Physical Activity-Related Metabolites Are Associated with Mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) Study
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
2.2. Metabolites and Physical Activity
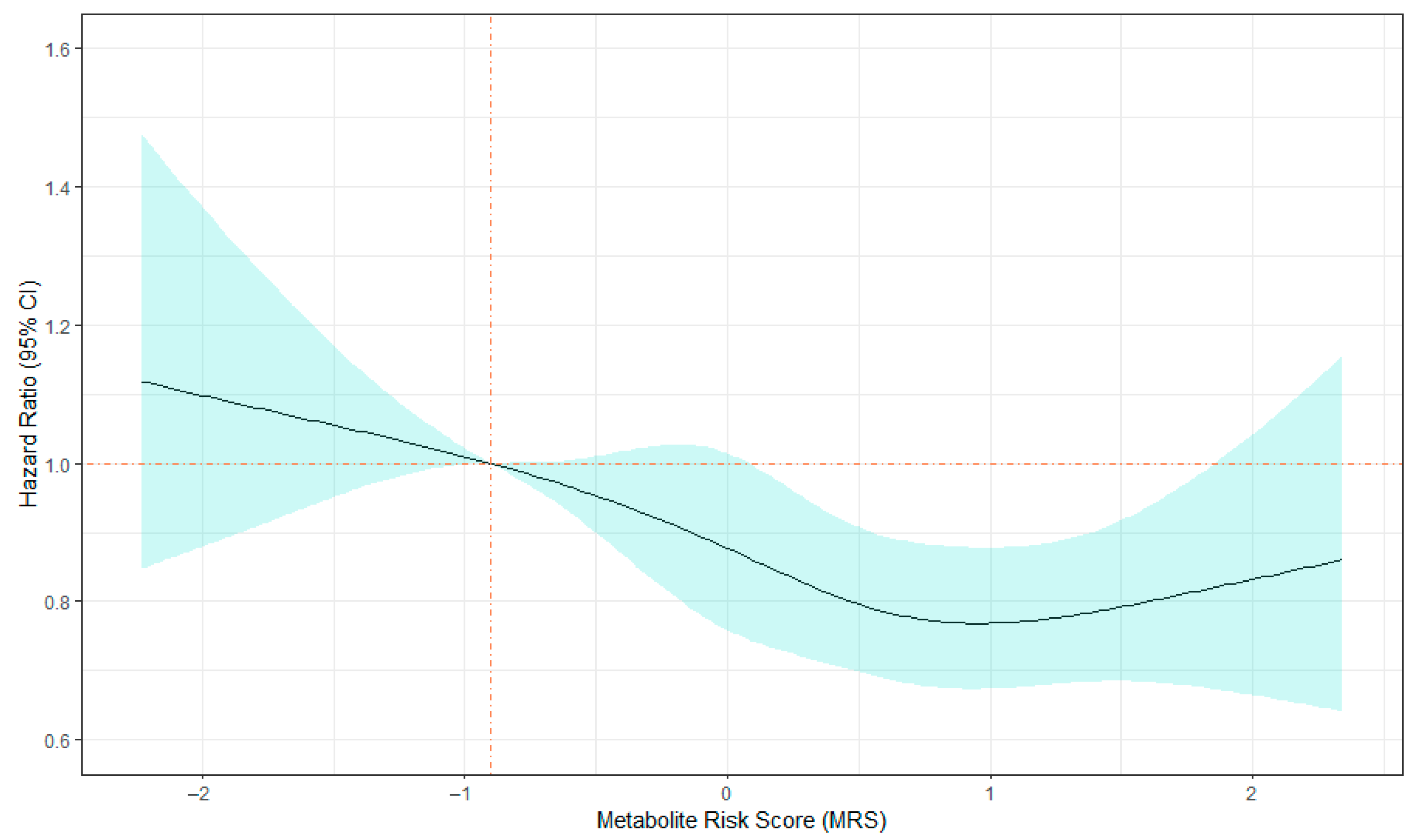
2.3. MRS and All-Cause Mortality
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Assessment of Physical Activity, All-Cause Mortality, and Covariates
4.3. Metabolomic Profiling
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, C.E.; Keadle, S.K.; Troiano, R.P.; Kahle, L.; Koster, A.; Brychta, R.J.; Van Domelen, D.; Caserotti, P.; Chen, K.Y.; Harris, T.B.; et al. Accelerometer-measured dose-response for physical activity, sedentary time, and mortality in US adults. Am. J. Clin. Nutr. 2016, 104, 1424–1432. [Google Scholar] [CrossRef]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; De Gonzalez, A.B.; Visvanathan, K.; Matthews, C.E. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Intern. Med. 2015, 175, 959–967. [Google Scholar] [CrossRef]
- Ekelund, U.; Ward, H.A.; Norat, T.; Luan, J.; May, A.M.; Weiderpass, E.; Sharp, S.J.; Overvad, K.; Østergaard, J.N.; Tjønneland, A.; et al. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: The European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am. J. Clin. Nutr. 2015, 101, 613–621. [Google Scholar] [CrossRef]
- Fishman, E.; Steeves, J.A.; Zipunnikov, V.; Koster, A.; Berrigan, D.; Harris, T.A.; Murphy, R. Association between Objectively Measured Physical Activity and Mortality in NHANES. Med. Sci. Sports Exerc. 2016, 48, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Gebel, K.; Ding, D.; Chey, T.; Stamatakis, E.; Brown, W.J.; Bauman, A. Effect of Moderate to Vigorous Physical Activity on All-Cause Mortality in Middle-aged and Older Australians. JAMA Intern. Med. 2015, 175, 970–977. [Google Scholar] [CrossRef]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130,000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Saint-Maurice, P.F.; Troiano, R.P.; Matthews, C.E.; Kraus, W.E. Moderate-to-vigorous physical activity and all-cause mortality: Do bouts matter? J. Am. Heart Assoc. 2018, 7, e007678. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, E.; Gale, J.; Bauman, A.; Ekelund, U.; Hamer, M.; Ding, D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019, 73, 2062–2072. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Zethelius, B.; McKeigue, P.M.; Lithell, H.O. Changes in physical activity are associated with changes in metabolic cardiovascular risk factors. Diabetologia 2001, 44, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Lakka, T.; Laaksonen, D.E. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, J.T.; Ivey, F.M.; Ryan, A.S.; Martel, G.F.; Hurlbut, D.E.; Metter, J.E.; Fozard, J.L.; Fleg, J.L.; Hurley, B.F. Effect of strength training on resting metabolic rate and physical activity: Age and gender comparisons. Med. Sci. Sports Exerc. 2001, 33, 532–541. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P. Relationship between body composition changes and changes in physical function and metabolic risk factors in aging. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 523–528. [Google Scholar] [PubMed]
- Bar-Or, O.; Baranowski, T. Physical Activity, Adiposity, and Obesity among Adolescents. Pediatr. Exerc. Sci. 1994, 6, 348–360. [Google Scholar] [CrossRef]
- DiPietro, L. Physical activity, body weight, and adiposity: An epidemiologic perspective. Exerc. Sport Sci. Rev. 1995, 23, 275–303. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef]
- Hu, F.B.; Willett, W.C.; Li, T.; Stampfer, M.J.; Colditz, G.A.; Manson, J.E. Adiposity as Compared with Physical Activity in Predicting Mortality among Women. N. Engl. J. Med. 2004, 351, 2694–2703. [Google Scholar] [CrossRef]
- Mason, K.E.; Pearce, N.; Cummins, S. Associations between fast food and physical activity environments and adiposity in mid-life: Cross-sectional, observational evidence from UK Biobank. Lancet Public Health 2018, 3, e24–e33. [Google Scholar] [CrossRef]
- Must, A.; Tybor, D.J. Physical activity and sedentary behavior: A review of longitudinal studies of weight and adiposity in youth. Int. J. Obes. 2005, 29, S84–S96. [Google Scholar] [CrossRef]
- Gagnon, D.D.; Dorman, S.; Ritchie, S.; Mutt, S.J.; Stenbäck, V.; Walkowiak, J.; Herzig, K.-H. Multi-Day Prolonged Low- to Moderate-Intensity Endurance Exercise Mimics Training Improvements in Metabolic and Oxidative Profiles without Concurrent Chromosomal Changes in Healthy Adults. Front. Physiol. 2019, 10, 1123. [Google Scholar] [CrossRef]
- Helge, J.W.; Damsgaard, R.; Overgaard, K.; Andersen, J.L.; Donsmark, M.; Dyrskog, S.E.; Hermansen, K.; Saltin, B.; Daugaard, J.R. Low-intensity training dissociates metabolic from aerobic fitness. Scand. J. Med. Sci. Sports 2007, 18, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Houmard, J.A.; Tanner, C.J.; Slentz, C.A.; Duscha, B.D.; McCartney, J.S.; Kraus, W.E. Effect of the volume and intensity of exercise training on insulin sensitivity. J. Appl. Physiol. 2004, 96, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.N.; Boca, S.M.; Shu, X.O.; Stolzenberg-Solomon, R.Z.; Matthews, C.E.; Hsing, A.W.; Tan, Y.T.; Ji, B.-T.; Chow, W.-H.; Cai, Q.; et al. Metabolomics in Epidemiology: Sources of Variability in Metabolite Measurements and Implications. Cancer Epidemiol. Biomark. Prev. 2013, 22, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, L.; Vinaixa, M.; Murillo, S.; Samino, S.; Rodriguez, M.A.; Beltran, A.; Lerin, C.; Davison, G.; Correig, X.; Novials, A. Metabolomics Approach for Analyzing the Effects of Exercise in Subjects with Type 1 Diabetes Mellitus. PLoS ONE 2012, 7, e40600. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lin, W.-T.; Hsu, F.-L.; Tsai, P.-W.; Hou, C.-C. Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur. J. Appl. Physiol. 2009, 108, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Soininen, P.; Kangas, A.J.; Würtz, P.; Suna, T.; Ala-Korpela, M. Quantitative Serum Nuclear Magnetic Resonance Metabolomics in Cardiovascular Epidemiology and Genetics. Circ. Cardiovasc. Genet. 2015, 8, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, B.; Alexander, D.; Mosley, T.H.; Heiss, G.; Nettleton, J.A.; Boerwinkle, E. Metabolomics and incident hypertension among blacks: The atherosclerosis risk in communities study. Hypertension 2013, 62, 398–403. [Google Scholar] [CrossRef]
- Kujala, U.M.; Mäkinen, V.-P.; Heinonen, I.; Soininen, P.; Kangas, A.J.; Leskinen, T.; Rahkila, P.; Würtz, P.; Kovanen, V.; Cheng, S.; et al. Long-term Leisure-time Physical Activity and Serum Metabolome. Circulation 2012, 127, 340–348. [Google Scholar] [CrossRef]
- Floegel, A.; Wientzek, A.; Bachlechner, U.; Jacobs, S.; Drogan, D.; Prehn, C.; Adamski, J.; Krumsiek, J.; Schulze, M.B.; Pischon, T.; et al. Linking diet, physical activity, cardiorespiratory fitness and obesity to serum metabolite networks: Findings from a population-based study. Int. J. Obes. 2014, 38, 1388–1396. [Google Scholar] [CrossRef]
- Palmnäs, M.S.A.; Kopciuk, K.A.; Shaykhutdinov, R.A.; Robson, P.J.; Mignault, D.; Rabasa-Lhoret, R.; Vogel, H.J.; Csizmadi, I. Serum Metabolomics of Activity Energy Expenditure and its Relation to Metabolic Syndrome and Obesity. Sci. Rep. 2018, 8, 3308. [Google Scholar] [CrossRef]
- Xiao-Ou, S.; Moore, S.C.; Keadle, S.K.; Xiang, Y.-B.; Zheng, W.; Peters, T.M.; Leitzmann, M.F.; Ji, B.-T.; Sampson, J.N.; Shu, X.-O.; et al. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int. J. Epidemiol. 2016, 45, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Siegrist, J. Physical Activity and Risk of Cardiovascular Disease—A Meta-Analysis of Prospective Cohort Studies. Int. J. Environ. Res. Public Health 2012, 9, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Nayor, M.; Shah, R.V.; Miller, P.E.; Blodgett, J.B.; Tanguay, M.; Pico, A.R.; Murthy, V.L.; Malhotra, R.; Houstis, N.E.; Deik, A.; et al. Metabolic Architecture of Acute Exercise Response in Middle-Aged Adults in the Community. Circulation 2020, 142, 1905–1924. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M. Vitamin C: Effects of Exercise and Requirements with Training. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 125–151. [Google Scholar] [CrossRef]
- Almanza-Aguilera, E.; Brunius, C.; Bernal-Lopez, M.R.; Garcia-Aloy, M.; Madrid-Gambin, F.; Tinahones, F.J.; Gómez-Huelgas, R.; Landberg, R.; Andres-Lacueva, C. Impact in Plasma Metabolome as Effect of Lifestyle Intervention for Weight-Loss Reveals Metabolic Benefits in Metabolically Healthy Obese Women. J. Proteome Res. 2018, 17, 2600–2610. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Dai, Z.; Chung, S.K.; Miao, D.; Lau, K.S.; Chan, A.W.; Kung, A.W. Sodium/myo-inositol cotransporter 1 and myo-inositol are essential for osteogenesis and bone formation. J. Bone Miner. Res. 2011, 26, 582–590. [Google Scholar] [CrossRef]
- Vittorio Vittorio Unfer for The Experts Group on Inositol in Basic and Clinical Research; Carlomagno, G.; Dante, G.; Facchinetti, F. Effects of myo-inositol in women with PCOS: A systematic review of randomized controlled trials. Gynecol. Endocrinol. 2012, 28, 509–515. [Google Scholar]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef]
- Ix, J.H.; de Boer, I.H.; Wassel, C.L.; Criqui, M.H.; Shlipak, M.G.; Whooley, M.A. Urinary creatinine excretion rate and mortality in persons with coronary artery disease: The Heart and Soul Study. Circulation 2010, 121, 1295. [Google Scholar] [CrossRef]
- Baxmann, A.C.; Ahmed, M.S.; Marques, N.C.; Menon, V.B.; Pereira, A.B.; Kirsztajn, G.M.; Heilberg, I.P. Influence of Muscle Mass and Physical Activity on Serum and Urinary Creatinine and Serum Cystatin C. Clin. J. Am. Soc. Nephrol. 2008, 3, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Light, A.R.; Hoppel, C.L.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Hughen, R.W.; Keim, N.L.; et al. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp. Physiol. 2016, 102, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Van Schaftingen, E.; Draye, J.P.; Van Hoof, F. Coenzyme specificity of mammalian liver d-glycerate dehydrogenase. Eur. J. Biochem. 1989, 186, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Lamb, G.D.; Westerblad, H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 2011, 589, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Hurley, B.F.; Hanson, E.D.; Sheaff, A.K. Strength Training as a Countermeasure to Aging Muscle and Chronic Disease. Sports Med. 2011, 41, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Yokochi, N.; Nishimura, S.; Yoshikane, Y.; Ohnishi, K.; Yagi, T. Identification of a new tetrameric pyridoxal 4-dehydrogenase as the second enzyme in the degradation pathway for pyridoxine in a nitrogen-fixing symbiotic bacterium, Mesorhizobium loti. Arch. Biochem. Biophys. 2006, 452, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Frei, B.; Fulgoni, V.L.; Weaver, C.; Zeisel, S.H.; Fulgoni, V.L. Contribution of Dietary Supplements to Nutritional Adequacy in Race/Ethnic Population Subgroups in the United States. Nutrients 2017, 9, 1295. [Google Scholar] [CrossRef]
- Pusceddu, I.; Herrmann, W.; Kleber, M.E.; Scharnagl, H.; Hoffmann, M.M.; Winklhofer-Roob, B.M.; März, W.; Herrmann, M. Subclinical inflammation, telomere shortening, homocysteine, vitamin B6, and mortality: The Ludwigshafen Risk and Cardiovascular Health Study. Eur. J. Nutr. 2020, 59, 1399–1411. [Google Scholar] [CrossRef]
- Parry, T. Paper chromatography of 56 amino compounds using phenol and butanol-acetic acid as solvents: With illustrative chromatograms of normal and abnormal urines. Clin. Chim. Acta 1957, 2, 115–125. [Google Scholar] [CrossRef]
- Li, K.J.; Borresen, E.C.; Jenkins-Puccetti, N.; Luckasen, G.; Ryan, E.P. Navy Bean and Rice Bran Intake Alters the Plasma Metabolome of Children at Risk for Cardiovascular Disease. Front. Nutr. 2018, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Weinstein, S.J.; Moore, S.C.; Derkach, A.; Hua, X.; Liao, L.M.; Gu, F.; Mondul, A.M.; Sampson, J.N.; Albanes, D. Serum Metabolomic Profiling of All-Cause Mortality: A Prospective Analysis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study Cohort. Am. J. Epidemiol. 2018, 187, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Culleton, B.F.; Larson, M.G.; Wilson, P.W.F.; Evans, J.C.; Parfrey, P.S.; Levy, D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999, 56, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-R.; Coresh, J.; Inker, L.A.; Levey, A.S.; Zheng, Z.; Rebholz, C.M.; Tin, A.; Appel, L.J.; Chen, J.; Sarnak, M.J.; et al. Serum metabolites are associated with all-cause mortality in chronic kidney disease. Kidney Int. 2018, 94, 381–389. [Google Scholar] [CrossRef]
- Ekelund, U.; Brage, S.; Froberg, K.; Harro, M.; Anderssen, S.A.; Sardinha, L.B.; Riddoch, C.; Andersen, L.B. TV Viewing and Physical Activity Are Independently Associated with Metabolic Risk in Children: The European Youth Heart Study. PLoS Med. 2006, 3, e488. [Google Scholar] [CrossRef]
- O’Donovan, G.; Stensel, D.J.; Hamer, M.; Stamatakis, E. The association between leisure-time physical activity, low HDL-cholesterol and mortality in a pooled analysis of nine population-based cohorts. Eur. J. Epidemiol. 2017, 32, 559–566. [Google Scholar] [CrossRef]
- Rauh, M.J.D.; Hovell, M.F.; Hofstetter, C.R.; Sallis, J.F.; Gleghorn, A. Reliability and Validity of Self-Reported Physical Activity in Latinos. Int. J. Epidemiol. 1992, 21, 966–971. [Google Scholar] [CrossRef]
- Rousham, E.K.; Clarke, P.E.; Gross, H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. Eur. J. Clin. Nutr. 2005, 60, 393–400. [Google Scholar] [CrossRef]
- Hertogh, E.M.; Monninkhof, E.M.; Schouten, E.G.; Peeters, P.H.; Schuit, A.J. Validigy of the modified Baeche questionnaires: Comparison with energy expenditure according to the doubly labeled water method. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 30. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Ainsworth, B.E.; Hartman, T.J.; Leon, A.S. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med. Sci. Sports Exerc. 1993, 25, 81–91. [Google Scholar] [CrossRef]
- Aric Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. Am. J. Epidemiol. 1989, 129, 687–702. [Google Scholar] [CrossRef]
- Bell, E.J.; Lutsey, P.L.; Windham, B.G.; Folsom, A.R. Physical activity and cardiovascular disease in African Americans in ARIC. Med. Sci. Sports Exerc. 2013, 45, 901. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.T.; Ainsworth, B.E.; Wu, H.-C.; Jacobs, D.R., Jr.; Leon, A.S. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int. J. Epidemiol. 1995, 24, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Arnett, D.K.; Bank, A.; Liao, D.; Evans, G.W.; Evenson, K.R.; Stevens, J.; Sorlie, P.; Folsom, A.R. Arterial distensibility and physical activity in the ARIC study. Med. Sci. Sports Exerc. 2001, 33, 2065–2071. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D. Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Ohta, T.; Masutomi, N.; Tsutsui, N.; Sakairi, T.; Mitchell, M.; Milburn, M.V.; Ryals, J.A.; Beebe, K.D.; Guo, L. Untargeted Metabolomic Profiling as an Evaluative Tool of Fenofibrate-Induced Toxicology in Fischer 344 Male Rats. Toxicol. Pathol. 2009, 37, 521–535. [Google Scholar] [CrossRef]
- Yu, B.; Heiss, G.; Alexander, D.; Grams, M.E.; Boerwinkle, E. Associations Between the Serum Metabolome and All-Cause Mortality Among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2016, 183, 650–656. [Google Scholar] [CrossRef]
- Chin, K.; Zhao, D.; Tibuakuu, M.; Martin, S.S.; Ndumele, C.E.; Florido, R.; Windham, B.G.; Guallar, E.; Lutsey, P.L.; Michos, E.D. Physical Activity, Vitamin D, and Incident Atherosclerotic Cardiovascular Disease in Whites and Blacks: The ARIC Study. J. Clin. Endocrinol. Metab. 2017, 102, 1227–1236. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, C.; Nambi, V.; Morrison, A.C.; Folsom, A.R.; Ballantyne, C.M.; Yu, B. Metabolomic Pattern Predicts Incident Coronary Heart Disease: Findings from the Atherosclerosis Risk in Communities Study. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 38. [Google Scholar] [CrossRef]

| Characteristics | Poor PA n = 1823 | Intermediate PA n = 805 | Ideal PA n = 1174 | p Value * |
|---|---|---|---|---|
| Age, years | 53.52 (5.7) | 53.63 (5.7) | 53.69 (5.8) | 0.41 |
| African Americans, n (%) | 1375 (75.4) | 434 (53.9) | 535 (45.6) | <0.001 |
| Male, n (%) | 649 (35.6) | 281 (34.9) | 594 (50.6) | <0.001 |
| BMI, kg/m2 | 29.64 (6.4) | 28.33 (5.4) | 27.63 (5.0) | <0.001 |
| Smoking | ||||
| Never smoker, n (%) | 835 (45.8) | 397 (49.3) | 453 (38.6) | <0.001 |
| Former smoker, n (%) | 427 (23.4) | 219 (27.2) | 418 (35.6) | |
| Current smoker, n (%) | 561 (30.8) | 189 (23.5) | 303 (25.8) | |
| LTPA, MET⋅hr⋅wk−1 | 0 (0) | 5.37 (3.0) | 22.12 (11.3) | <0.001 |
| Diabetes, n (%) | 291 (16.0) | 88 (10.9) | 138 (11.7) | <0.001 |
| Cardiovascular disease, n (%) | 190 (10.4) | 80 (9.9) | 146 (12.4) | 0.13 |
| eGFR, mL/min/1.73 m2 | 101.56 (18.4) | 98.70 (17.1) | 95.67 (17.0) | <0.001 |
| HDL, mmol/L | 1.3 (0.4) | 1.38 (0.4) | 1.37 (0.4) | 0.15 |
| Triglycerides, mmol/L | 1.26 (0.6) | 1.34 (0.7) | 1.34 (0.7) | <0.001 |
| Total cholesterol, mmol/L | 5.53 (1.1) | 5.58 (1.1) | 5.53 (1.1) | 0.92 |
| SBP, mmHg | 127.06 (21.8) | 123.60 (20.6) | 121.70 (19.5) | <0.001 |
| Death, n (%) | 980 (53.8) | 376 (46.7) | 572 (48.7) | 0.001 |
| MRS | 28.65 (7.3) | 30.32 (7.2) | 31.84 (7.4) | <0.001 |
| Physical Activity * | β (95% CI) | p Value |
|---|---|---|
| MRS and LTPA | 0.012 (0.0010, 0.015) | 2.65 × 10−20 |
| MRS and 2018 Physical Activity Guidelines | ||
| Intermediate vs. poor | 0.184 (0.105, 0.263) | 5.34 × 10−6 |
| Ideal vs. poor | 0.374 (0.302, 0.446) | 5.52 × 10−24 |
| All-cause mortality † | Hazard Ratio (95% CI) | p Value |
| MRS (per SD change) | 0.90 (0.85, 0.95) | 3.86 × 10−5 |
| MRS quintiles | ||
| Q2 vs. Q1 | 0.95 (0.83, 1.09) | 0.48 |
| Q3 vs. Q1 | 0.85 (0.74, 0.98) | 0.03 |
| Q4 vs. Q1 | 0.76 (0.66, 0.88) | 0.0002 |
| Q5 vs. Q1 | 0.78 (0.67, 0.92) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, G.; Hegde, S.M.; Palta, P.; Boerwinkle, E.; Gabriel, K.P.; Yu, B. Physical Activity-Related Metabolites Are Associated with Mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) Study. Metabolites 2021, 11, 59. https://doi.org/10.3390/metabo11010059
Xu J, Liu G, Hegde SM, Palta P, Boerwinkle E, Gabriel KP, Yu B. Physical Activity-Related Metabolites Are Associated with Mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) Study. Metabolites. 2021; 11(1):59. https://doi.org/10.3390/metabo11010059
Chicago/Turabian StyleXu, Jun, Guning Liu, Sheila M. Hegde, Priya Palta, Eric Boerwinkle, Kelley P. Gabriel, and Bing Yu. 2021. "Physical Activity-Related Metabolites Are Associated with Mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) Study" Metabolites 11, no. 1: 59. https://doi.org/10.3390/metabo11010059
APA StyleXu, J., Liu, G., Hegde, S. M., Palta, P., Boerwinkle, E., Gabriel, K. P., & Yu, B. (2021). Physical Activity-Related Metabolites Are Associated with Mortality: Findings from the Atherosclerosis Risk in Communities (ARIC) Study. Metabolites, 11(1), 59. https://doi.org/10.3390/metabo11010059





