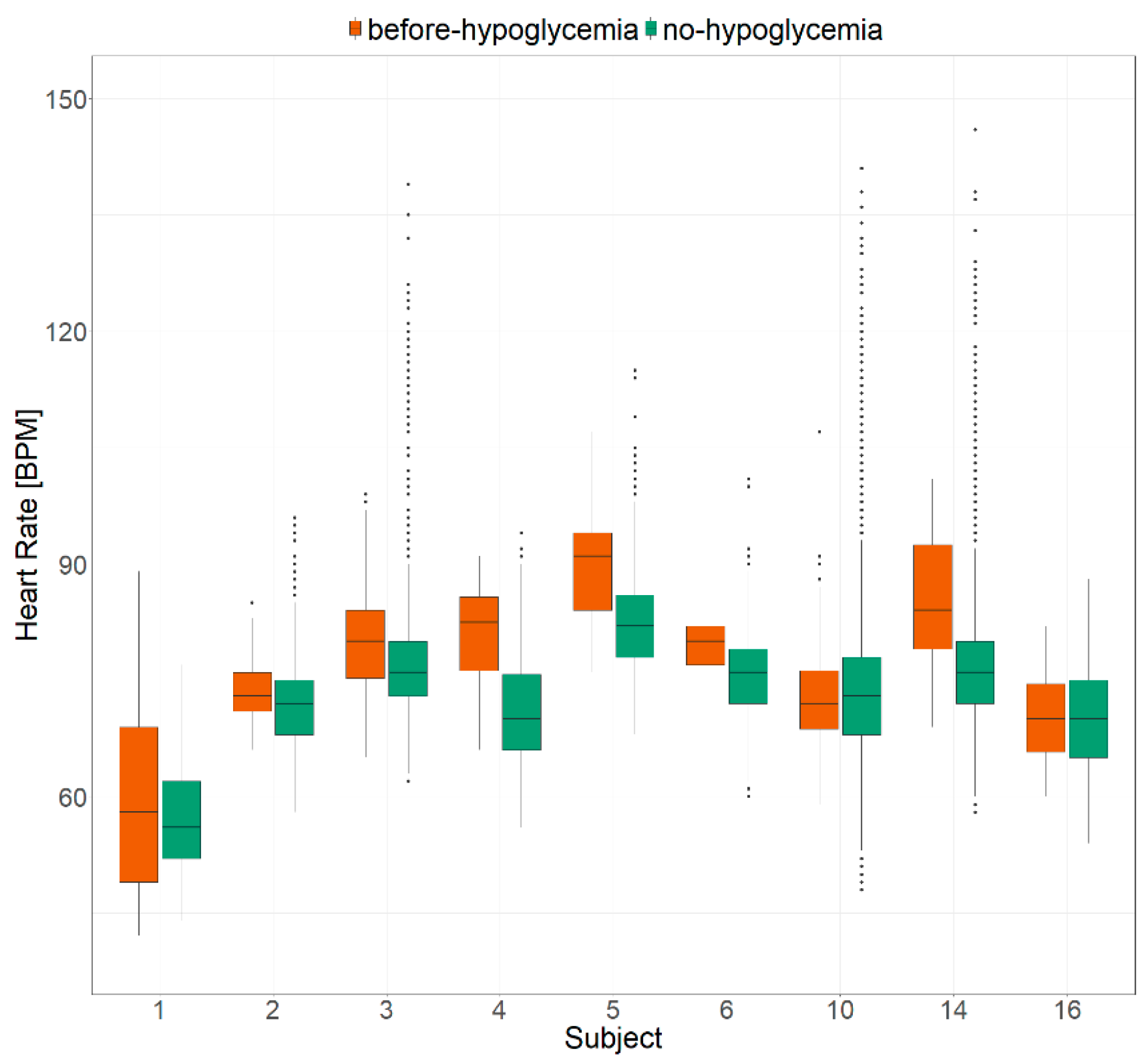
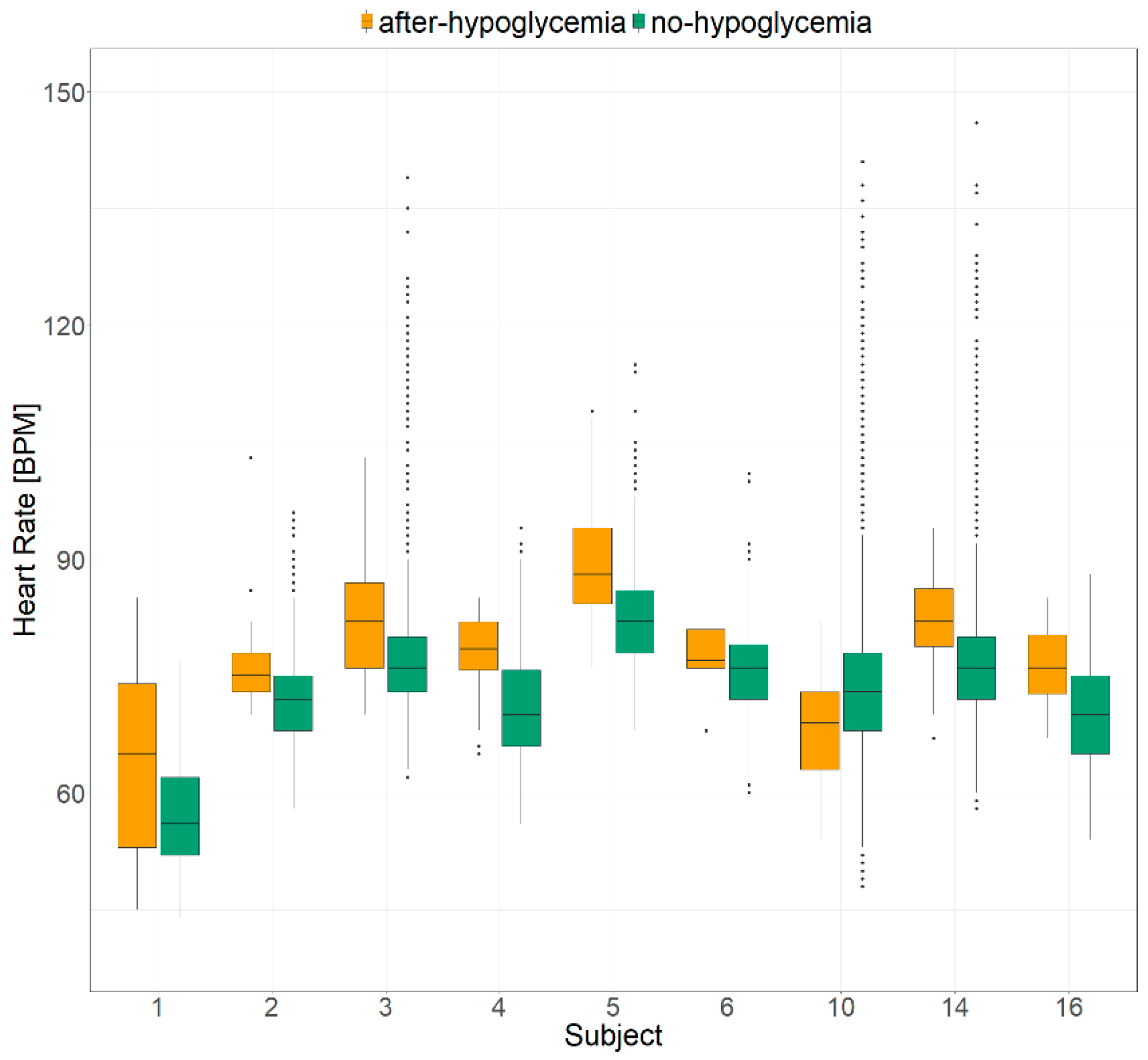
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Anthropometric and Clinical Assessment
4.3. Advanced Intelligent Distant—Glucose Monitoring (AID-GM)
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGill, D.E.; Levitsky, L.L. Management of Hypoglycemia in Children and Adolescents with Type 1 Diabetes Mellitus. Curr. Diabetes Rep. 2016, 16, 88. [Google Scholar] [CrossRef] [PubMed]
- Shaefer, C.; Hinnen, D.; Sadler, C. Hypoglycemia and Diabetes: Increased Need for Awareness. Curr. Med. Res. Opin. 2016, 32, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Awoniyi, O.; Rehman, R.; Dagogo-Jack, S. Hypoglycemia in Patients with Type 1 Diabetes: Epidemiology, Pathogenesis, and Prevention. Curr. Diabetes Rep. 2013, 13, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Cryer, P.E. Hypoglycemia in Type 1 Diabetes Mellitus. Endocrinol. Metab. Clin. N. Am. 2010, 39, 641–654. [Google Scholar] [CrossRef]
- Gill, G.V.; Woodward, A.; Casson, I.F.; Weston, P.J. Cardiac Arrhythmia and Nocturnal Hypoglycaemia in Type 1 Diabetes—The “dead in Bed” Syndrome Revisited. Diabetologia 2009, 52, 42–45. [Google Scholar] [CrossRef]
- Tu, E.; Twigg, S.M.; Semsarian, C. Sudden Death in Type 1 Diabetes: The Mystery of the ‘Dead in Bed’ Syndrome. Int. J. Cardiol. 2010, 138, 91–93. [Google Scholar] [CrossRef]
- Hsieh, A.; Twigg, S.M. The Enigma of the Dead-in-Bed Syndrome: Challenges in Predicting and Preventing This Devastating Complication of Type 1 Diabetes. J. Diabetes Complicat. 2014, 28, 585–587. [Google Scholar] [CrossRef]
- Woodward, A.; Weston, P.; Casson, I.F.; Gill, G.V. Nocturnal Hypoglycaemia in Type 1 Diabetes—Frequency and Predictive Factors. QJM 2009, 102, 603–607. [Google Scholar] [CrossRef]
- Koltin, D.; Daneman, D. Dead-in-Bed Syndrome—A Diabetes Nightmare. Pediatric Diabetes 2008, 9, 504–507. [Google Scholar] [CrossRef]
- Gagnum, V.; Stene, L.C.; Jenssen, T.G.; Berteussen, L.M.; Sandvik, L.; Joner, G.; Njølstad, P.R.; Skrivarhaug, T. Causes of Death in Childhood-Onset Type 1 Diabetes: Long-Term Follow-Up. Diabet. Med. 2017, 34, 56–63. [Google Scholar] [CrossRef]
- Pop-Busui, R. Cardiac Autonomic Neuropathy in Diabetes: A Clinical Perspective. Diabetes Care 2010, 33, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.L.; Frystyk, J.; Tarnow, L.; Fleischer, J. Are Changes in Heart Rate Variability During Hypoglycemia Confounded by the Presence of Cardiovascular Autonomic Neuropathy in Patients with Diabetes? Diabetes Technol. Ther. 2017, 19, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, L.J.; Lees, T.; Clifton-Bligh, R.; Lal, S. Association Between Heart Rate Variability Measures and Blood Glucose Levels: Implications for Noninvasive Glucose Monitoring for Diabetes. Diabetes Technol. Ther. 2016, 18, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.L.; Henriksen, M.M.; Tarnow, L.; Thorsteinsson, B.; Pedersen-Bjergaard, U.; Fleischer, J. Validation of an Algorithm for Predicting Hypoglycemia From Continuous Glucose Measurements and Heart Rate Variability Data. J. Diabetes Sci. Technol. 2019, 13, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Salvi, E.; Bosoni, P.; Tibollo, V.; Kruijver, L.; Calcaterra, V.; Sacchi, L.; Bellazzi, R.; Larizza, C. Patient-Generated Health Data Integration and Advanced Analytics for Diabetes Management: The AID-GM Platform. Sensors 2020, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Owens, D.R. The Application of Simple Metrics in the Assessment of Glycaemic Variability. Diabetes Metab. 2018, 44, 313–319. [Google Scholar] [CrossRef]
- Los, E.; Wilt, A.S. Diabetes mellitus type 1 in children. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Marks, A.; Wilson, V.; Crisp, J. The Management of Type 1 Diabetes in Primary School: Review of the Literature. Issues Compr. Pediatric Nurs. 2013, 36, 98–119. [Google Scholar] [CrossRef]
- Silver, B.; Ramaiya, K.; Andrew, S.B.; Fredrick, O.; Bajaj, S.; Kalra, S.; Charlotte, B.M.; Claudine, K.; Makhoba, A. EADSG Guidelines: Insulin Therapy in Diabetes. Diabetes Ther. 2018, 9, 449–492. [Google Scholar] [CrossRef]
- Mazarello Paes, V.; Barrett, J.K.; Taylor-Robinson, D.C.; Chesters, H.; Charalampopoulos, D.; Dunger, D.B.; Viner, R.M.; Stephenson, T.J. Effect of Early Glycemic Control on HbA1c Tracking and Development of Vascular Complications after 5 Years of Childhood Onset Type 1 Diabetes: Systematic Review and Meta-Analysis. Pediatric Diabetes 2019, 20, 494–509. [Google Scholar] [CrossRef]
- Subramanian, S.; Hirsch, I.B. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes Mellitus: Implications of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study 30-Year Follow-Up. Endocrinol. Metab. Clin. N. Am. 2018, 47, 65–79. [Google Scholar] [CrossRef]
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide Estimates of Incidence, Prevalence and Mortality of Type 1 Diabetes in Children and Adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef] [PubMed]
- Evans-Cheung, T.C.; Bodansky, H.J.; Parslow, R.C.; Feltbower, R.G. Mortality and Acute Complications in Children and Young Adults Diagnosed with Type 1 Diabetes in Yorkshire, UK: A Cohort Study. Diabet. Med. 2018, 35, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Cardwell, C.R.; Black, C.J.; McCance, D.R.; Patterson, C.C. Excess Mortality in Type 1 Diabetes Diagnosed in Childhood and Adolescence: A Systematic Review of Population-Based Cohorts. Acta Diabetol. 2015, 52, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, G.; Källén, B. Mortality in Childhood-Onset Type 1 Diabetes: A Population-Based Study. Diabetes Care 2005, 28, 2384–2387. [Google Scholar] [CrossRef] [PubMed]
- McNally, P.G.; Lawrence, I.G.; Panerai, R.B.; Weston, P.J.; Thurston, H. Sudden Death in Type 1 Diabetes. Diabetes Obes. Metab. 1999, 1, 151–158. [Google Scholar] [CrossRef]
- Heller, S. Dead in Bed. Diabet. Med. 1999, 16, 782–785. [Google Scholar]
- Harris, N.D.; Heller, S.R. Sudden Death in Young Patients with Type 1 Diabetes: A Consequence of Disease, Treatment or Both? Diabet. Med. 1999, 16, 623–625. [Google Scholar] [CrossRef]
- Weston, P.J.; Gill, G.V. Is Undetected Autonomic Dysfunction Responsible for Sudden Death in Type 1 Diabetes Mellitus? The ‘Dead in Bed’ Syndrome Revisited. Diabet. Med. 1999, 16, 626–631. [Google Scholar] [CrossRef]
- Lawrence, I.G.; Weston, P.J.; Bennett, M.A.; McNally, P.G.; Burden, A.C.; Thurston, H. Is Impaired Baroreflex Sensitivity a Predictor or Cause of Sudden Death in Insulin-Dependent Diabetes Mellitus? Diabet. Med. 1997, 14, 82–85. [Google Scholar] [CrossRef]
- Heller, S.R. Abnormalities of the Electrocardiogram during Hypoglycaemia: The Cause of the Dead in Bed Syndrome? Int J. Clin. Pract. Suppl. 2002, 129, 27–32. [Google Scholar]
- Koivikko, M.L.; Kenttä, T.; Salmela, P.I.; Huikuri, H.V.; Perkiömäki, J.S. Changes in Cardiac Repolarisation during Spontaneous Nocturnal Hypoglycaemia in Subjects with Type 1 Diabetes: A Preliminary Report. Acta Diabetol. 2017, 54, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.M.; Daphna-Iken, D.; Chen, Y.S.; VanderWeele, J.; Jethi, K.; Fisher, S.J. Severe Hypoglycemia-Induced Lethal Cardiac Arrhythmias Are Mediated by Sympathoadrenal Activation. Diabetes 2013, 62, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Due-Andersen, R.; Hoi-Hansen, T.; Larroude, C.E.; Olsen, N.V.; Kanters, J.K.; Boomsma, F.; Pedersen-Bjergaard, U.; Thorsteinsson, B. Cardiac Repolarization during Hypoglycaemia in Type 1 Diabetes: Impact of Basal Renin-Angiotensin System Activity. Europace 2008, 10, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Bertachi, A.; Viñals, C.; Biagi, L.; Contreras, I.; Vehí, J.; Conget, I.; Giménez, M. Prediction of Nocturnal Hypoglycemia in Adults with Type 1 Diabetes under Multiple Daily Injections Using Continuous Glucose Monitoring and Physical Activity Monitor. Sensors 2020, 20, 1705. [Google Scholar] [CrossRef]
- Resalat, N.; Hilts, W.; Youssef, J.E.; Tyler, N.; Castle, J.R.; Jacobs, P.G. Adaptive Control of an Artificial Pancreas Using Model Identification, Adaptive Postprandial Insulin Delivery, and Heart Rate and Accelerometry as Control Inputs. J. Diabetes Sci. Technol. 2019, 13, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Mba, C.M.; Nganou-Gnindjio, C.-N.; Azabji-Kenfack, M.; Mfeukeu-Kuate, L.; Dehayem, M.Y.; Mbanya, J.C.; Sobngwi, E. Short Term Optimization of Glycaemic Control Using Insulin Improves Sympatho-Vagal Tone Activities in Patients with Type 2 Diabetes. Diabetes Res. Clin. Pract. 2019, 157, 107875. [Google Scholar] [CrossRef] [PubMed]
- Elvebakk, O.; Tronstad, C.; Birkeland, K.I.; Jenssen, T.G.; Bjørgaas, M.R.; Gulseth, H.L.; Kalvøy, H.; Høgetveit, J.O.; Martinsen, Ø.G. A Multiparameter Model for Non-Invasive Detection of Hypoglycemia. Physiol. Meas. 2019, 40, 085004. [Google Scholar] [CrossRef]
- Olde Bekkink, M.; Koeneman, M.; de Galan, B.E.; Bredie, S.J. Early Detection of Hypoglycemia in Type 1 Diabetes Using Heart Rate Variability Measured by a Wearable Device. Diabetes Care 2019, 42, 689–692. [Google Scholar] [CrossRef]
- Massa, G.G.; Gys, I.; Op‘t Eyndt, A.; Bevilacqua, E.; Wijnands, A.; Declercq, P.; Zeevaert, R. Evaluation of the FreeStyle® Libre Flash Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. HRP 2018, 89, 189–199. [Google Scholar] [CrossRef]
- FreeStyle Libre 14-Day System|Glucose Sensor & Reader|FreeStyleLibre.us. Available online: https://www.freestylelibre.us/ (accessed on 25 October 2020).
- Fitbit Official Site for Activity Trackers & More. Available online: https://www.fitbit.com/global/us/home (accessed on 25 October 2020).
- Marshall, W.A.; Tanner, J.M. Variations in Pattern of Pubertal Changes in Girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, L.; Capozzi, D.; Bellazzi, R.; Larizza, C. JTSA: An Open Source Framework for Time Series Abstractions. Comput. Methods Programs Biomed. 2015, 121, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Wilcox.Test Function|R Documentation. Available online: https://www.rdocumentation.org/packages/stats/versions/3.6.2/topics/wilcox.test/ (accessed on 25 October 2020).
| Characteristic | Summary Statistics |
|---|---|
| Sex | Female: 9 (52.94%) |
| Male: 8 (47.06%) | |
| Pubertal stage | Pre-pubertal: 5 (29.41%) |
| Peri-pubertal: 7 (41.18%) | |
| Post-pubertal: 5 (29.41%) | |
| Age (years) | 12.30 ± 4.33 |
| Diabetes duration (years) | 5.11 ± 4.18 |
| Insulin dose (IU/Kg/die) | 0.84 ± 0.24 |
| HbA1c (%) | 8.21 ± 1.29 |
| HbA1c (mmol/mol) | 66.12 ± 14.16 |
| BMI (Kg/m2) | 19.88 ± 5.06 |
| Systolic arterial pressure (mmHg) | 105.53 ± 11.01 |
| Diastolic arterial pressure (mmHg) | 67.65 ± 6.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Bosoni, P.; Sacchi, L.; Zuccotti, G.V.; Mannarino, S.; Bellazzi, R.; Larizza, C. Continuous Glucose and Heart Rate Monitoring in Young People with Type 1 Diabetes: An Exploratory Study about Perspectives in Nocturnal Hypoglycemia Detection. Metabolites 2021, 11, 5. https://doi.org/10.3390/metabo11010005
Calcaterra V, Bosoni P, Sacchi L, Zuccotti GV, Mannarino S, Bellazzi R, Larizza C. Continuous Glucose and Heart Rate Monitoring in Young People with Type 1 Diabetes: An Exploratory Study about Perspectives in Nocturnal Hypoglycemia Detection. Metabolites. 2021; 11(1):5. https://doi.org/10.3390/metabo11010005
Chicago/Turabian StyleCalcaterra, Valeria, Pietro Bosoni, Lucia Sacchi, Gian Vincenzo Zuccotti, Savina Mannarino, Riccardo Bellazzi, and Cristiana Larizza. 2021. "Continuous Glucose and Heart Rate Monitoring in Young People with Type 1 Diabetes: An Exploratory Study about Perspectives in Nocturnal Hypoglycemia Detection" Metabolites 11, no. 1: 5. https://doi.org/10.3390/metabo11010005
APA StyleCalcaterra, V., Bosoni, P., Sacchi, L., Zuccotti, G. V., Mannarino, S., Bellazzi, R., & Larizza, C. (2021). Continuous Glucose and Heart Rate Monitoring in Young People with Type 1 Diabetes: An Exploratory Study about Perspectives in Nocturnal Hypoglycemia Detection. Metabolites, 11(1), 5. https://doi.org/10.3390/metabo11010005







