Abstract
The increasing global emergence of multidrug resistant (MDR) pathogens is categorized as one of the most important health problems. Therefore, the discovery of novel antimicrobials is of the utmost importance. Lichens provide a rich source of natural products including unique polyketides and polyphenols. Many of them display pharmaceutical benefits. The aim of this study was directed towards the characterization of sunflower oil extracts from the fruticose lichen, Usnea barbata. The concentration of the major polyketide, usnic acid, was 1.6 mg/mL extract as determined by NMR analysis of the crude mixture corresponding to 80 mg per g of the dried lichen. The total phenolics and flavonoids were determined by photometric assays as 4.4 mg/mL (gallic acid equivalent) and 0.27 mg/mL (rutin equivalent) corresponding to 220 mg/g and 13.7 mg/g lichen, respectively. Gram-positive (e.g., Enterococcus faecalis) and Gram-negative bacteria, as well as clinical isolates of infected chickens were sensitive against these extracts as determined by agar diffusion tests. Most of these activities increased in the presence of zinc salts. The data suggest the potential usage of U. barbata extracts as natural additives and mild antibiotics in animal husbandry, especially against enterococcosis in poultry.
1. Introduction
The emergence of multidrug resistant (MDR) pathogenic bacteria poses a worsening and general health problem [1]. This phenomenon was mainly caused by excessive use of antimicrobials as therapeutics and preventive tools in the animal industry and large-scale poultry operations. This challenge has led to a constant search for the most suitable alternatives for in-feed antimicrobials [2,3]. The WHO has recently rated the phenomenon of MDR among the most important problems threatening human health, as there are large gaps in the existing surveillance of antimicrobial resistance in many parts of the world [4].
In large poultry operations, antimicrobials have been extensively used as therapeutics and growth promoters [5,6,7]. However, the indiscriminate usage of antimicrobials and the increasing levels of antibiotic residuals in animal products, particularly in meat and poultry products, can have serious effects on human health [8,9]. Among the most important MDR bacteria, the “ESKAPE” group encompassing Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter strains can escape the biocidal action of most antimicrobial agents [10]. In the poultry industry, a steadily increasing level of MDR bacteria has also been reported, for example of Escherichia coli [11], Campylobacter [12,13,14], Salmonella [15,16], and methicillin-resistant S. aureus (MRSA) [17,18].
Recent achievements in phytochemical and phytopharmacological sciences have facilitated clarification of the composition and biological activities of many medicinal plants (for an example, see [19]). Plant extracts and essential oils have been used since ancient times for the treatment of various diseases and have also been established in modern medicine mainly because of their availability and lower toxicity as compared with related synthetic drugs (for an example, see [20]).
Lichens are worldwide symbiotic associations of fungi with microalgae or cyanobacteria [21], and, next to plants, they represent an important source of biologically active natural compounds [22,23]. For now, more than 800 lichen-specific secondary metabolites have been described and many of them exhibit beneficial bioactivities. These unique secondary metabolites usually represent 0.1–10% and sometimes even 30% of the dry vegetable mass of lichens, thus, providing a rich source for these natural compounds.
Due to their bioactivities, lichens have been used for centuries in traditional medicine. Nowadays, it is known that many of their unique compounds exhibit antimicrobial, antitumor, antimutagenic, antifungal, or antiviral effects (for some examples, see [24,25,26,27,28]), and therefore lichens are considered to be a treasure trove for natural compounds which can be further developed for applications in modern medicine. In the poultry industry, in vitro studies have shown that the crude polysaccharide fraction from Parmelia perlata has antiviral activities against RNA viruses including yellow fever virus, poliovirus, and infectious bursal disease virus [29]. Notably, however, it has also been shown that the antimicrobial activities of lichen extracts significantly varied according to the species and the method of extraction [30,31]. Therefore, careful quality control of lichen products is crucial prior to their usage.
Usnea is a widespread and well-known genus of lichens found in many countries. Indeed, Usnea barbata commonly known as “old man’s beard”, “beard lichen” or “treemoss” has been used in traditional medicine for the treatment of diarrhea, ulcers, urinary infections, tuberculosis, pneumonia, stomachache, and cattle fungal disease [32]. In about 500 Usnea species including U. barbata, the polyketide usnic acid (UA), a dibenzofuran derivative existing in two enantiomeric forms, represents the most important secondary metabolite [26,33,34,35]. More specifically, (+)-UA (Figure 1) is the predominant form in Usnea species [36,37].
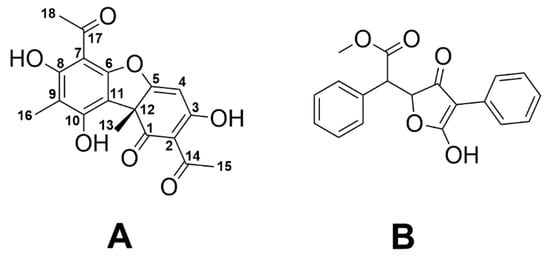
Figure 1.
Chemical structures of (+)-usnic acid (A) and vulpinic acid (B).
UA is one of the most extensively studied lichen metabolites with controversial results related to its benefits, apparently depending on the method of extraction and the lichen species [38,39]. The polyketide has been claimed to serve weight loss in humans, although hepatotoxic effects were also reported [40,41,42]. In addition, UA exhibits antibacterial, antiviral, antifungal, antiprotozoal, insecticidal, anti-inflammatory, and cytotoxic activities (reviewed in [26]). Notably, UA also inhibits the growth of Gram-positive bacteria including S. aureus, Enterococcus faecalis, E. faecium, and some anaerobic species similar to vulpinic acid, another well-known antimicrobial lichen metabolite (Figure 1) [43]. It has also been reported that UA is potent for the treatment of trichomoniasis in pigeons [44].
Previous reports have shown that some salts of UA were more efficient than free acid. For example, sodium UA displayed a superior acaricidal activity [45]. Under aqueous conditions, usnates are more soluble than the free acid with no toxicity reported [46]. As an example, zinc salts of UA have been used as adjuvants for the effective treatment of human papillomavirus with only minor side effects [47].
Herein, we report a simple and low-cost method for extracting U. barbata using sunflower oil in the cold. We show that this extract is characterized by high amounts of UA, polyphenols, and flavonoids per gram of lichen as compared with commercial CO2 extracts of the same lichen. The sunflower oil extract and its zinc salt very efficiently inhibited the growth especially of E. faecalis and displayed moderate antimicrobial activities also against other Gram-negative and Gram-positive reference pathogens, as well as against bacterial isolates (e.g., E. faecalis) from infected chickens.
2. Results
2.1. NMR-Based Determination of Usnic Acid in Oily Extracts of U. barbata
For a rapid identification and quantification of UA in crude extracts of U. barbata, we used 1H-NMR spectroscopy of the mixture that relied on the detection of specific and well separated signals for UA in the concurrent presence of abundant lipid signals from the extractant (Figure 2D). The NMR signals of UA were unequivocally assigned by two-dimensional NMR experiments, as reported earlier [48]. It turned out that the downfield shifted 1H singlet at 13.33 ppm due to the OH group at C-8 of UA (for carbon numbering, see Figure 1) was most useful for quantifying the polyketide in the complex mixture (cf. Figure 2B for a 1H-NMR spectrum of pure UA). In our procedure, the intensity of this signal was referenced to the intensity of the 1H-NMR signal at 6.89 ppm for the -CH=CH-protons of the internal reference, dimethyl fumarate (DMFum), which was added to the NMR sample at a known concentration (cf. Figure 2A for a 1H-NMR spectrum of pure DMFum).
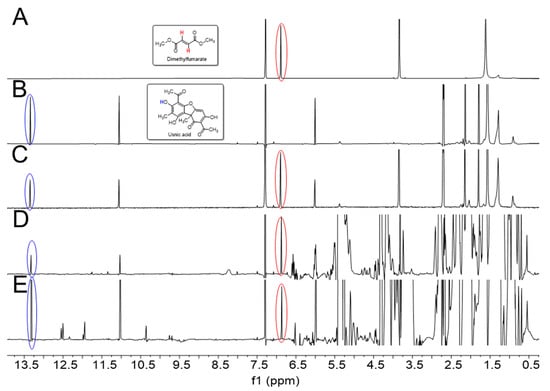
Figure 2.
(A) 1H-NMR spectrum of dimethyl fumarate (DMFum). The singlet signal (6.89 ppm) of the alkene moiety is indicated by the red circle; (B) 1H-NMR spectrum of usnic acid (UA). The well separated singlet signal of the OH proton at C-8 (13.33 ppm) is indicated by the blue circle; (C) 1H-NMR spectrum of a 1:1 molar mixture (DMFum/UA), showing the expected integral ratio of the DMFum and UA signals of approximately 2:1; (D) 1H-NMR spectrum of U. barbata extract using sunflower oil as the extractant; (E) 1H-NMR spectrum of a commercial U. barbata CO2 extract (Flavex®). All spectra (except for (B)) are scaled to the same height of the DMFum signals.
The spectrum of a 1:1 molar mixture of DMFum/UA displayed in Figure 2C shows the validity of the quantitative approach. The integral ratio of the signals due to DMFum and UA in this spectrum was determined as 2:1.02, quite accurately reflecting the relative numbers of protons for these signals provided by the respective compounds in equimolar amounts, i.e., two for DMFum and one for UA. As another control, Figure 2E shows the NMR spectrum of Flavex®, a commercial CO2 extract of U. barbata containing about 38 mg UA per mL, according to the manufacturer’s information. Here, the spectral region from 0.5 to 5.5 ppm is characterized by intense signals due to lipids (e.g., sunflower oil and polyglyceryl-3-palmitic acid) which are used by the manufacturer to dissolve the CO2 extract. Even in this complex mixture, the signals for the OH protons at position 3 (11.05 ppm) and 8 (13.33 ppm) of UA turned out to be well separated and the OH-8 signal could again be subjected to quantitative analysis, as described above. Using Equation (2) for calculation (see Materials and Methods) revealed a concentration of 31.5 ± 1.2 mg UA per ml Flavex® sample which was in the expected range.
The 1H-NMR spectrum of the crude sunflower oil extract of U. barbata is shown in Figure 2D. The spectrum was also dominated by huge signals due to the lipids from the sunflower oil, but the OH signals of UA were again well resolved allowing to quantify the content of UA. The detected normalized integral values I (UA) of OH at C-8 for UA in three replicates (A–C), each normalized to the integral of the internal standard (DMFum = 1.00), were as follows: sample A, I (UA) = 0.36; sample B, I (UA) = 0.34; and sample C, I (UA) = 0.33. Using Equation (2), the concentration of UA was determined as 1.6 ± 0.09 mg per mL extract. This corresponds to an amount of 80.4 ± 3.5 mg UA/g dry lichen.
2.2. Estimation of Total Phenolic and Flavonoid Contents
The total phenolic content in the extract was estimated by a colorimetric assay using gallic acid as a reference. In the concentration range of 5–50 µg gallic acid per mL, the assay had a linear response with a regression coefficient (R2) = 0.9986, a slope (m) = 0.0027 and an intercept = −0.0143. Using this equation, the total phenolics were determined as 4.4 mg ± 1.4 mg/mL extract corresponding to 220 mg/g dried lichen.
The total flavonoid content of the extract was measured using the aluminum chloride colorimetric assay using rutin as a reference. The rutin solution (5–100 µg/mL) conformed to Beer’s Law at 510 nm with a regression co-efficient (R2) = 0.9946. The plot had a slope (m) = 0.0011 and an intercept = 0.0131. On the basis of this, the total flavonoids were determined as 0.27 mg ± 0.11 mg/mL extract, corresponding to 13.7 mg/g dried lichen.
2.3. Antimicrobial Activity
Disc diffusion tests revealed antimicrobial activities against Gram-positive and Gram-negative bacteria (Figure 3). In these tests, we used 50 µL of the U. barbata sunflower oil extract which was 1:1 (v/v) diluted in DMSO (corresponding to ca. 40 µg UA per assay) or the respective amounts of the precipitated zinc salt. Most importantly, both samples were highly effective (inhibition zone ≥ 20 mm) against Enterococcus faecalis (Figure 4A). Here, inhibition due to the reference antibiotic, gentamycin, was comparable when present at approximately equimolar amounts of UA. Moreover, the extract exhibited a moderate antimicrobial activity (inhibition zone = 14–19 mm) against Bacillus cereus, S. aureus, Streptococcus mutans, and Enterobacter cloacae. Lower activity (inhibition zone = 8–13 mm) was observed for B. subtilis, S. epidermidis, Micrococcus spp, E. coli, and Proteus vulgaris (Figure 4A). The zinc salt precipitate was more effective (with the exception of E. coli), but still only moderately active (inhibition zone = 14–19 mm) against these bacteria (Figure 4A). The zinc precipitate and the extract, both, did not exhibit any antifungal effects against Aspergillus fumigatus and Candida albicans.
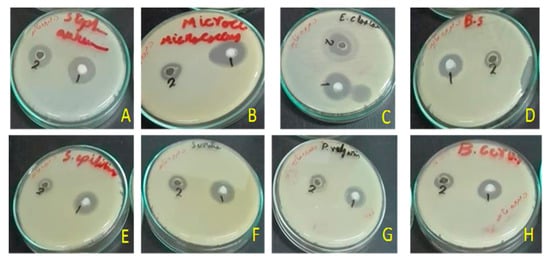
Figure 3.
Antimicrobial activity of the zinc salt of extract (1) and the total sunflower-oil extract (2) of U. barbata. (A) Staphylococcus aureus (RCMB010010); (B) Micrococcus sp. (RCMB 028); (C) Enterobacter cloacae (RCMB 001, ATCC 23355); (D) Bacillus subtilis (RCMB 015); (E) Staphylococcus epidermidis (RCMB 009); (F) Micrococcus spp (RCMB 028); (G) Proteus vulgaris (RCMB 004, ATCC 13315); (H) Bacillus cereus (RCMB 027).
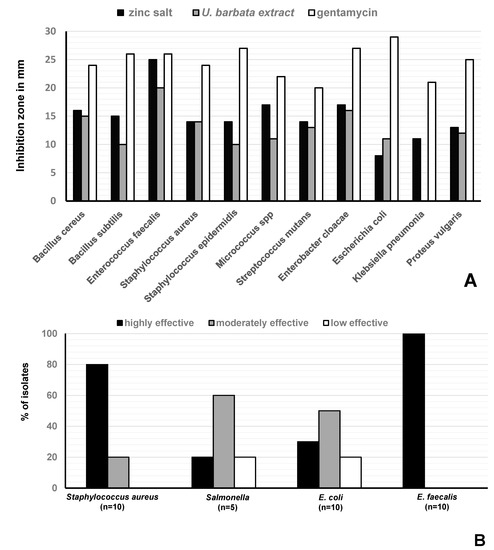
Figure 4.
Antimicrobial activity of U. barbata preparations. (A) Size of the inhibition zone of the sunflower-oil U. barbata extract and its zinc salt on Gram-positive and Gram-negative reference strains; (B) In vitro effect of the salt on S. aureus (n = 10), Salmonella spp (n = 5), E. coli (n = 10), and E. faecalis (n = 10) strains isolated from chickens. Not effective (very small inhibition zone) ≤8 mm, low effective (small inhibition zone) = 8–13, moderately effective (medium inhibition zone) = 14–19 mm, and highly effective (large inhibition zone) ≥20 mm. Sunflower oil was used as negative control and did not cause a zone of inhibition.
The zinc salt exhibited different antibacterial effects on bacterial strains isolated from poultry, i.e., against S. aureus strains (n = 10), Salmonella strains (n = 5), E. faecalis strains (n = 10), and E. coli strains (n = 10) with n = number of isolates (Figure 4B, Table 1). It was highly effective (inhibition zone ≥ 20 mm) against all (100%) tested E. faecalis strains. It was moderately effective (inhibition zone = 14–19 mm) and less effective (inhibition zone = 8–13 mm) on 8/10 (80%), and 2/10 (20%) of the tested S. aureus strains, respectively. It was highly effective, moderately effective, and less effective against 1/5 (20%), 3/5 (60%), and 1/5 (30%) of the tested Salmonella spp, respectively. Finally, it was highly effective, moderately effective, and less effective against 30%, 50%, and 20% of the tested E. coli strains, respectively (Figure 4B).

Table 1.
Target strains used in this study, their origin and culture conditions.
2.4. Cytotoxicity
Two concentrations of the extract, i.e., 10 or 100 µL of the extract in 1 mL DMEM, corresponding to 16 or 160 µg UA per mL of the resulting mixtures, respectively, and respective amounts of its zinc salt precipitate, were analyzed using cell viability assays with MCF-7 and HEPG-2 cells (Table 2). On the one hand, treatment of MCF-7 and HEPG-2 with U. barbata extract (10 µL/mL) for 24 h induced 97 and 93% viability, respectively. However, the higher concentration (100 µL/mL) induced only 31 and 46% viability, respectively. On the other hand, treatment of MCF-7 and HEPG-2 with the prepared zinc salt of the U. barbata extract at concentrations of 10 µL/mL, induced 90 and 98% viability, respectively. At higher concentrations of the zinc salt (100 µL/mL), the viability decreased to 44 and 6%, respectively.

Table 2.
In vitro cytotoxic assay using breast cell line MCF-7 and hepatoblastoma HEPG-2 human cancer cell lines.
3. Discussion
The emergence of MDR bacterial strains in the human food chain is still a main concern for public health and food safety authorities worldwide. The search for proper alternatives that fulfill the demands for antimicrobials without developing resistance is the topic of many research studies. Here, we have focused on the well-known antibiotic properties of extracts of the lichen U. barbata containing the polyketide UA in about 2% of the dried lichen, according to literature [39,52]. Most previous studies with U. barbata used methanol or ethyl acetate as solvents [53] or supercritical CO2 for the extraction. For the analysis of UA and other components, high performance liquid chromatography (HPLC) [54,55], and ultra-high-performance liquid chromatography (UHPLC) were typically used [56].
In the present study, U. barbata was extracted by an alternative mild procedure, in which the lichen material was treated for three months by cold sunflower oil. For the detection of UA, we exploited quantitative NMR spectroscopy of the crude extract. It turned out that NMR spectroscopy was appropriate to assess the target compound in the crude metabolite mixture without any prior chromatographic procedures.
Using the protocol described in the Materials and Method section, we could unequivocally quantify the amounts of UA as 1.6 ± 0.09 mg per mL extract corresponding to 80.4 ± 3 mg per g lichen (i.e., about 8% of the dried lichen material). In the commercial U. barbata extract using supercritical CO2 as extractant (Flavex® Naturextrakte GmbH, Rehlingen-Siersburg, Germany), about 32 mg UA per mL extract were determined, corresponding to about 13 mg UA per g lichen, assuming that 2–3 kg of lichen were used for the preparation of 1 L Flavex® extract according to the manufacturer. On this basis, the yields of UA are significantly higher when extracting U. barbata with sunflower oil in the cold for a long period. It should also be noted that the purity of UA seems to be higher in the sunflower oil extract when comparing the high-field regions (>9 ppm) of the NMR spectra (compare Figure 2D,E). These findings probably reflect that the yield of UA is correlated with the solubility of this compound in the solvent used for extraction [38,57,58], the temperature, and the time assigned to the operation [34]. However, the UA content in extracted lichens certainly also depends on additional more complex parameters including the geographical origin of the lichen and the time of sampling.
The antimicrobial and cytotoxic activities of UA have been the topics of many previous studies for more than 50 years [26,33,34]. The efficacy of UA against S. aureus, E. faecium, and E. faecalis has also been reported earlier [43,59] and it has been shown that UA significantly inhibited the formation of bacterial biofilms on polymer surfaces [60]. In the present study, the antimicrobial and antimycotic activities of the U. barbata sunflower-oil extract and its zinc salt precipitate were assessed against several Gram-positive and Gram-negative bacteria, as well as against two fungal strains and compared with reference antibiotic and antimycotic agents (gentamycin and ketoconazole, respectively). Interestingly, no antimycotic activities against the tested fungi could be observed in contrast to literature data [55]. However, it should be noted that the antimycotic effect on only two available strains was analyzed in our study.
In sharp contrast, significant inhibition of bacterial growth could be observed for all bacteria under study. Compared to the high antimicrobial effect of similar amounts of the reference antibiotic gentamycin (inhibition zone ≥ 20 mm), the U. barbata sunflower-oil extract and its zinc salt exhibited lower, but moderate antibacterial effects (inhibition zone = 14–19 mm) against several Gram-positive and Gram-negative bacteria including S. aureus and E. cloacae, respectively (for a notable exception, see below).
In addition, the antimicrobial activities of the extract and its zinc salt were evaluated against bacterial isolates from infected chicken. Concerning S. aureus, Salmonella, and E. coli isolates, the inhibition appeared diverse from no to moderate inhibition. On this basis, no generalized assertion about the antibacterial activities and mechanisms of the extract or its zinc salt against these pathogenic species isolated from poultry could be drawn.
However, high activities (inhibition zone ≥ 20 mm) were observed against the reference strain of E. faecalis and against all (10/10) clinical isolates of E. faecalis from chickens. This finding is of special importance since this Gram-positive bacterium is known to cause several clinical findings in poultry such as first-week mortality in chicks [61], amyloid arthropathy in layers [62,63], and pulmonary hypertension syndrome in broilers [64], as well as hepatic granulomas in turkey poults [65]. Moreover, poultry could be a source of MDR enterococci [66,67] that could result in nosocomial infections in humans such as urinary tract infections, bacteraemia or endocarditis [68,69,70,71].
Our results also indicate that most antimicrobial activities of the extract could be increased in the presence of zinc salts. This was consistent with the finding that sodium salts of UA exhibited potent antimicrobial activities against vancomycin-resistant enterococci and MRSA [72]. Further investigations are certainly needed to optimize a most effective salt combination and, also to verify the molecular mechanisms of the observed bioactivities.
In addition to the antimicrobial effects, cytotoxic activities of UA and UA derivatives are known from previous studies. It has been shown that both UA enantiomers have cytotoxic and apoptotic activities on Chinese hamster lung fibroblast-like (V79) and human lung carcinoma epithelial-like (A549) cell lines [73]. Moreover, it has been demonstrated that UA derivatives have cytotoxic and apoptotic activities on a mouse lymphocytic leukemia cell line (L1210) and Chinese hamster ovary (CHO) cell lines [74]. Furthermore, it has been reported that UA derivatives inhibited the growth of human hepatoma HepG2 cells [75].
Zinc ions are known to protect HEPG2 against oxidative stress and DNA damage induced by mycotoxins increasing cell viability [76]. In our study, a significant cytotoxicity (p < 0.0001) effect of the zinc precipitate of the lichen extract on HEPG2 at high doses (100 µL/mL) was found as compared with the total extract. This could be attributed to the zinc ions of this precipitate. In line with this hypothesis, recently, it was found that zinc accumulation induces oxidative stress and subsequent apoptosis of HEPG2 due to mitochondrial dysfunction [77].
In the presence of high amounts of polyphenols and flavonoids, probably serving as antioxidant, radical scavenging compounds in U. barbata extracts, the cytotoxic effects of UA could be modulated. Therefore, in vitro cytotoxic tests using the sulphorhodamin B (SRB) assay were carried out showing that both the U. barbata sunflower oil extract and its zinc salt significantly inhibited the proliferation of MCF-7 and HEPG-2 cells in a dose-dependent manner. Interestingly, the effect of the zinc salt was, again, more potent as compared with the cytotoxic activities of the whole extract. Further in vivo investigations are required to confirm these preliminary data and to ensure the activity and safety of these compounds as potential cytotoxic agents, especially at high concentrations.
4. Conclusions
In the present study, we established a rapid NMR method to directly detect and quantify UA incrude oily extracts of Usnea. It appears that higher yields of UA per g of dry lichen can be obtained when using cold sunflower oil for a long period as extractant as compared with extracts using supercritical CO2. The oil extract and its zinc salt exhibited high antimicrobial activities against E. faecalis and moderate activities against other Gram-positive or Gram-negative bacteria. Especially, the high activity observed against E. faecalis and 10 of 10 clinical isolates of this pathogen from infected chickens is promising and underlines the potential usage of Usnea sunflower oil extracts or its zinc salt as natural feed additives for poultry, particularly to treat or prevent enterococcosis. Nevertheless, antibiograms could still be required to determine the doses and specificities of Usnea extracts before beginning a potential therapy of infected chickens or other animals.
5. Materials and Methods
5.1. Materials and Extraction of U. barbata
U. barbata raw materials, originated from Mexico, were obtained from Heinrich Klenk GmbH, Schwebheim, Germany (Figure 5). Air-dried and cleaned lichen materials were ground in a household blender. The lichen samples (20 g) were separately blended with 1 L of Bio-sunflower oil (OPW Ingredients GmbH, Niederkrüchten, Germany) at room temperature, in the dark, for 3 months, and then filtered. This extract was used to determine the concentrations of UA, total phenolics and flavonoids, as well as for screening of antimicrobial and cytotoxicity activities.

Figure 5.
The lichen U. barbata used in this study. Whole (left); Macerated (middle); Powder (right).
A commercial U. barbata CO2 extract dissolved in oil (Flavex® Naturextrakte GmbH, Rehlingen-Siersburg, Germany) was used as a positive control for UA determination. According to the producer, 2–3 kg of U. barbata raw material, originated from Macedonia, were used to produce 1 L extract containing 38–42 mg/mL (3.8–4.2%) UA. In these samples, the U. barbata CO2 extract is dissolved in 70% sunflower oil and 25% polyglyceryl-3-palmitate. Sunflower oil (OPW Ingredients GmbH, Niederkrüchten, Germany) was used as a placebo control.
5.2. Preparation of a Zinc Salt Precipitate
A total of 25 g of the total extract in 250 mL of water was heated at 50 °C. A volume of 5 mL of 1 M NaOH (0.005 mol) was added and 0.4 g (0.005 mol) of ZnO in 40 mL of water was added, and the resulting suspension was stirred, for 30 min, at 50 °C. Then, the pH was adjusted to 7.3 by addition of aqueous HCl. The resulting precipitate was filtered at reduced pressured and washed with dH2O to yield the zinc salt of UA and other acids [78].
5.3. Quantification of UA by NMR
For identification and quantification of UA in the extract, three replicates (A–C) of the sunflower oil extract were analyzed. For each replicate, a volume of 100 µL was transferred into a standard 5 mm NMR tube which was, then, filled with 100 µL of a solution of fumaric acid dimethylester (DMFum) in CDCl3 (1 mg/mL) and 360 µL of CDCl3. This mixture was analyzed by 1H-NMR spectroscopy using a Bruker Avance III spectrometer operating at 500 MHz and a temperature of 27 °C. For typical measurements, 256 scans were collected. The acquisition time was 3.2 s, the relaxation delay was 10 s, and the data size was 32 k. Control measurements with a relaxation delay of 30 s resulted in the same ratios of UA and DMFum integrals. Before Fourier transformation, the FIDs were multiplied with a mild Gaussian curve (corresponding to a lb value of −1 and a gf value of 0.01, using the MestreNova software). The phases and baselines were manually corrected for each spectrum. For a typical spectrum of the extract and an UA reference (0.1 mg in the sample), see Figure 2B,D, respectively. The observed integrals for the well-resolved and well-separated signals at 6.89 ppm (-CH=CH- protons of DMFum) and at 13.33 ppm (OH at C-8 of UA) [43], then, served to quantify the polyketide. To calculate the amount of UA, Equation (1) was used:
m (UA) = mass of UA; m (DMFum) = mass of dimethylfumarate; I (UA) = NMR integral for UA; I (DMFum) = NMR integral for dimethylfumarate; N (UA) = proton count for the NMR signal of UA; N (DMFum) = proton count for the NMR signal of dimethylfumarate; M (UA) = molar mass of UA; M (DMFum) = molar mass of dimethylfumarate
Equation (1) was simplified to Equation (2):
Equation (2) already contains the value of the NMR integral of DMFum normalized to 1.00, the number of protons causing the respective signals (DMFum = 2 and UA = 1), and the molar masses of the two compounds (DMFum = 144 g/mol and UA = 344 g/mol), and the mass of DMFum added as the internal reference (100 µg).
As a control, we calculated the recovery of UA in a reference mixture, where we added 172 µL of a standard UA solution (1 mg/mL in CDCl3) corresponding to 172 µg of UA and 72 µL of a standard solution of DMFum (1 mg/mL in CDCl3). On the basis of the 1H-NMR analysis and using Equation (2), we calculated an amount of 175 µg of UA in this sample (recovery, 102%).
5.4. Quantification of Total Phenolics and Flavonoid
The total phenolic content of the total extract was determined by the Folin–Ciocalteu method [79]. Gallic acid was used as a standard, and the total phenolic content was expressed in terms of gallic acid equivalents (GAE). The absorbance was monitored at 725 nm. The total flavonoid content was determined by a colorimetric assay based on the formation of an aluminum chloride complex. Rutin was used as a standard, and the flavonoid content was determined in terms of rutin equivalents (RE). The absorbance of the reaction mixture was measured at 510 nm [80]. All procedures were performed in 6 replicates. The phenolic and flavonoid contents of the extract were expressed as mg GAE or RE per gram of dried sample.
5.5. Antibiograms
The antimicrobial activities of the total extract and the prepared zinc salt of the extract were evaluated using the well-established agar diffusion method [81] against different reference microorganisms including Gram-positive bacteria (Bacillus cereus (RCMB 027), Bacillus subtilis (RCMB 015), Enterococcus faecalis (ATCC 29212), Staphylococcus aureus (RCMB010010), Staphylococcus epidermidis (RCMB 009), Micrococcus spp (RCMB 028), Streptococcus mutans (RCMB 017, and ATCC 25175)), Gram-negative bacteria (Enterobacter cloacae (RCMB 001, ATCC 23355), Escherichia coli (RCMB 010052, ATCC 25955), Klebsiella pneumonia (RCMB 003, ATCC 13883), and Proteus vulgaris (RCMB 004, ATCC 13315)), and yeasts and fungi (Candida albicans (RCMB 005003, ATCC 10231), and Aspergillus fumigatus (RCMB 002002)).
Additionally, the antimicrobial activity was assessed for Gram-positive and Gram-negative bacterial species isolated from poultry such as Staphylococcus aureus (n = 10), Salmonella (n = 5), Enterococcus faecalis (n = 10), and E. coli (n = 10) (n = number of isolates). The cultural origin of these chicken isolates and their culture conditions are summarized in Table 1.
The inoculum suspensions were prepared from colonies grown overnight on an agar plate and inoculated into Mueller–Hinton broth (fungi using malt broth). The extract or the salt precipitate was dissolved in dimethyl sulfoxide (DMSO) (20 mL extract/20 mL DMSO). From this solution, 50 µL corresponding to ca. 40 µg of UA were dropped onto the plate. The inhibition zone was measured around each well after 24 h, at 37 °C. Controls using DMSO were adequately done. The activities were evaluated according to the size of inhibition zone as described by Bismarck et al. [82] as follows: not effective ≤8 mm, low effective = 8–13 mm, moderately effective = 14–19 mm, and highly effective ≥20 mm. Readymade antibiotic discs containing gentamycin (10 µg, Oxoid, Wesel, Germany) or ketoconazole (15 µg, Rosco Diagnostica, Taastrup, Denmark) were used as positive controls for antibacterial and antifungal activities, respectively.
5.6. Cytotoxicity
To assess cytotoxicity of the extract and its salt precipitate, the human breast cell line MCF-7 and hepatoblastoma cell line HEPG2 (Leibniz Institute DSMZ - German Collection of Microorganisms and Cell Cultures, Braunschweig Germany) were used. Cells were maintained in DMEM media supplemented with 100 mg/mL of streptomycin, 100 units/mL of penicillin, and 10% of heat-inactivated fetal bovine serum in humidified, 5% (v/v) CO2 atmosphere at 37 °C. Cell viability was assessed by the sulforhodamine B (SRB) assay. Briefly, aliquots of 100 µL cell suspension (5 × 103 cells) filled into 96-well plates were incubated in complete media for 24 h. Cells were treated with another aliquot of 100 µL medium containing the extract and its salt under study at final concentrations of 10 and 100 µg/mL, respectively. After 72 h of exposure, cells were fixed by replacing media with 150 µL of 10% TCA and incubated at 4 °C for 1 h. The TCA solution was removed, and the cells were washed 5 times with distilled water. Aliquots of 70 µL SRB solution (0.4%, w/v) were added and incubated in a dark place at room temperature for 10 min. Plates were washed 3 times with 1% acetic acid and allowed to air-dry overnight. Then, 150 µL of TRIS (10 mM) were added to dissolve protein-bound SRB stain; the absorbance was measured at 540 nm using a BMG LABTECH®-FLUOstar Omega microplate reader (Ortenberg, Germany).
5.7. Statistical Analysis
Data of cytotoxicity are shown as means with standard errors (SEM). Unpaired Students t test was used to identify significant differences between means. The statistical analysis was carried out with GraphPad Prism 4 (GaphPad Software, La Jolla, CA, USA). In all cases, p < 0.05 was assumed to indicate significant differences.
Author Contributions
Conceptualization, A.A.S., W.E., K.-R.T., and M.H.; methodology, S.B.; M.A.A.F., R.T., M.E.-S., A.E., T.G., and C.H.; validation, A.A.S., W.E., and C.H.; formal analysis, A.A.S., W.E., and C.H.; data curation, A.A.S., W.E., and C.H.; writing—original draft preparation, A.A.S., W.E., S.B., M.A.A.F., T.G., and C.H.; writing—review and editing, A.A.S. and W.E.; visualization, investigation, and supervision, A.A.S. and W.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We gratefully acknowledge all workers at PerNaturam GmbH, Gödenroth, Germany for their support during materials preparations. W.E. and C.H. thank the Hans-Fischer-Gesellschaft e.V. for continuous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Tarabees, R.; Gafar, K.M.; El-Sayed, M.S.; Shehata, A.A.; Ahmed, M. Effects of dietary supplementation of probiotic mix and prebiotic on growth performance, cecal microbiota composition, and protection against Escherichia coli O78 in broiler chickens. Probiotics Antimicrob Proteins 2019, 11, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Tarabees, R.; El-Sayed, M.S.; Shehata, A.A.; Diab, M.S. Effects of the probiotic candidate E. faecalis-1, the poulvac E. coli vaccine, and their combination on growth performance, caecal microbial composition, immune response, and protection against E. coli O78 challenge in broiler chickens. Probiotics Antimicrob Proteins 2019. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Landoni, M.F.; Albarellos, G. The use of antimicrobial agents in broiler chickens. Vet. J. 2015, 205, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Leger, D.; Carson, C. Review of antimicrobial therapy of selected bacterial diseases in broiler chickens in Canada. Can. Vet. J. 2012, 53, 1289–1300. [Google Scholar]
- Pagel, S.W.; Gautier, P. Use of antimicrobial agents in livestock. Rev. Sci. Tech. 2012, 31, 145–188. [Google Scholar] [CrossRef]
- Goetting, V.; Lee, K.A.; Tell, L.A. Pharmacokinetics of veterinary drugs in laying hens and residues in eggs: A review of the literature. J. Vet. Pharmacol. Ther. 2011, 34, 521–556. [Google Scholar] [CrossRef]
- Reig, M.; Toldra, F. Veterinary drug residues in meat: Concerns and rapid methods for detection. Meat. Sci. 2008, 78, 60–67. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert. Rev. Anti. Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Montoro-Dasi, L.; Villagra, A.; Sevilla-Navarro, S.; Perez-Gracia, M.T.; Vega, S.; Marin, C. The dynamic of antibiotic resistance in commensal Escherichia coli throughout the growing period in broiler chickens: Fast-growing vs. slow-growing breeds. Poult. Sci. 2020, 99, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Drame, O.; Leclair, D.; Parmley, E.J.; Deckert, A.; Ouattara, B.; Daignault, D.; Ravel, A. Antimicrobial resistance of Campylobacter in broiler chicken along the food chain in Canada. Foodborne Pathog. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Sahibzada, S.; Hewson, K.; Laird, T.; Abraham, R.; Pavic, A.; Truswell, A.; Lee, T.; O’Dea, M.; Jordan, D. Emergence of fluoroquinolone-resistant Campylobacter jejuni and Campylobacter coli among Australian chickens in the absence of fluoroquinolone use. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Fani, F.; Aminshahidi, M.; Firoozian, N.; Rafaatpour, N. Prevalence, antimicrobial resistance, and virulence-associated genes of Campylobacter isolates from raw chicken meat in Shiraz, Iran. Iran J. Vet. Res. 2019, 20, 283–288. [Google Scholar]
- Shehata, A.A.; Shereen, B.; Elrazek, A.A.; Sultan, H.; Elsayed, M.S.A.E.; Tarabees, R.; Talat, S.; Moharam, I.; Said, A.; Mohsen, W.A.; et al. Characterization of Salmonella enterica isolated from poultry hatcheries and commercial broiler chickens. Pak. Vet. J. 2019, 39, 515–520. [Google Scholar] [CrossRef]
- Asfaw Ali, D.; Tadesse, B.; Ebabu, A. Prevalence and antibiotic resistance pattern of Salmonella isolated from caecal contents of exotic chicken in Debre Zeit and Modjo, Ethiopia. Int. J. Microbiol. 2020, 2020, 1910630. [Google Scholar] [CrossRef]
- Bernier-Lachance, J.; Arsenault, J.; Usongo, V.; Parent, E.; Labrie, J.; Jacques, M.; Malouin, F.; Archambault, M. Prevalence and characteristics of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada. PLoS ONE 2020, 15, e0227183. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Jauro, S.; Musa, J.A.; Lekko, Y.M.; Salihu, S.I.; Danchuwa, H.A. Phenotypic detection of methicillin-resistant Staphylococcus aureus in village chickens from poultry markets in Maiduguri, Nigeria. J. Adv. Vet. Anim. Res. 2019, 6, 163–167. [Google Scholar] [CrossRef]
- Kuhn, S.; Colreavy-Donnelly, S.; Santana de Souza, J.; Borges, R.M. An integrated approach for mixture analysis using MS and NMR techniques. Faraday Discuss. 2019, 218, 339–353. [Google Scholar] [CrossRef]
- Uma, K.; Huang, X.; Kumar, B.A. Antifungal effect of plant extract and essential oil. Chin. J. Integr. Med. 2017, 23, 233–239. [Google Scholar] [CrossRef]
- Aschenbrenner, I.A.; Cernava, T.; Berg, G.; Grube, M. Understanding microbial multi-species symbioses. Front. Microbiol. 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Huneck, S. New results on the chemistry of lichen substances. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 2001; pp. 1–276. [Google Scholar]
- Molnar, K.; Farkas, E. Current results on biological activities of lichen secondary metabolites: A review. Z Nat. C J. Biosci. 2010, 65, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Felczykowska, A.; Pastuszak-Skrzypczak, A.; Pawlik, A.; Bogucka, K.; Herman-Antosiewicz, A.; Guzow-Krzeminska, B. Antibacterial and anticancer activities of acetone extracts from in vitro cultured lichen-forming fungi. BMC Complement. Altern. Med. 2017, 17, 300. [Google Scholar] [CrossRef] [PubMed]
- Studzinska-Sroka, E.; Holderna-Kedzia, E.; Galanty, A.; Bylka, W.; Kacprzak, K.; Cwiklinska, K. In vitro antimicrobial activity of extracts and compounds isolated from Cladonia uncialis. Nat. Prod. Res. 2015, 29, 2302–2307. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef]
- Boustie, J.; Tomasi, S.; Grube, M. Bioactive lichen metabolites: Alpine habitats as an untapped source. Phytochem. Rev. 2011, 10, 287–307. [Google Scholar] [CrossRef]
- Xu, M.; Heidmarsson, S.; Olafsdottir, E.S.; Buonfiglio, R.; Kogej, T.; Omarsdottir, S. Secondary metabolites from cetrarioid lichens: Chemotaxonomy, biological activities and pharmaceutical potential. Phytomedicine 2016, 23, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Esimone, C.O.; Ofokansi, K.C.; Adikwu, M.U.; Ibezim, E.C.; Abonyi, D.O.; Odaibo, G.N.; Olaleye, D.O. In vitro evaluation of the antiviral activity of extracts from the lichen Parmelia perlata (L.) Ach. against three RNA viruses. J. Infect. Dev. Ctries. 2007, 1, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, B.; Misic, M.; Sukdolak, S. Evaluation of antimicrobial activity of the lichens Lasallia pustulata, Parmelia sulcata, Umbilicaria crustulosa, and Umbilicaria cylindrica. Mikrobiologiia 2007, 76, 817–821. [Google Scholar] [CrossRef]
- Rankovic, B.; Misic, M.; Sukdolak, S. Antimicrobial activity of extracts of the lichens Cladonia furcata, Parmelia caperata, Parmelia pertusa, Hypogymnia physodes and Umbilicaria polyphylla. Br. J. Biomed. Sci. 2007, 64, 143–148. [Google Scholar] [CrossRef]
- Prateeksha, P.; Paliya, B.S.; Bajpai, R.; Jadaun, V.; Kumar, J.; Kumar, S.; Upreti, D.K.; Singh, B.R.; Nayaka, S.; Joshi, Y.; et al. The genus Usnea: A potent phytomedicine with multifarious ethnobotany, phytochemistry and pharmacology. RSC Adv. 2016, 6, 21672–21696. [Google Scholar] [CrossRef]
- Ingolfsdottir, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Cocchietto, M.; Skert, N.; Nimis, P.L.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Upreti, D.; Tewari, L. Secondary metabolite variability in lichen genus Usnea in India: A potential source for bioprospection. J. Environ. Sci. Technol. 2015, 2, 44–55. [Google Scholar]
- Kinoshita, Y.; Yamamoto, Y.; Yoshimura, I.; Kurokawa, T.; Huneck, S. Distribution of optical isomers of usnic and isousnic acids analyzed by high performance liquid chromatography. Hattori Bot. Lab. 1997, 83, 173–178. [Google Scholar]
- Lucarini, R.; Tozatti, M.; De, A.; Salloum, O.; Crotti, A.E.; Silva, M.; Gimenez, V.; Groppo, M.; Januario, A.H.; Martins, C.H.; et al. Antimycobacterial activity of Usnea steineri and its major constituent (+)-usnic acid. Afr. J. Biotechnol. 2012, 11, 4636–4639. [Google Scholar] [CrossRef]
- Zugic, A.; Jeremic, I.; Isakovic, A.; Arsic, I.; Savic, S.; Tadic, V. Evaluation of anticancer and antioxidant activity of a commercially available CO2 supercritical extract of Old Man’s Beard (Usnea barbata). PLoS ONE 2016, 11, e0146342. [Google Scholar] [CrossRef]
- Ivanovic, J.; Meyer, F.; Misic, D.; Asanin, J.; Jaeger, P.; Zizovic, I.; Eggers, R. Influence of different pre-treatment methods on isolation of extracts with strong antibacterial activity from lichen Usnea barbata using carbon dioxide as a solvent. J. Supercrit. Fluids 2013, 76, 1–9. [Google Scholar] [CrossRef]
- Hsu, L.M.; Huang, Y.S.; Chang, F.Y.; Lee, S.D. ‘Fat burner’ herb, usnic acid, induced acute hepatitis in a family. J. Gastroenterol. Hepatol. 2005, 20, 1138–1139. [Google Scholar] [CrossRef]
- Sonko, B.J.; Schmitt, T.C.; Guo, L.; Shi, Q.; Boros, L.G.; Leakey, J.E.; Beger, R.D. Assessment of usnic acid toxicity in rat primary hepatocytes using 13C isotopomer distribution analysis of lactate, glutamate and glucose. Food Chem. Toxicol. 2011, 49, 2968–2974. [Google Scholar] [CrossRef]
- Yellapu, R.K.; Mittal, V.; Grewal, P.; Fiel, M.; Schiano, T. Acute liver failure caused by ’fat burners’ and dietary supplements: A case report and literature review. Can. J. Gastroenterol. 2011, 25, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Lauterwein, M.; Oethinger, M.; Belsner, K.; Peters, T.; Marre, R. In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (-)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob. Agents Chemother. 1995, 39, 2541–2543. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, M.; Ding, D.; Tan, T.; Yan, B. Effect of Cladonia alpestris on Trichomonas vaginalis in vitro. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 1995, 13, 126–129. [Google Scholar] [PubMed]
- Shang, X.; Miao, X.; Lv, H.; Wang, D.; Zhang, J.; He, H.; Yang, Z.; Pan, H. Acaricidal activity of usnic acid and sodium usnic acid against Psoroptes cuniculi in vitro. Parasitol. Res. 2014, 113, 2387–2390. [Google Scholar] [CrossRef]
- Martins, M.C.; Silva, M.C.; Silva, L.R.; Lima, V.L.; Pereira, E.C.; Falcao, E.P.; Melo, A.M.; da Silva, N.H. Usnic acid potassium salt: An alternative for the control of Biomphalaria glabrata (Say, 1818). PLoS ONE 2014, 9, e111102. [Google Scholar] [CrossRef]
- Scirpa, P.; Scambia, G.; Masciullo, V.; Battaglia, F.; Foti, E.; Lopez, R.; Villa, P.; Malecore, M.; Mancuso, S. A zinc sulfate and usnic acid preparation used as post-surgical adjuvant therapy in genital lesions by Human Papillomavirus. Minerva Ginecol. 1999, 51, 255–260. [Google Scholar]
- Kuhn, V.; Geisberger, T.; Huber, C.; Beck, A.; Eisenreich, W. A facile in vivo procedure to analyze metabolic pathways in intact lichens. New Phytol. 2019, 224, 1657–1667. [Google Scholar] [CrossRef]
- Shehata, A.A.; Schrödl, W.; Aldin, A.A.; Hafez, H.M.; Krüger, M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr. Microbiol. 2013, 66, 350–358. [Google Scholar] [CrossRef]
- Shehata, A.A.; Sultan, H.; Hafez, H.M.; Krüger, M. Safety and efficacy of a metabolic drift live attenuated Salmonella gallinarum vaccine against fowl typhoid. Avian Dis. 2013, 57, 29–35. [Google Scholar] [CrossRef]
- Krüger, M.; Shehata, A.A.; Schrödl, W.; Rodloff, A. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum. Anaerobe 2013, 20, 74–78. [Google Scholar] [CrossRef]
- Cansaran, D.; Kahya, D.; Yurdakulola, E.; Atakol, O. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. Z. Naturforsch. C J. Biosci. 2006, 61, 773–776. [Google Scholar] [CrossRef]
- Idamokoro, E.M.; Masika, P.J.; Muchenje, V.; Falta, D.; Green, E. In-vitro antibacterial sensitivity of Usnea barbata lichen extracted with methanol and ethyl-acetate against selected Staphylococcus species from milk of cows with mastitis. Archiv. Fur. Tierzucht. 2014, 57, 1–9. [Google Scholar] [CrossRef]
- Popovici, V.; Bucur, L.; Popescu, A.; Caraiane, A.; Badea, V. Determination of the content in usnic acid and polyphenols from the extracts of Usnea barbata L. and the evaluation of their antioxidant activity. Farmacia 2018, 66, 337–341. [Google Scholar]
- Rankovic, B.; Kosanic, M.; Stanojkovic, T.; Vasiljevic, P.; Manojlovic, N. Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int. J. Mol. Sci. 2012, 13, 14707–14722. [Google Scholar] [CrossRef] [PubMed]
- Popovici, V.; Bucur, L.; Costache, T.; Gherghel, D.; Vochița, G.; Mihai, C.T.; Rotinberg, P.; Schroder, V.; florin ciprian, B.; Badea, V. Studies on preparation and UHPLC analysis of the Usnea barbata (L) F.H.Wigg dry acetone extract. Rev. Roum. Chim. 2019, 70, 3775–3777. [Google Scholar] [CrossRef]
- Cansaran-Duman, D.; Aras, S.; Atakol, O. Determination of usnic acid content in some lichen species found in Anatolia. JABS 2008, 23, 41–44. [Google Scholar]
- Cansaran, D.; Cetin, D.; Halici, M.G.; Atakol, O. Determination of usnic acid in some Rhizoplaca species from Middle Anatolia and their antimicrobial activities. Z. Naturforsch. C J. Biosci. 2006, 61, 47–51. [Google Scholar] [CrossRef]
- Shibata, S.; Ukita, T.; Tamura, T.; Miura, Y. Relation between chemical constitutions and antibacterial effects of usnic acid and its derivatives. Jpn. J. Med. 1948, 1, 152–155. [Google Scholar] [CrossRef]
- Francolini, I.; Norris, P.; Piozzi, A.; Donelli, G.; Stoodley, P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob Agents Chemother 2004, 48, 4360–4365. [Google Scholar] [CrossRef]
- Olsen, R.H.; Frantzen, C.; Christensen, H.; Bisgaard, M. An investigation on first-week mortality in layers. Avian. Dis. 2012, 56, 51–57. [Google Scholar] [CrossRef]
- Blanco, A.E.; Barz, M.; Icken, W.; Cavero, D.; Mazaheri, A.; Voss, M.; Schmutz, M.; Preisinger, R. Twenty years of amyloid arthropathy research in chickens. World Poult. Sci. J. 2016, 72, 495–508. [Google Scholar] [CrossRef]
- Tankson, J.D.; Thaxton, J.P.; Vizzier-Thaxton, Y. Pulmonary hypertension syndrome in broilers caused by Enterococcus faecalis. Infect. Immun. 2001, 69, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, R.H.; Petersen, A.; Christensen, H.; Bisgaard, M. Multilocus sequence typing of Enterococcus faecalis isolates demonstrating different lesion types in broiler breeders. Avian Pathol. 2010, 39, 435–440. [Google Scholar] [CrossRef]
- Moore, W.E.C.; Gross, W.B. Liver granulomas of turkeys: Causative agents and mechanism of infection. Avian Dis. 1968, 12, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Maasjost, J.; Mühldorfer, K.; Cortez de Jäckel, S.; Hafez, H.M. Antimicrobial susceptibility patterns of Enterococcus faecalis and Enterococcus faecium isolated from poultry flocks in Germany. Avian Dis. 2015, 59, 143–148. [Google Scholar] [CrossRef]
- Maasjost, J.; Lüschow, D.; Kleine, A.; Hafez, H.M.; Mühldorfer, K. Presence of virulence genes in Enterococcus species isolated from meat turkeys in Germany does not correlate with chicken embryo lethality. Biomed. Res. Int. 2019, 2019, 6147695. [Google Scholar] [CrossRef] [PubMed]
- NNIS. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992-June 2001, issued August 2001. Am. J. Infect. Control. 2001, 29, 404–421. [Google Scholar] [CrossRef]
- Singh, K.V.; Nallapareddy, S.R.; Sillanpaa, J.; Murray, B.E. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 2010, 6, e1000716. [Google Scholar] [CrossRef]
- Singh, K.V.; Nallapareddy, S.R.; Murray, B.E. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J. Infect. Dis. 2007, 195, 1671–1677. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Thomas, V.C.; Narayanan, S.; Olson, S.; Fleming, S.D.; Hancock, L.E. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect. Immun. 2010, 78, 4936–4943. [Google Scholar] [CrossRef]
- Elo, H.; Matikainen, J.; Pelttari, E. Potent activity of the lichen antibiotic (+)-usnic acid against clinical isolates of vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Naturwissenschaften 2007, 94, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T.; Tuylu, B.A.; Turk, H. In vitro cytotoxic activities of (+)-usnic acid and (-)-usnic acid on V79, A549, and human lymphocyte cells and their non-genotoxicity on human lymphocytes. Nat. Prod. Res. 2006, 20, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Bazin, M.A.; Le Lamer, A.C.; Delcros, J.G.; Rouaud, I.; Uriac, P.; Boustie, J.; Corbel, J.C.; Tomasi, S. Synthesis and cytotoxic activities of usnic acid derivatives. Bioorg. Med. Chem. 2008, 16, 6860–6866. [Google Scholar] [CrossRef]
- Yu, X.; Guo, Q.; Su, G.; Yang, A.; Hu, Z.; Qu, C.; Wan, Z.; Li, R.; Tu, P.; Chai, X. Usnic acid derivatives with cytotoxic and antifungal activities from the lichen Usnea longissima. J. Nat. Prod. 2016, 79, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, Y.; Xu, W.; Luo, Y.; Hao, J.; Shen, X.L.; Yang, X.; Li, X.; Huang, K. Zinc protects HepG2 cells against the oxidative damage and DNA damage induced by ochratoxin A. Toxicol. Appl. Pharmacol. 2013, 268, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Lin, D.; Wang, J.; Li, P.; Liu, W. Apoptosis in HepG2 cells induced by zinc pyrithione via mitochondrial dysfunction pathway: Involvement of zinc accumulation and oxidative stress. Ecotoxicol. Environ. Saf. 2018, 161, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Liedek, E.; Haegele, G. Zinc and/or Lead Salts of Carboxylic Acids and Their Use as Corrosion Inhibitors. U.S. Patent No. 4,830,775, 16 May 1989. [Google Scholar]
- Chun, O.K.; Kim, D.O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Hindler, J.A.; Howard, B.J.; Keiser, J.F. Antimicrobial Agents and Susceptibility Testing. In Clinical and Pathogenic Microbiology; Howard, B.J., Ed.; Mosby-Year Book Inc.: St. Louis, MO, USA, 1994. [Google Scholar]
- Bismarck, D.; Schneider, M.; Müller, E. Antibakterielle In-vitro-Wirksamkeit ätherischer Öle gegen veterinärmedizinisch relevante Keime klinischer Isolate von Hunden, Katzen und Pferden. Complement Med. Res. 2017, 24, 153–163. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).