Skeletal Muscle Angiopoietin-Like Protein 4 and Glucose Metabolism in Older Adults after Exercise and Weight Loss
Abstract
1. Introduction
2. Results
2.1. Effects of Exercise Plus Weight Loss
2.2. Comparisons between Men and Women
2.3. Correlations with ANGPTL4 mRNA
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Exercise and Weight Loss Intervention
4.3. Outcome Measures
4.3.1. VO2max and Body Composition
4.3.2. Oral Glucose Tolerance Test (OGTT) and Lipids
4.3.3. Glucose Clamp and Skeletal Muscle Biopsies
4.3.4. Serum ANGPTL4
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grootaert, C.; Van de Wiele, T.; Verstraete, W.; Bracke, M.; Vanhoecke, B. Angiopoietin-like protein 4: Health effects, modulating agents and structure-function relationships. Expert. Rev. Proteom. 2012, 9, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Mandard, S.; Zandbergen, F.; van Straten, E.; Wahli, W.; Kuipers, F.; Muller, M.; Kersten, S. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 2006, 281, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Hjorth, M.; Langleite, T.M.; Lee, S.; Holen, T.; Bindesboll, C.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Kielland, A.; et al. Regulation of angiopoietin-like protein 4 production during and after exercise. Physiol. Rep. 2014, 2, e12109. [Google Scholar] [CrossRef] [PubMed]
- Catoire, M.; Alex, S.; Paraskevopulos, N.; Mattijssen, F.; Evers-van Gogh, I.; Schaart, G.; Jeppesen, J.; Kneppers, A.; Mensink, M.; Voshol, P.J.; et al. Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise. Proc. Natl. Acad. Sci. USA 2014, 111, E1043–E1052. [Google Scholar] [CrossRef]
- Ingerslev, B.; Hansen, J.S.; Hoffmann, C.; Clemmesen, J.O.; Secher, N.H.; Scheler, M.; de Angelis, M.H.; Häring, H.U.; Pedersen, B.K.; Weigert, C.; et al. Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP. Mol. Metab. 2017, 6, 1286–1295. [Google Scholar] [CrossRef]
- Dutton, S.; Trayhurn, P. Regulation of angiopoietin-like protein 4/fasting-induced adipose factor (Angptl4/FIAF) expression in mouse white adipose tissue and 3T3-L1 adipocytes. Br. J. Nutr. 2008, 100, 18–26. [Google Scholar] [CrossRef][Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- La Paglia, L.; Listi, A.; Caruso, S.; Amodeo, V.; Passiglia, F.; Bazan, V.; Fanale, D. Potential Role of ANGPTL4 in the Cross Talk between Metabolism and Cancer through PPAR Signaling Pathway. PPAR Res. 2017, 2017, 8187235. [Google Scholar] [CrossRef]
- Köster, A.; Chao, B.Y.; Mosior, M.; Ford, A.; Gonzalez-DeWhitt, P.A.; Hale, J.E.; Li, D.; Qiu, Y.; Fraser, C.C.; Yang, D.D.; et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: Regulation of triglyceride metabolism. Endocrinology 2005, 146, 4943–4950. [Google Scholar] [CrossRef]
- Wang, C.Y.; Liu, K.H.; Tsai, M.L.; Ho, M.Y.; Yeh, J.K.; Hsieh, I.C.; Wen, M.-S.; Yeh, T.-S. FTO variants are associated with ANGPTL4 abundances and correlated with body weight reduction after bariatric surgery. Obes. Res. Clin. Pract. 2020, 14, 257–263. [Google Scholar] [CrossRef]
- Clasen, B.F.; Krusenstjerna-Hafstrom, T.; Vendelbo, M.H.; Thorsen, K.; Escande, C.; Moller, N.; Pedersen, S.B.; Jørgensen, J.O.L.; Jessen, N. Gene expression in skeletal muscle after an acute intravenous GH bolus in human subjects: Identification of a mechanism regulating ANGPTL4. J. Lipid Res. 2013, 54, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Lam, M.C.; Chan, K.W.; Wang, Y.; Zhang, J.; Hoo, R.L.; Xu, J.Y.; Chen, B.; Chow, W.-S.; Tso, A.W.K.; et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 6086–6091. [Google Scholar] [CrossRef] [PubMed]
- Barja-Fernandez, S.; Moreno-Navarrete, J.M.; Folgueira, C.; Xifra, G.; Sabater, M.; Castelao, C.; Fern, J.; Leis, R.; Diéguez, C.; Casanueva, F.F.; et al. Plasma ANGPTL-4 is Associated with Obesity and Glucose Tolerance: Cross-Sectional and Longitudinal Findings. Mol. Nutr. Food Res. 2018, 62, e1800060. [Google Scholar] [CrossRef]
- Van Raalte, D.H.; Brands, M.; Serlie, M.J.; Mudde, K.; Stienstra, R.; Sauerwein, H.P.; Kersten, S.; Diamant, M. Angiopoietin-like protein 4 is differentially regulated by glucocorticoids and insulin in vitro and in vivo in healthy humans. Exp. Clin. Endocrinol. Diabetes 2012, 120, 598–603. [Google Scholar] [CrossRef]
- Chang, H.; Kwon, O.; Shin, M.S.; Kang, G.M.; Leem, Y.H.; Lee, C.H.; Kim, S.J.; Roh, E.; Kim, H.-K.; Youn, B.-S. Role of Angptl4/Fiaf in exercise-induced skeletal muscle AMPK activation. J. Appl. Physiol. (1985) 2018, 125, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Li, X.; Geng, Y.; Cui, H.; Jin, C.; Wang, P.; Li, Y.; Yang, Y. Tongxinluo attenuates reperfusion injury in diabetic hearts by angiopoietin-like 4-mediated protection of endothelial barrier integrity via PPAR-alpha pathway. PLoS ONE. 2018, 13, e0198403. [Google Scholar] [CrossRef] [PubMed]
- Georgiadi, A.; Boekschoten, M.V.; Muller, M.; Kersten, S. Detailed transcriptomics analysis of the effect of dietary fatty acids on gene expression in the heart. Physiol. Genom. 2012, 44, 352–361. [Google Scholar] [CrossRef]
- Smati, S.; Regnier, M.; Fougeray, T.; Polizzi, A.; Fougerat, A.; Lasserre, F.; Lukowicz, C.; Tramunt, B.; Guillaume, M.; Burnol, A.-F.; et al. Regulation of hepatokine gene expression in response to fasting and feeding: Influence of PPAR-alpha and insulin-dependent signalling in hepatocytes. Diabetes Metab. 2020, 46, 129–136. [Google Scholar] [CrossRef]
- Staiger, H.; Haas, C.; Machann, J.; Werner, R.; Weisser, M.; Schick, F.; Machicao, F.; Stefan, N.; Fritsche, A.; Häring, H.-U. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes 2009, 58, 579–589. [Google Scholar] [CrossRef]
- Montagner, A.; Polizzi, A.; Fouche, E.; Ducheix, S.; Lippi, Y.; Lasserre, F.; Barquissau, V.; Régnier, M.; Lukowicz, C.; Benhamed, F.; et al. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016, 65, 1202–1214. [Google Scholar] [CrossRef]
- Chan, S.M.; Sun, R.Q.; Zeng, X.Y.; Choong, Z.H.; Wang, H.; Watt, M.J.; Ye, J.-M. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes 2013, 62, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes 2014, 37 (Suppl. 1), S81–S90. [Google Scholar]
- Cullberg, K.B.; Christiansen, T.; Paulsen, S.K.; Bruun, J.M.; Pedersen, S.B.; Richelsen, B. Effect of weight loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity (Silver Spring) 2013, 21, 454–460. [Google Scholar] [CrossRef]
- Van der Kolk, B.W.; Vink, R.G.; Jocken, J.W.E.; Roumans, N.J.T.; Goossens, G.H.; Mariman, E.C.M.; van Baak, M.A.; Blaak, E.E. Effect of diet-induced weight loss on angiopoietin-like protein 4 and adipose tissue lipid metabolism in overweight and obese humans. Physiol. Rep. 2018, 6, e13735. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Burgess, S.C.; Ge, H.; Wong, K.K.; Nassem, R.H.; Garry, D.J.; Sherry, A.D.; Malloy, C.R.; Berger, J.P.; Li, C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc. Natl. Acad. Sci. USA 2005, 102, 1767–1772. [Google Scholar] [CrossRef]
- Peters, J.M.; Hennuyer, N.; Staels, B.; Fruchart, J.C.; Fievet, C.; Gonzalez, F.J.; Auwerx, J. Alterations in lipoprotein metabolism in peroxisome proliferator-activated receptor alpha-deficient mice. J. Biol. Chem. 1997, 272, 27307–27312. [Google Scholar] [CrossRef] [PubMed]
- Van der Kolk, B.W.; Goossens, G.H.; Jocken, J.W.; Kersten, S.; Blaak, E.E. Angiopoietin-Like Protein 4 and Postprandial Skeletal Muscle Lipid Metabolism in Overweight and Obese Prediabetics. J. Clin. Endocrinol. Metab. 2016, 101, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Ortmeyer, H.K.; Sorkin, J.D. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E145–E152. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2186–2191. [Google Scholar] [CrossRef]
- Ryan, A.S.; Nicklas, B.J.; Berman, D.M. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity (Silver Spring) 2006, 14, 1064–1072. [Google Scholar] [CrossRef]
- Ryan, A.S.; Ge, S.; Blumenthal, J.B.; Serra, M.C.; Prior, S.J.; Goldberg, A.P. Aerobic Exercise and Weight Loss Reduce Vascular Markers of Inflammation and Improve Insulin Sensitivity in Obese Women. J. Am. Geriatr. Soc. 2014, 62, 607–614. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Li, G.; Blumenthal, J.B.; Ortmeyer, H.K. Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults. Obesity (Silver Spring) 2013, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | Pre AEX + WL | Post AEX + WL |
|---|---|---|
| Weight (kg) | 91.6 ± 2.8 | 84.6 ± 2.7 ‡ |
| BMI (kg/m2) | 31.3 ± 0.7 | 28.5 ± 0.7 ‡ |
| Waist circumference (cm) | 102.1 ± 2.7 | 96.9 ± 2.8 ‡ |
| Hip circumference (cm) (n = 21) | 114.9 ± 2.5 | 110.4 ± 2.2 ‡ |
| Percent body fat | 39.2 ± 1.5 | 34.994 ± 1.7 ‡ |
| Fat mass (kg) | 35.8 ± 1.8 | 29.8 ± 1.8 ‡ |
| Fat-free mass (kg) | 55.9 ± 2.2 | 55.4 ± 2.3 |
| VO2max (mL/kg/min) | 21.8 ± 1.0 | 26.81 ± 1.3 ‡ |
| VO2max (L/min) | 2.00 ± 0.11 | 2.26 ± 0.12 ‡ |
| Fasting glucose (mmol/L) | 5.41 ± 0.10 | 5.25 ± 0.09 |
| 120-min postprandial glucose (mmol/L) | 8.82 ± 0.4 | 7.35 ± 0.37 |
| GlucoseAUC (mmol/L/120 min) | 980 ± 37 | 926 ± 36 † |
| GlucoseAUC (mmol/L/180 min) | 1260 ± 51 | 1194 ± 42 * |
| Fasting insulin (pmol/L) | 87 ± 7 | 69 ± 5 ‡ |
| InsulinAUC (pmol/L/120 min) | 53,098 ± 4004 | 44,011 ± 3178 ‡ |
| InsulinAUC (pmol/L/180 min) | 67,294 ± 5264 | 55,994 ± 4316 ‡ |
| Glucose utilization (mg/kg/min) | 5.13 ± 0.31 | 6.60 ± 0.33 ‡ |
| Glucose utilization (µmol/kg FFM/min) | 48.01 ± 3.29 | 56.50 ± 2.86 ‡ |
| Insulin sensitivity (mg/kg/min/min/pM) | 0.026 ± 0.002 | 0.036 ± 0.002 ‡ |
| Insulin sensitivity (µmol/kg FFM/min/pM) | 0.044 ± 0.004 | 0.054 ± 0.003 ‡ |
| Muscle ANGPTL4 expression (AU) | 0.801 ± 0.115 | 0.888 ± 0.144 |
| Muscle PPARα expression (AU) (n = 9) | 16.15 ± 2.163 | 15.62 ± 2.469 |
| Serum ANGPTL4 (pg/mL) | 313.6 ± 35.0 | 331.6 ± 35.9 |
| Visceral fat area (cm2) | 160.1 ± 14.7 | 127.5 ± 12.5 ‡ |
| Abdominal subcutaneous fat area (cm2) | 356.6 ± 19.0 | 301.2 ± 21.3 ‡ |
| Mid-thigh low density lean tissue area (cm2) | 23.6 ± 1.6 | 22.8 ± 1.7 |
| Mid-thigh muscle area (cm2) | 90.4 ± 5.8 | 94.7 ± 5.0 |
| Mid-thigh subcutaneous fat (cm2) | 117.4 ± 11.1 | 103.1 ± 9.7 |
| Mid-thigh muscle attenuation (HU) (n=15) | 36.5 ± 2.4 | 38.6 ± 2.8 * |
| TG (mg/dL) | 123 ± 7 | 101 ± 6 |
| Total Cholesterol (mg/dL) | 184 ± 6 | 176 ± 5 † |
| HDL-Cholesterol (mg/dL) | 44.7 ± 1.8 | 47.5 ± 1.8 † |
| LDL-Cholesterol (mg/dL) | 115.5 ± 4.9 | 108.1 ± 4.6 † |
| Characteristic | Women | Men | p Value |
|---|---|---|---|
| Weight (kg) | 82.7 ± 4.0 | 100.1 ± 2.9 | 0.010 |
| BMI (kg/m2) | 31.1 ± 1.2 | 31.6 ± 0.7 | 0.747 |
| Percent body fat | 45.6 ± 1.4 | 33.1 ± 1.3 | 0.000 |
| Fat mass (kg) | 38.6 ± 3.0 | 33.7 ± 2.0 | 0.194 |
| FFM (kg) | 44.7 ± 1.3 | 67.2 ± 1.7 | 0.000 |
| VO2max (mL/kg/min) | 19.8 ± 1.2 | 24.2 ± 1.4 | 0.022 |
| Fasting glucose (mmol/L) | 5.41 ± 0.10 | 5.25 ± 0.09 | 0.182 |
| Fasting insulin (pmol/L) | 87 ± 13 | 87 ± 7 | 0.953 |
| Glucose utilization (mg/kg/min) | 5.7 ± 0.4 | 4.5 ± 0.4 (n = 15) | 0.051 |
| Glucose utilization (µmol/kg FFM/min) | 58.3 ± 4.2 | 36.4 ± 3.1 (n = 15) | 0.000 |
| Insulin sensitivity (mg/kg/min/min/pM) | 0.029 ± 0.003 | 0.021 ± 0.002 (n = 13) | 0.048 |
| Insulin sensitivity (µmol/kg FFM/min/pM) | 0.053 ± 0.005 | 0.032 ± 0.003 (n = 13) | 0.001 |
| TG (mg/dL) | 127 ± 9 | 120 ± 11 | 0.607 |
| Total Cholesterol (mg/dL) | 201 ± 9 | 169 ± 6 | 0.005 |
| HDL-cholesterol (mg/dL) | 50.1 ± 2.8 | 39.7 ± 1.5 | 0.003 |
| LDL-cholesterol (mg/dL) | 125.9 ± 7.6 | 105.6 ± 5.3 | 0.038 |
| Muscle ANGPTL4 expression (AU) | 1.174 ± 0.167 | 0.507 ± 0.119 | 0.003 |
| Muscle PPARα expression (AU) | 10.60 ± 1.353 (n = 9) | 20.46 ± 3.036 (n = 9) | 0.013 |
| Serum ANGPTL4 (pg/mL) | 374.3 ± 71.4 (n = 15) | 309.2 ± 31.5 (n = 15) | 0.415 |
| All Participants | r | p |
|---|---|---|
| Percent body fat (n = 35) | 0.64 | 0.000 |
| Fat mass (n = 35) | 0.48 | 0.004 |
| Fat-free mass (n = 35) | −0.45 | 0.007 |
| Mid-thigh muscle area (n = 33) | −0.56 | 0.001 |
| Mid-thigh subcutaneous fat area (n = 33) | 0.52 | 0.002 |
| Visceral fat area (n = 34) | 0.23 | 0.196 |
| Total cholesterol (n = 35) | 0.43 | 0.010 |
| HDL-cholesterol (n = 35) | 0.35 | 0.037 |
| LDL-cholesterol (n = 35) | 0.37 | 0.027 |
| Triglyceride (n = 35) | 0.07 | 0.717 |
| VO2max (mL/kg/min) (n = 35) | −0.57 | 0.001 |
| M (µmol/kg FFM/min) (n = 32) | 0.38 | 0.033 |
| GlucoseAUC (mmol/L/120 min) (n = 34) | −0.01 | 0.965 |
| GlucoseAUC (mmol/L/180 min) (n = 34) | 0.12 | 0.500 |
| InsulinAUC (pmol/L/120 min) (n = 34) | 0.28 | 0.115 |
| InsulinAUC (pmol/L/180 min) (n = 33) | 0.18 | 0.327 |
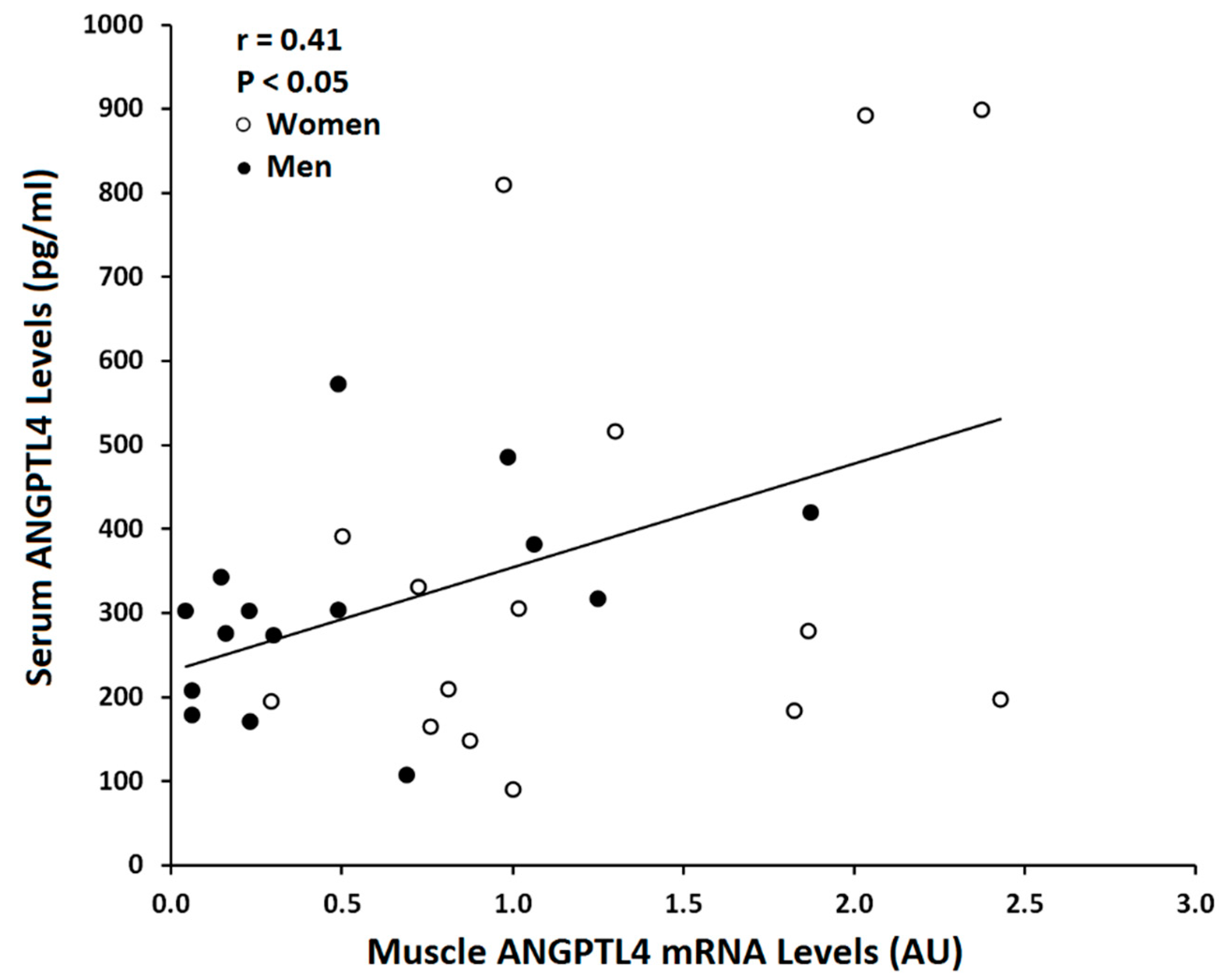
| Serum ANGPTL4 (n = 30) | 0.41 | 0.025 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Zhang, H.; Ryan, A.S. Skeletal Muscle Angiopoietin-Like Protein 4 and Glucose Metabolism in Older Adults after Exercise and Weight Loss. Metabolites 2020, 10, 354. https://doi.org/10.3390/metabo10090354
Li G, Zhang H, Ryan AS. Skeletal Muscle Angiopoietin-Like Protein 4 and Glucose Metabolism in Older Adults after Exercise and Weight Loss. Metabolites. 2020; 10(9):354. https://doi.org/10.3390/metabo10090354
Chicago/Turabian StyleLi, Guoyan, Hefang Zhang, and Alice S. Ryan. 2020. "Skeletal Muscle Angiopoietin-Like Protein 4 and Glucose Metabolism in Older Adults after Exercise and Weight Loss" Metabolites 10, no. 9: 354. https://doi.org/10.3390/metabo10090354
APA StyleLi, G., Zhang, H., & Ryan, A. S. (2020). Skeletal Muscle Angiopoietin-Like Protein 4 and Glucose Metabolism in Older Adults after Exercise and Weight Loss. Metabolites, 10(9), 354. https://doi.org/10.3390/metabo10090354






