Hyperoxic Exposure Caused Lung Lipid Compositional Changes in Neonatal Mice
Abstract
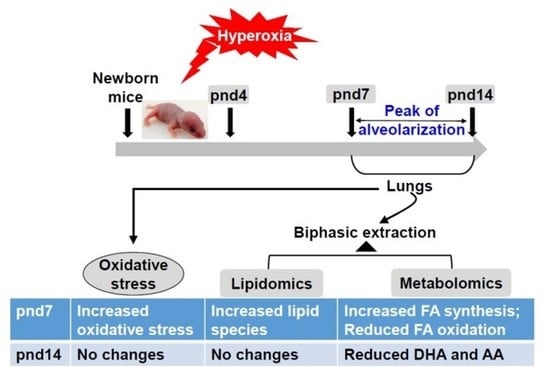
1. Introduction
2. Results
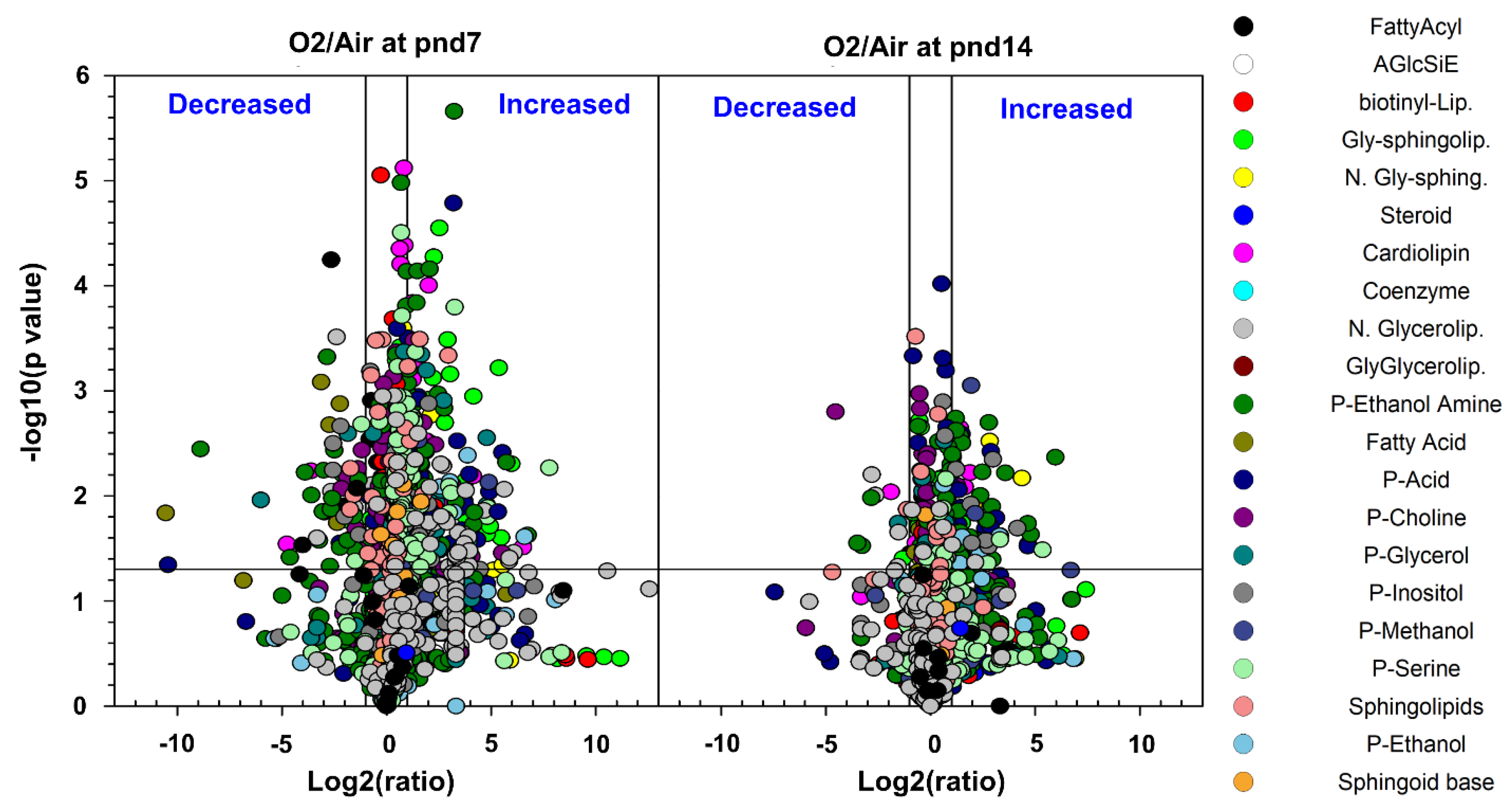
2.1. Overview and Analysis of the Pulmonary Lipidome in Response to Neonatal Hyperoxia
2.2. Neonatal Hyperoxia-Induced Alteration in Specific Lipid Categories
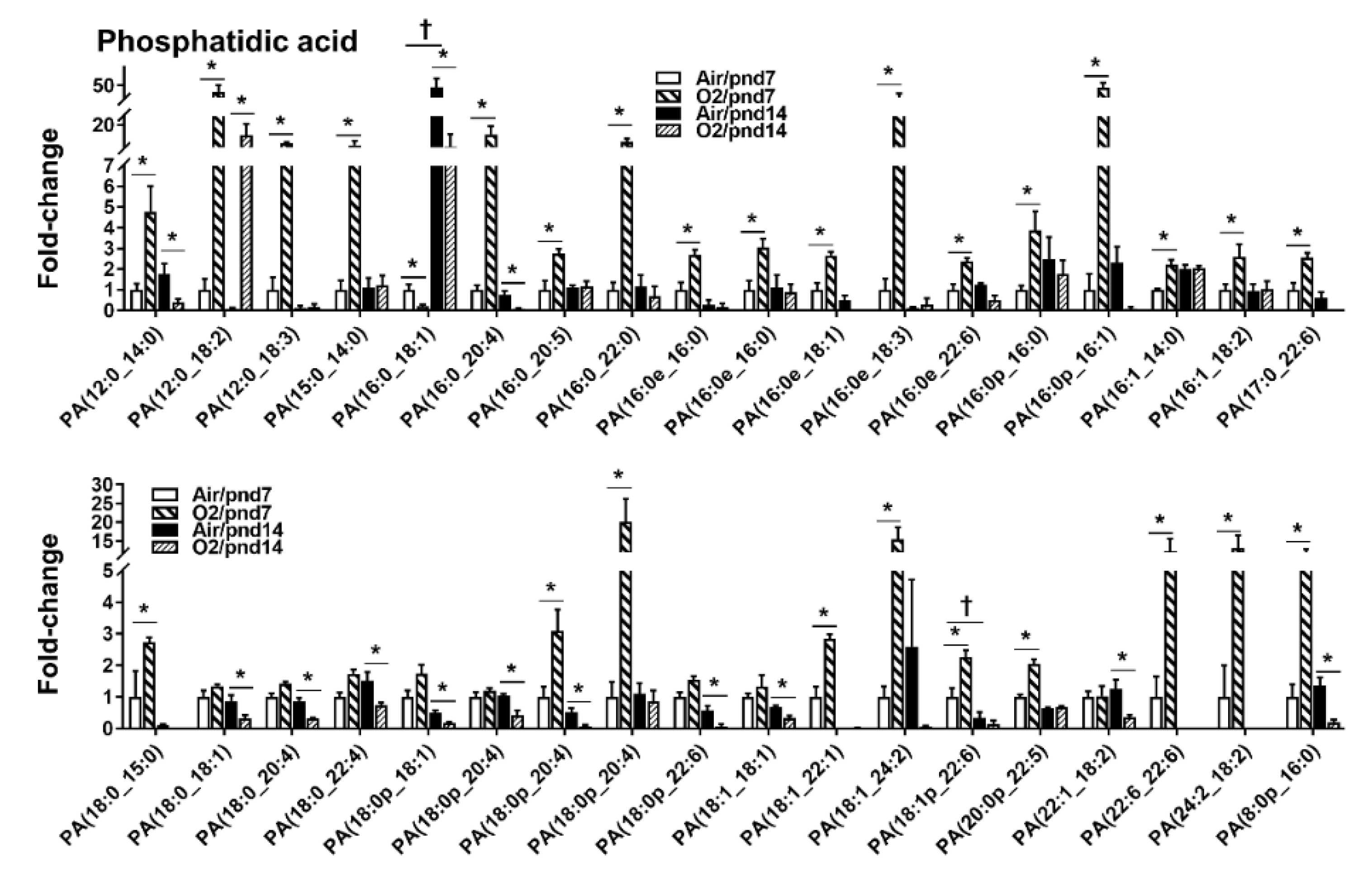
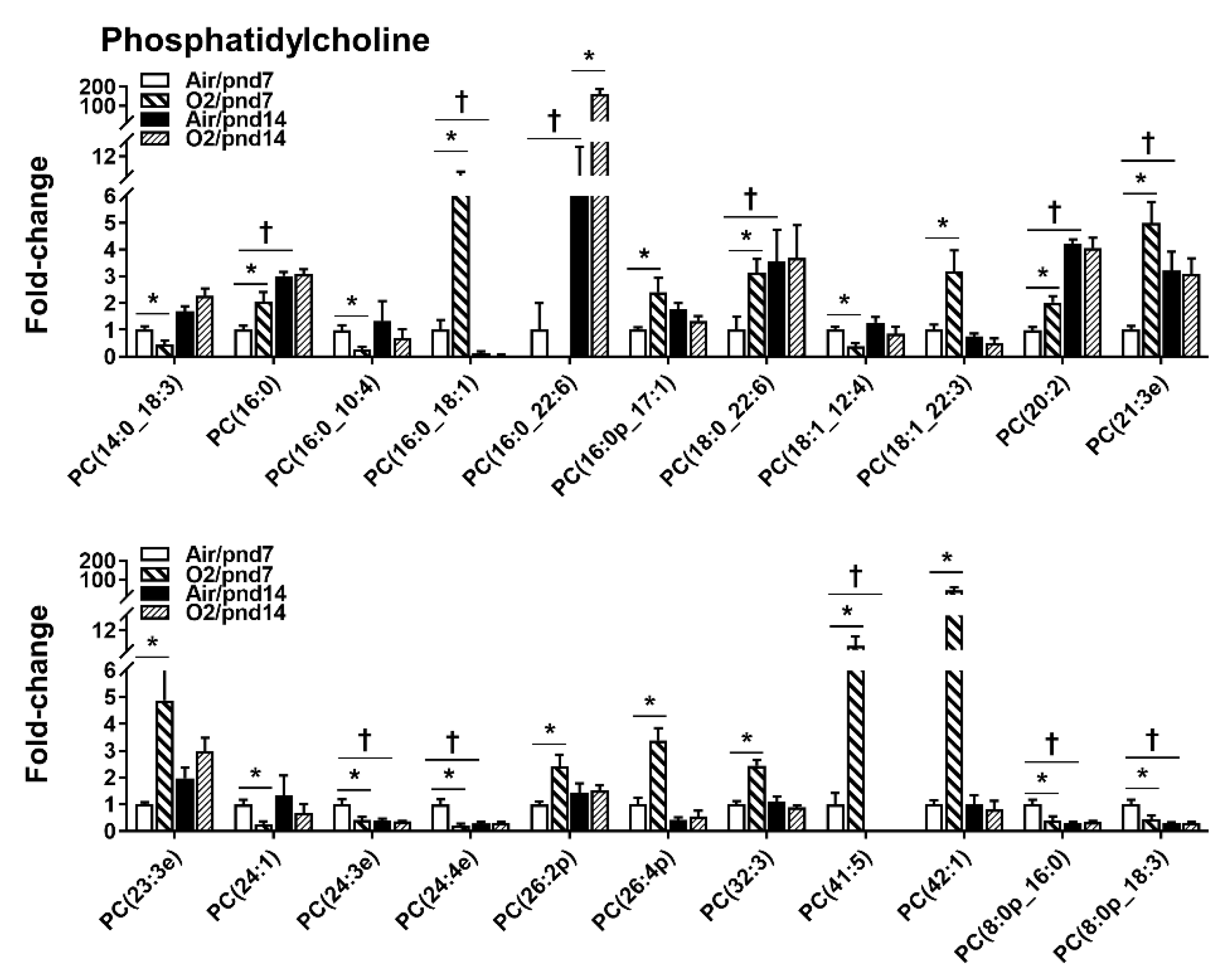
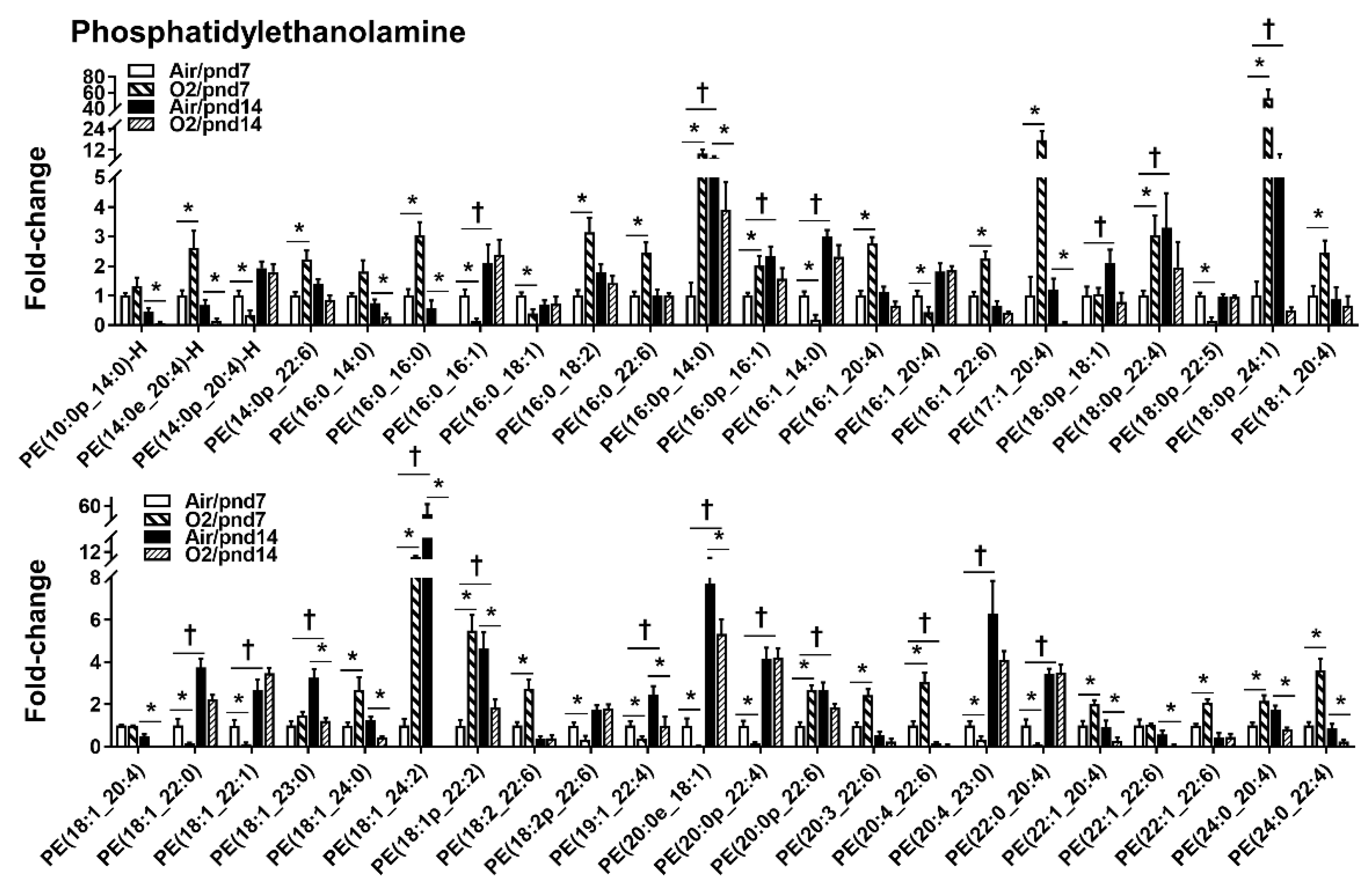
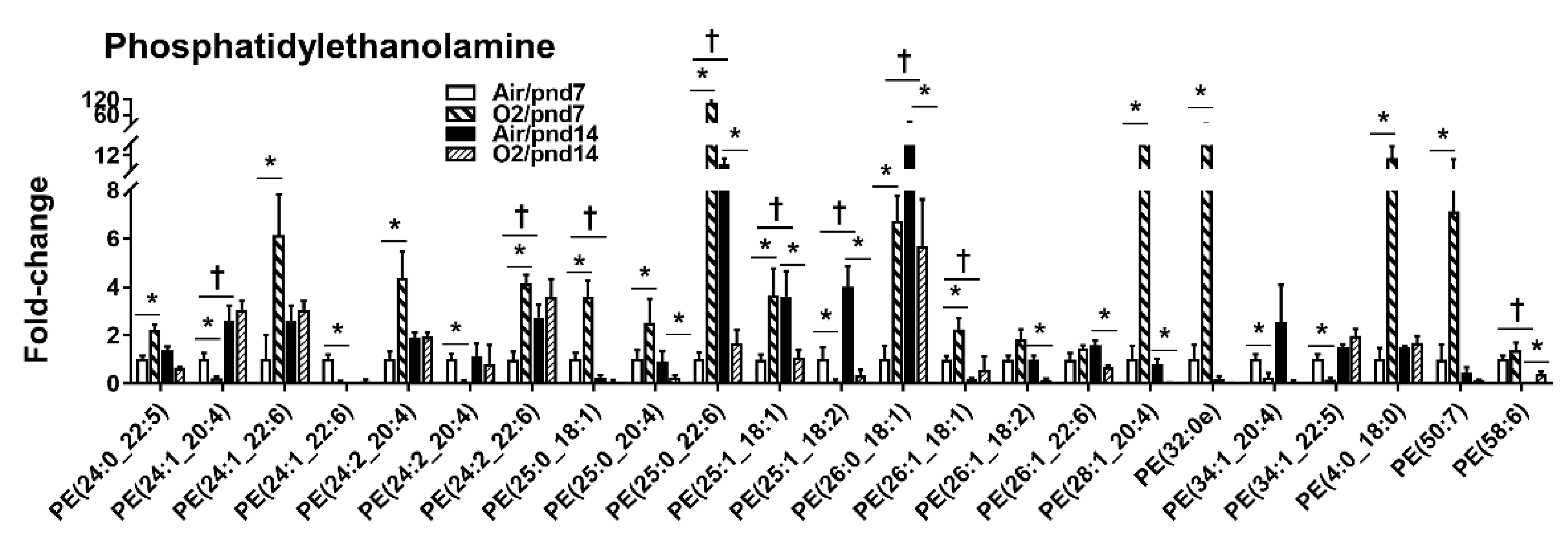
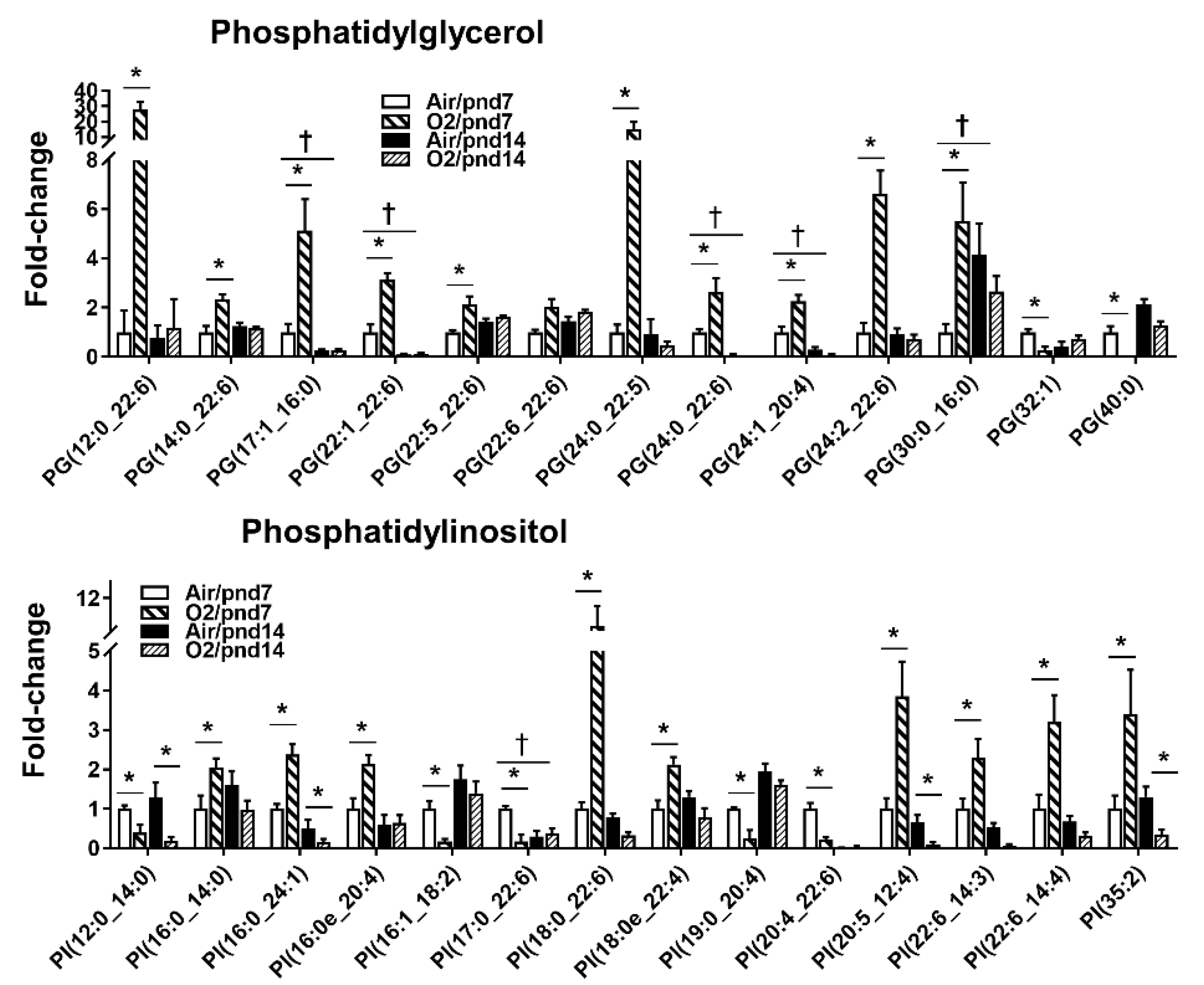
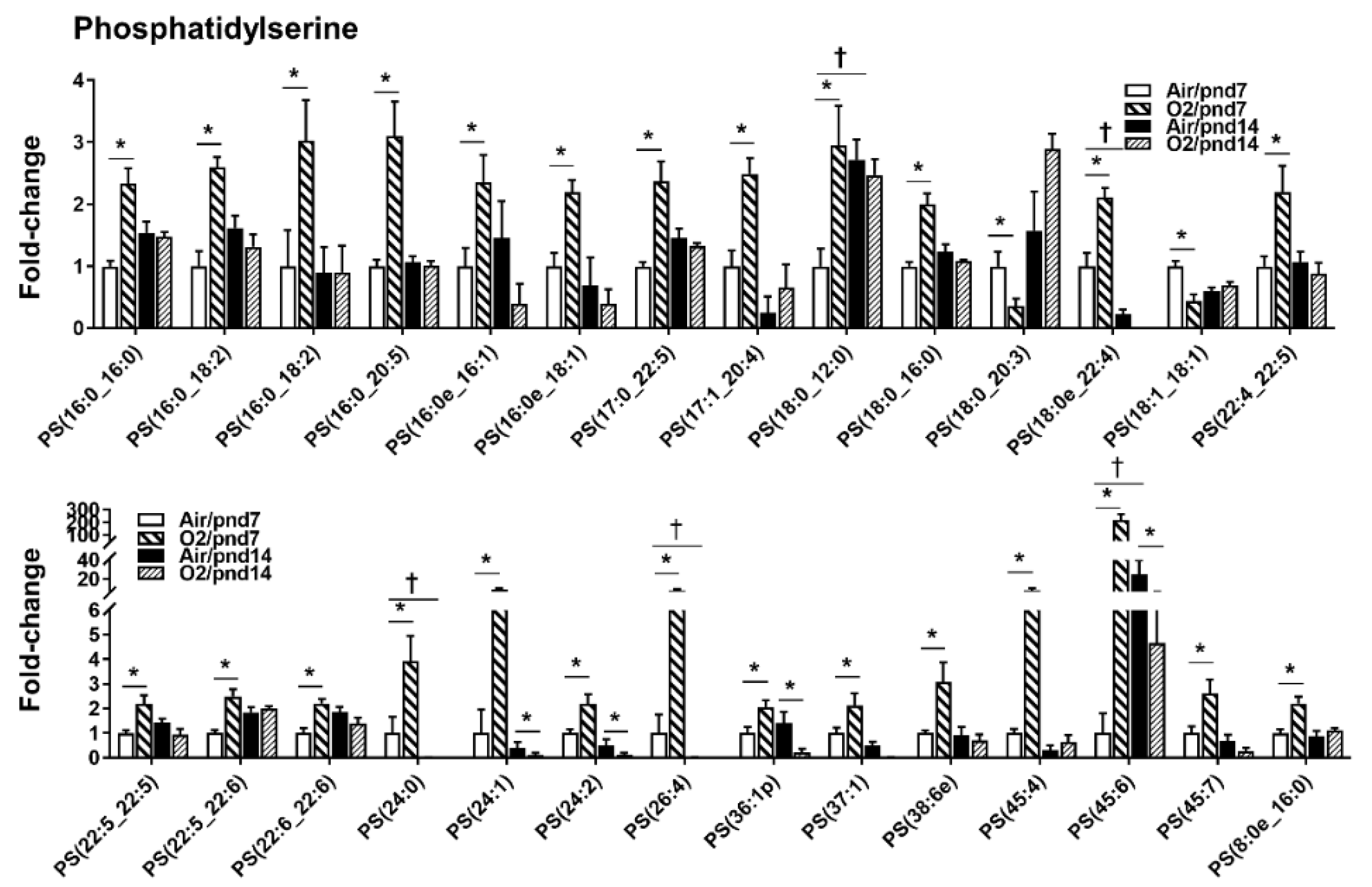
2.2.1. Glycerophospholipids
2.2.2. Lysophospholipids
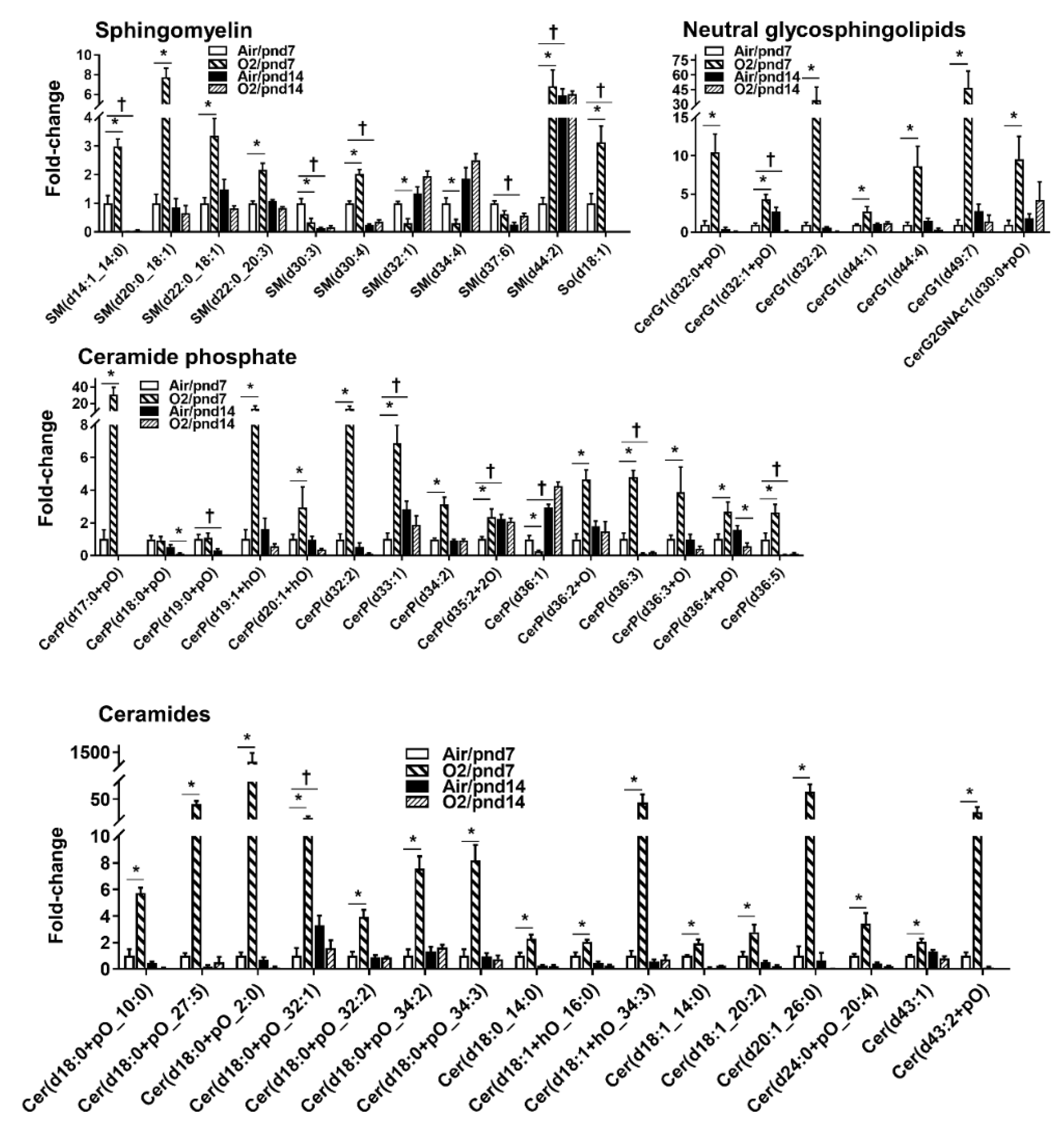
2.2.3. Sphingolipids
2.2.4. Glycerolipids
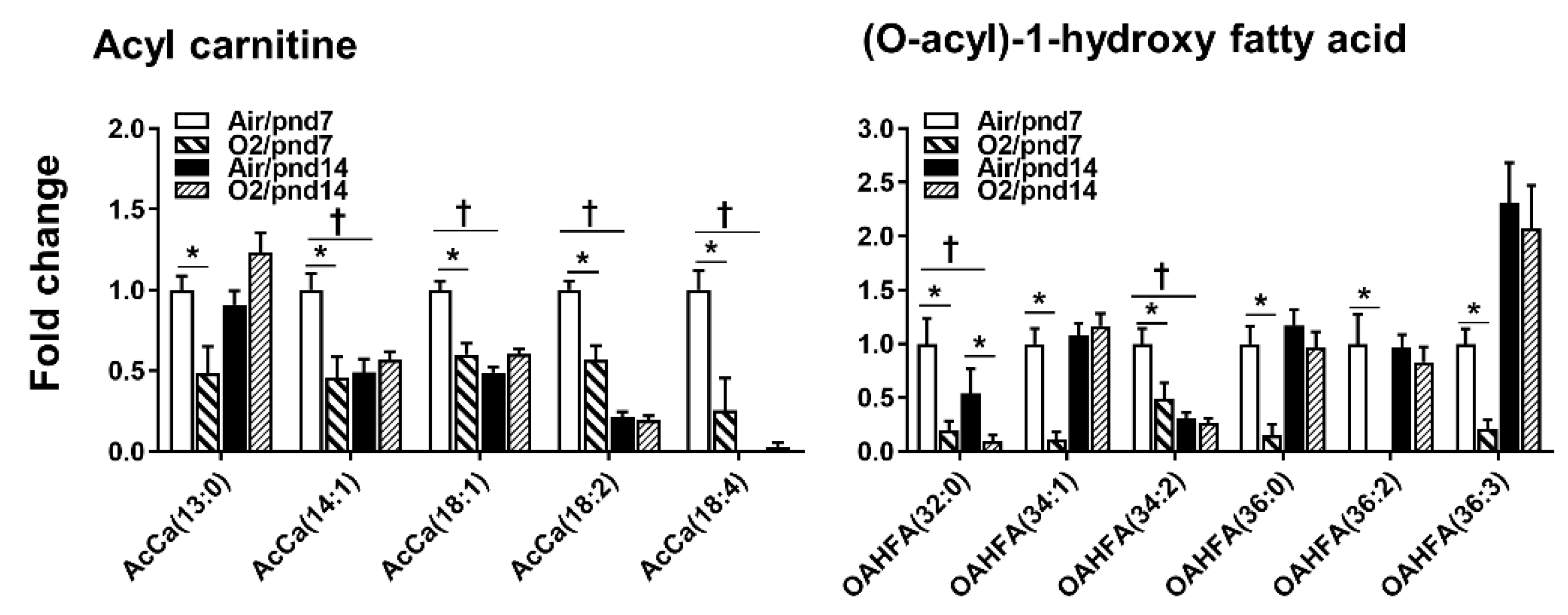
2.2.5. Fatty Acids
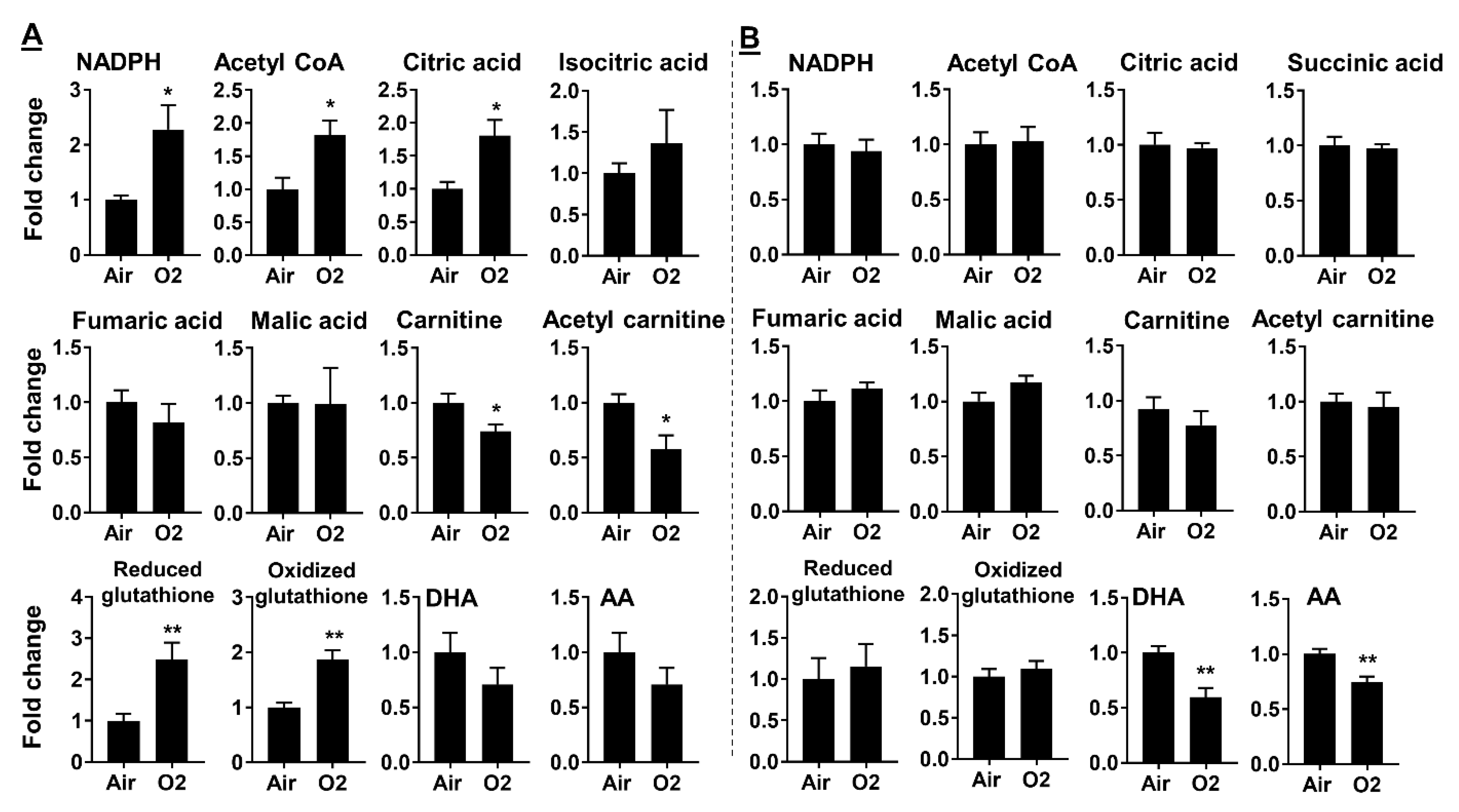
2.3. Neonatal Hyperoxia Dynamically Altered Metabolites for Fatty Acid Synthesis and Oxidation
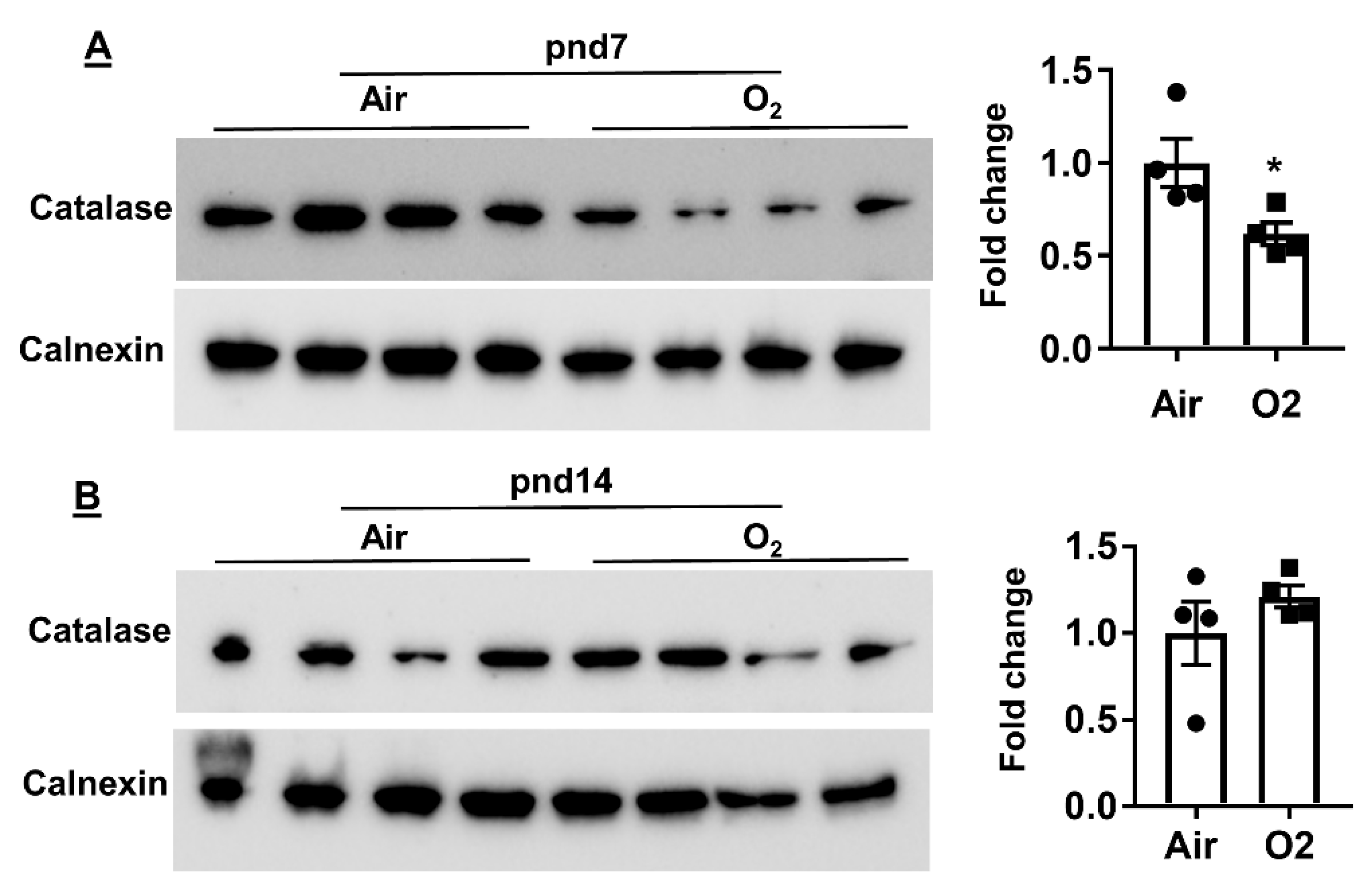
2.4. Neonatal Hyperoxia Increased Oxidative Stress in Mouse Lungs at pnd7 but Not pnd14
3. Discussion
4. Methods
4.1. Hyperoxic Exposure
4.2. Biphasic Extraction of Lung Tissues for Lipidomics and Metabolomics
4.3. Lipidomics Analysis
4.4. Metabolomics Assay
4.5. Measurement of Protein by Western Blot
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| AcCa | Acyl carnitine |
| BPD | Bronchopulmonary dysplasia |
| Cer | Ceramide |
| CerP | Ceramide phosphate |
| CL | Cardiolipin |
| DG | Diglyceride |
| DHA | Docosahexaenoic acid |
| LC–MS | Liquid chromatography–mass spectrometry |
| LPA | Lysophosphatidic acid |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphatidylethanolamine |
| LPG | Lysophosphatidylglycerol |
| LPS | Lysophosphatidylserine |
| OAHFA | O-acyl-1-hydroxy fatty acid |
| Pnd | Postnatal day |
| PA | Phosphatidic acid |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| SM | Sphingomyelin |
| TG | Triglyceride |
References
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Gong, J.; Peterson, A.L.; Lu, X.; Zhang, P.; Dennery, P.A. Fatty Acid Oxidation Protects against Hyperoxia-induced Endothelial Cell Apoptosis and Lung Injury in Neonatal Mice. Am. J. Respir. Cell Mol. Biol. 2019, 60, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Bhandari, V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L936–L947. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Thébaud, B. Animal models of bronchopulmonary dysplasia. The term rat models. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L948–L958. [Google Scholar] [CrossRef] [PubMed]
- Veldhuizen, R.; Nag, K.; Orgeig, S.; Possmayer, F. The role of lipids in pulmonary surfactant. Biochim. Biophys. Acta 1998, 1408, 90–108. [Google Scholar] [CrossRef]
- Ballard, P.L.; Keller, R.L.; Truog, W.E.; Chapin, C.; Horneman, H.; Segal, M.R.; Ballard, R.A.; Investigators, T. Surfactant status and respiratory outcome in premature infants receiving late surfactant treatment. Pediatr. Res. 2018, 85, 305–311. [Google Scholar] [CrossRef]
- Bancalari, E.; Jain, D. Bronchopulmonary dysplasia: 50 years after the original description. Neonatology 2019, 115, 384–391. [Google Scholar] [CrossRef]
- Hendricks-Muñoz, K.D.; Xu, J.; Voynow, J.A. Tracheal aspirate VEGF and sphingolipid metabolites in the preterm infant with later development of bronchopulmonary dysplasia. Pediatr. Pulmonol. 2018, 53, 1046–1052. [Google Scholar] [CrossRef]
- Van Mastrigt, E.; Zweekhorst, S.; Bol, B.; Tibboel, J.; van Rosmalen, J.; Samsom, J.N.; Kroon, A.A.; de Jongste, J.C.; Reiss, I.K.M.; Post, M.; et al. Ceramides in tracheal aspirates of preterm infants: Marker for bronchopulmonary dysplasia. PLoS ONE 2018, 13, e0185969. [Google Scholar] [CrossRef]
- Falciglia, H.S.; Johnson, J.R.; Sullivan, J.; Hall, C.F.; Miller, J.D.; Riechmann, G.C.; Falciglia, G.A. Role of antioxidant nutrients and lipid peroxidation in premature infants with respiratory distress syndrome and bronchopulmonary dysplasia. Am. J. Perinatol. 2003, 20, 97–108. [Google Scholar] [CrossRef]
- Dautel, S.E.; Kyle, J.E.; Clair, G.; Sontag, R.L.; Weitz, K.K.; Shukla, A.K.; Nguyen, S.N.; Kim, Y.-M.; Zink, E.M.; Luders, T.; et al. Lipidomics reveals dramatic lipid compositional changes in the maturing postnatal lung. Sci. Rep. 2017, 7, 40555. [Google Scholar] [CrossRef] [PubMed]
- Karnati, S.; Garikapati, V.; Liebisch, G.; Van Veldhoven, P.P.; Spengler, B.; Schmitz, G.; Baumgart-Vogt, E. Quantitative lipidomic analysis of mouse lung during postnatal development by electrospray ionization tandem mass spectrometry. PLoS ONE 2018, 13, e0203464. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A.; Kramer, C.M.; Alpert, S.E. Effect of fatty acid profiles on the susceptibility of cultured rabbit tracheal epithelial cells to hyperoxic injury. Am. J. Respir. Cell Mol. Biol. 1990, 3, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Mund, S.I.; Stampanoni, M.; Schittny, J.C. Developmental alveolarization of the mouse lung. Dev. Dyn. 2008, 237, 2108–2116. [Google Scholar] [CrossRef]
- Perez, M.; Robbins, M.E.; Revhaug, C.; Saugstad, O.D. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free. Radic. Biol. Med. 2019, 142, 61–72. [Google Scholar] [CrossRef]
- Momoeda, K.; Hirota, K.; Utsuki, T.; Tsuchida, Y.; Hanaoka, K.; Iwamori, M. Developmental changes of neutral glycosphingolipids as receptors for pulmonary surfactant protein SP-A in the alveolar epithelium of murine lung. J. Biochem. 1996, 119, 1189–1195. [Google Scholar] [CrossRef]
- Tordet, C.; Marin, L.; Dameron, F. Pulmonary di-and-triacylglycerols during the perinatal development of the rat. Experientia 1981, 37, 333–334. [Google Scholar] [CrossRef]
- Clair, G.; Bramer, L.; Kyle, J.; Zink, E.; Pryhuber, G.; Misra, R.; Ansong, C. Proteomics and lipidomics analysis of the developing post-natal human lung. Am. J. Respir. Crit. Care Med. 2020, 021, A5979. [Google Scholar]
- Yee, M.; Buczynski, B.W.; O’Reilly, M.A. Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells. Am. J. Respir. Cell Mol. Biol. 2014, 50, 757–766. [Google Scholar] [CrossRef]
- Rogers, L.K.; Tipple, T.E.; Nelin, L.D.; Welty, S.E. Differential responses in the lungs of newborn mouse pups exposed to 85% or >95% oxygen. Pediatr. Res. 2009, 65, 33–38. [Google Scholar] [CrossRef]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Tyurin, V.A.; Jiang, J.; Tyurina, Y.Y.; Ritov, V.B.; Amoscato, A.A.; Osipov, A.N.; Belikova, N.A.; Kapralov, A.A.; Kini, V.; et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Methods 2005, 1, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.B.; Durairaj, L.; Chen, B.B.; McVerry, B.J.; Ryan, A.J.; Donahoe, M.; Waltenbaugh, A.K.; O’Donnell, C.P.; Henderson, F.C.; Etscheidt, C.A.; et al. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat. Med. 2010, 16, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Tyurina, Y.Y.; Tyurin, V.A.; Kaynar, A.M.; Kapralova, V.I.; Wasserloos, K.; Li, J.; Mosher, M.; Wright, L.; Wipf, P.; Watkins, S.; et al. Oxidative lipidomics of hyperoxic acute lung injury: Mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am. J. Physiol. Cell. Lung Mol. Physiol. 2010, 299, L73–L85. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yeganeh, B.; Ermini, L.; Post, M. Sphingolipids as cell fate regulators in lung development and disease. Apoptosis 2015, 20, 740–757. [Google Scholar] [CrossRef]
- Petrache, I.; Berdyshev, E.V. Ceramide Signaling and Metabolism in Pathophysiological States of the Lung. Annu. Rev. Physiol. 2016, 78, 463–480. [Google Scholar] [CrossRef]
- Suryadevara, V.; Fu, P.; Ebenezer, D.L.; Berdyshev, E.; Bronova, I.A.; Huang, L.S.; Harijith, A.; Natarajan, V. Sphingolipids in Ventilator Induced Lung Injury: Role of Sphingosine-1-Phosphate Lyase. Int. J. Mol. Sci. 2018, 19, 114. [Google Scholar] [CrossRef]
- Tibboel, J.; Joza, S.; Reiss, I.; de Jongste, J.C.; Post, M. Amelioration of hyperoxia-induced lung injury using a sphingolipid-based intervention. Eur. Respir. J. 2013, 42, 776–784. [Google Scholar] [CrossRef]
- McGrath-Morrow, S.A.; Stahl, J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am. J. Respir. Cell Mol. Biol. 2001, 25, 150–155. [Google Scholar] [CrossRef]
- Russo, D.; Capolupo, L.; Loomba, J.S.; Sticco, L.; D’Angelo, G. Glycosphingolipid metabolism in cell fate specification. J. Cell Sci. 2018, 131, jcs219204. [Google Scholar] [CrossRef]
- Ter Horst, S.A.J.; Fijlstra, M.; Sengupta, S.; Walther, F.J.; Wagenaar, G.T.M. Spatial and temporal expression of surfactant proteins in hyperoxia-induced neonatal rat lung injury. BMC Pulm. Med. 2006, 6, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wareham, J.A.; Ferlauto, J.J.; Wells, D.H.; Newell, R.W. Hypertriglyceridemia in infants with bronchopulmonary dysplasia. J. Pediatr. 1989, 114, 458–460. [Google Scholar] [CrossRef]
- Zhao, H.; Dennery, P.A.; Yao, H. Metabolic reprogramming in the pathogenesis of chronic lung diseases, including BPD, COPD, and pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L544–L554. [Google Scholar] [CrossRef] [PubMed]
- Noel, H.; Pande, S.V. An essential requirement of cardiolipin for mitochondrial carnitine acylcarnitine translocase activity. Lipid requirement of carnitine acylcarnitine translocase. Eur. J. Biochem. 1986, 155, 99–102. [Google Scholar] [CrossRef]
- Ali, M.; Heyob, K.M.; Velten, M.; Tipple, T.E.; Rogers, L.K. DHA suppresses chronic apoptosis in the lung caused by perinatal inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L441–L448. [Google Scholar] [CrossRef]
- Martin, C.R.; DaSilva, D.A.; Cluette-Brown, J.E.; DiMonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011, 159, 743–749. [Google Scholar] [CrossRef]
- Miraldi, E.R.; Sharfi, H.; Friedline, R.H.; Johnson, H.; Zhang, T.; Lau, K.S.; Ko, H.J.; Curran, T.G.; Haigis, K.M.; Yaffe, M.B.; et al. Molecular network analysis of phosphotyrosine and lipid metabolism in hepatic PTP1b deletion mice. Integr. Biol. 2013, 5, 940–963. [Google Scholar] [CrossRef]

| Class of Lipids | Subclass of Lipids | pnd7 | pnd14 | ||
|---|---|---|---|---|---|
| Air | O2 | Air | O2 | ||
| Glycerophospholipids | Phosphatidic acid (PA) | 151 | 154 | 154 | 152 |
| Phosphatidylcholine (PC) | 347 | 346 | 345 | 343 | |
| Phosphatidylethanolamine (PE) | 413 | 413 | 412 | 409 | |
| Phosphatidylethanol | 52 | 57 | 57 | 58 | |
| Phosphatidylglycerol (PG) | 147 | 146 | 149 | 149 | |
| Phosphatidylinositol (PI) | 120 | 120 | 113 | 115 | |
| Phosphatidylmethanol (PMe) | 35 | 35 | 35 | 34 | |
| Phosphatidylserine (PS) | 170 | 168 | 169 | 164 | |
| Phosphatidylethanol (PEth) | 52 | 57 | 57 | 58 | |
| Cardiolipin (CL) | 74 | 74 | 75 | 75 | |
| Sphingolipids | Sphingolipids | 88 | 88 | 88 | 88 |
| Sphingoid base | 12 | 12 | 11 | 11 | |
| Glycosphingolipids | 163 | 167 | 163 | 163 | |
| Neutral glycosphingolipids | 59 | 60 | 57 | 57 | |
| Glycerolipids | Neutral glycerolipid (e.g., TG, DG, and MG) | 150 | 163 | 156 | 155 |
| Glycoglycerolipid | 4 | 5 | 5 | 4 | |
| Fatty acyls | Fatty acid | 19 | 20 | 19 | 19 |
| Fatty acyl and other lipids | 57 | 58 | 49 | 49 | |
| Sterol lipids | Steroid | 4 | 6 | 4 | 4 |
| Derivatized lipids | 141 | 145 | 144 | 143 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, A.L.; Carr, J.F.; Ji, X.; Dennery, P.A.; Yao, H. Hyperoxic Exposure Caused Lung Lipid Compositional Changes in Neonatal Mice. Metabolites 2020, 10, 340. https://doi.org/10.3390/metabo10090340
Peterson AL, Carr JF, Ji X, Dennery PA, Yao H. Hyperoxic Exposure Caused Lung Lipid Compositional Changes in Neonatal Mice. Metabolites. 2020; 10(9):340. https://doi.org/10.3390/metabo10090340
Chicago/Turabian StylePeterson, Abigail L., Jennifer F. Carr, Xiangming Ji, Phyllis A. Dennery, and Hongwei Yao. 2020. "Hyperoxic Exposure Caused Lung Lipid Compositional Changes in Neonatal Mice" Metabolites 10, no. 9: 340. https://doi.org/10.3390/metabo10090340
APA StylePeterson, A. L., Carr, J. F., Ji, X., Dennery, P. A., & Yao, H. (2020). Hyperoxic Exposure Caused Lung Lipid Compositional Changes in Neonatal Mice. Metabolites, 10(9), 340. https://doi.org/10.3390/metabo10090340