The Cardiac Lipidome in Models of Cardiovascular Disease
Abstract
1. Introduction
1.1. Importance of Lipids in the Development of Cardiovascular Disease
1.2. Cardiac Lipid Composition
1.3. Cardiac Lipid Utilization
2. Models of Cardiovascular Disease
2.1. Cardiac Lipid Profiles in Experimental Myocardial Infarction Models
2.2. Cardiac Lipid Profiles in Animal Models of Obesity
2.3. Cardiac Lipid Profiles in Diabetic Cardiomyopathy Models
2.4. Lipid Profiles in Cardiac Hypertrophy
2.5. Lipid Profiles in Dilated Cardiomyopathy
2.6. Similarities in Cardiac Lipid Profiles in Models of Cardiovascular Disease
3. The Effect of Current and Novel Therapies on Cardiac Lipid Profiles
3.1. The Effect of Non-Pharmacological Interventions on Cardiac Lipid Profiles
3.2. The Effect of Commonly Prescribed CVD Medications on Cardiac Lipids
3.3. The Effect of Natural Health Products and Novel Drugs on Cardiac Lipid Profiles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 17 March 2020).
- Ference, B.A.; Graham, I.; Tokgozoglu, L.; Catapano, A.L. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, L.C.; Bakovic, M. Formation and Regulation of Mitochondrial Membranes. Int. J. Cell Biol. 2014, 2014, 709828. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Lipid Bilayer; Garland Science: New York, NY, USA, 2002; ISBN 0-8153-3218-1. [Google Scholar]
- Bing, R.J.; Siegel, A.; Ungar, I.; Gilbert, M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am. J. Med. 1954, 16, 504–515. [Google Scholar] [CrossRef]
- Van Der Vusse, G.J.; Van Bilsen, M.; Glatz, J.F.C. Cardiac fatty acid uptake and transport in health and disease. Cardiovasc. Res. 2000, 45, 279–293. [Google Scholar] [CrossRef]
- Nelson, R.H. Hyperlipidemia as a Risk Factor for Cardiovascular Disease. Prim. Care Clin. Off. Pract. 2013, 40, 195–211. [Google Scholar] [CrossRef]
- Leutner, M.; Göbl, C.; Wielandner, A.; Howorka, E.; Prünner, M.; Bozkurt, L.; Harreiter, J.; Prosch, H.; Schlager, O.; Charwat-Resl, S.; et al. Cardiometabolic Risk in Hyperlipidemic Men and Women. Int. J. Endocrinol. 2016, 2016, 2647865. [Google Scholar] [CrossRef]
- Köfeler, H.C.; Fauland, A.; Rechberger, G.N.; Trötzmüller, M. Mass spectrometry based lipidomics: An overview of technological platforms. Metabolites 2012, 2, 19–38. [Google Scholar] [CrossRef]
- Sysi-Aho, M.; Koikkalainen, J.; Seppänen-Laakso, T.; Kaartinen, M.; Kuusisto, J.; Peuhkurinen, K.; Kärkkäinen, S.; Antila, M.; Lauerma, K.; Reissell, E.; et al. Serum Lipidomics Meets Cardiac Magnetic Resonance Imaging: Profiling of Subjects at Risk of Dilated Cardiomyopathy. PLoS ONE 2011, 6, e15744. [Google Scholar] [CrossRef]
- Syme, C.; Czajkowski, S.; Shin, J.; Abrahamowicz, M.; Leonard, G.; Perron, M.; Richer, L.; Veillette, S.; Gaudet, D.; Strug, L.; et al. Glycerophosphocholine Metabolites and Cardiovascular Disease Risk Factors in Adolescents: A Cohort Study. Circulation 2016, 134, 1629–1636. [Google Scholar] [CrossRef]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef]
- Anjos, S.; Feiteira, E.; Cerveira, F.; Melo, T.; Reboredo, A.; Colombo, S.; Dantas, R.; Costa, E.; Moreira, A.; Santos, S.; et al. Lipidomics Reveals Similar Changes in Serum Phospholipid Signatures of Overweight and Obese Pediatric Subjects. J. Proteome Res. 2019, 18, 3174–3183. [Google Scholar] [CrossRef]
- Zalloua, P.; Kadar, H.; Hariri, E.; Abi Farraj, L.; Brial, F.; Hedjazi, L.; Le Lay, A.; Colleu, A.; Dubus, J.; Touboul, D.; et al. Untargeted Mass Spectrometry Lipidomics identifies correlation between serum sphingomyelins and plasma cholesterol. Lipids Health Dis. 2019, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Kohno, S.; Keenan, A.L.; Ntambi, J.M.; Miyazaki, M. Lipidomic insight into cardiovascular diseases. Biochem. Biophys. Res. Commun. 2018, 504, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Hazen, S.L. Seeking a unique lipid signature predicting cardiovascular disease risk. Circulation 2014, 129, 1799–1803. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.M.; Messineo, F.C. Lipid-membrane interactions and the pathogenesis of ischemic damage in the myocardium. Circ. Res. 1981, 48, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Uhlenbrock, K.; Gassenhuber, H.; Kostenis, E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell. Signal. 2002, 14, 941–953. [Google Scholar] [CrossRef]
- Ohanian, J.; Liu, G.; Ohanian, V.; Heagerty, A.M. Lipid second messengers derived from glycerolipids and sphingolipids, and their role in smooth muscle function. Acta Physiol. Scand. 1998, 164, 533–548. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Page, I.H.; Stare, F.J.; Corcoran, A.C.; Pollack, H.; Wilkinson, C.F. Atherosclerosis and the Fat Content of the Diet. Circulation 1957, 16, 163–178. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators, risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Boekholdt, S.M.; Kastelein, J.J.P. Lipid parameters for measuring risk of cardiovascular disease. Nat. Rev. Cardiol. 2011, 8, 197–206. [Google Scholar] [CrossRef]
- Bodor, G.S. Biochemical Markers of Myocardial Damage. J. Int. Fed. Clin. Chem. Lab. Med. 2016, 27, 95–111. [Google Scholar]
- Lipidomics-Standards-Initiative (LSI). Available online: https://lipidomics-standards-initiative.org/ (accessed on 18 March 2020).
- Liebisch, G.; Ahrends, R.; Arita, M.; Arita, M.; Bowden, J.A.; Ejsing, C.S.; Griffiths, W.J.; Holčapek, M.; Köfeler, H.; Mitchell, T.W.; et al. Lipidomics needs more standardization. Nat. Metab. 2019, 1, 745–747. [Google Scholar]
- ChemAxon. Marvin Was Used for Drawing and Displaying Chemical Structures. In Marvin V20.11.; Available online: https://chemaxon.com/products/marvin/download (accessed on 16 April 2020).
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting cardiac cellular composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef]
- Zhou, P.; Pu, W.T. Recounting cardiac cellular composition. Circ. Res. 2016, 118, 368–370. [Google Scholar] [CrossRef]
- Gray, G.M.; Macfarlane, M.G. Separation and composition of the phospholipids of ox heart. Biochem. J. 1958, 70, 409–425. [Google Scholar] [CrossRef]
- Wheeldon, L.W.; Schumert, Z.; Turner, D.A. Lipid composition of heart muscle homogenate. J. Lipid Res. 1965, 6, 481–489. [Google Scholar] [PubMed]
- Pradas, I.; Huynh, K.; Cabré, R.; Ayala, V.; Meikle, P.J.; Jové, M.; Pamplona, R. Lipidomics reveals a tissue-specific fingerprint. Front. Physiol. 2018, 9, 1165. [Google Scholar] [CrossRef]
- Crowe, M.O.; Walker, A. The ultraviolet absorption spectra and other physical data for cardiolipin, a new phospholipid, and lecithin isolated from beef heart. J. Opt. Soc. Am. 1945, 35, 800. [Google Scholar] [PubMed]
- Schlame, M.; Rua, D.; Greenberg, M.L. The biosynthesis and functional role of cardiolipin. Prog. Lipid Res. 2000, 39, 257–288. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; De Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Depre, C.; Vanoverschelde, J.L.J.; Taegtmeyer, H. Glucose for the heart. Circulation 1999, 99, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Brivet, M.; Boutron, A.; Slama, A.; Costa, C.; Thuillier, L.; Demaugre, F.; Rabier, D.; Saudubray, J.M.; Bonnefont, J.P. Defects in activation and transport of fatty acids. J. Inherit. Metab. Dis. 1999, 22, 428–441. [Google Scholar] [CrossRef]
- Son, N.H.; Basu, D.; Samovski, D.; Pietka, T.A.; Peche, V.S.; Willecke, F.; Fang, X.; Yu, S.Q.; Scerbo, D.; Chang, H.R.; et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Investig. 2018, 128, 4329–4342. [Google Scholar] [CrossRef]
- Bolisetty, S.; Jaimes, E. Mitochondria and Reactive Oxygen Species: Physiology and Pathophysiology. Int. J. Mol. Sci. 2013, 14, 6306–6344. [Google Scholar] [CrossRef]
- Allard, M.F. Energy substrate metabolism in cardiac hypertrophy. Curr. Hypertens. Rep. 2004, 6, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Young, M.E.; Lopaschuk, G.D.; Abel, E.D.; Brunengraber, H.; Darley-Usmar, V.; Des Rosiers, C.; Gerszten, R.; Glatz, J.F.; Griffin, J.L.; et al. Assessing Cardiac Metabolism. Circ. Res. 2016, 118, 1659–1701. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Hasanally, D.; Ravandi, A. Role of oxidized phospholipids in cardiovascular pathology. Clin. Lipidol. 2013, 8, 205–215. [Google Scholar] [CrossRef]
- Funasaki, H.; Gilbertson, J.R. Isolation and identification of cholesteryl alkyl ethers from bovine cardiac muscle. J. Lipid Res. 1968, 9, 766–768. [Google Scholar]
- Han, X.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef]
- Hsueh, W.; Isakson, P.C.; Needleman, P. Hormone selective lipase activation in the isolated rabbit heart. Prostaglandins 1977, 13, 1073–1091. [Google Scholar] [CrossRef]
- Chien, K.R.; Han, A.; Sen, A.; Buja, L.M.; Willerson, J.T. Accumulation of unesterified arachidonic acid in ischemic canine myocardium. Relationship to a phosphatidylcholine deacylation-reacylation cycle and the depletion of membrane phospholipids. Circ. Res. 1984, 54, 313–322. [Google Scholar] [CrossRef]
- Epps, D.E.; Schmid, P.C.; Natarajan, V.; Schmid, H.H.O. N-acylethanolamine accumulation in infarcted myocardium. Biochem. Biophys. Res. Commun. 1979, 90, 628–633. [Google Scholar] [CrossRef]
- Sousa, B.; Melo, T.; Campos, A.; Moreira, A.S.P.; Maciel, E.; Domingues, P.; Carvalho, R.P.; Rodrigues, T.R.; Girão, H.; Domingues, M.R.M. Alteration in Phospholipidome Profile of Myoblast H9c2 Cell Line in a Model of Myocardium Starvation and Ischemia. J. Cell. Physiol. 2016, 231, 2266–2274. [Google Scholar] [CrossRef]
- Nam, M.; Jung, Y.; Ryu, D.H.; Hwang, G.S. A metabolomics-driven approach reveals metabolic responses and mechanisms in the rat heart following myocardial infarction. Int. J. Cardiol. 2017, 227, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017, 2, e82922. [Google Scholar] [CrossRef] [PubMed]
- Menger, R.F.; Stutts, W.L.; Anbukumar, D.S.; Bowden, J.A.; Ford, D.A.; Yost, R.A. MALDI mass spectrometric imaging of cardiac tissue following myocardial infarction in a rat coronary artery ligation model. Anal. Chem. 2012, 84, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Halade, G.V.; Dorbane, A.; Ingle, K.A.; Kain, V.; Schmitter, J.M.; Rhourri-Frih, B. Comprehensive targeted and non-targeted lipidomics analyses in failing and non-failing heart. Anal. Bioanal. Chem. 2018, 410, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.D.; Hsu, F.F.; Ford, D.A. Electrospray ionization mass spectrometry analyses of nuclear membrane phospholipid loss after reperfusion of ischemic myocardium. J. Lipid Res. 2000, 41, 1585–1595. [Google Scholar] [PubMed]
- Halade, G.V.; Kain, V.; Tourki, B.; Jadapalli, J.K. Lipoxygenase drives lipidomic and metabolic reprogramming in ischemic heart failure. Metabolism 2019, 96, 22–32. [Google Scholar] [CrossRef]
- Lim, H.-Y.; Bodmer, R. Phospholipid homeostasis and lipotoxic cardiomyopathy. Fly (Austin) 2011, 5, 234–236. [Google Scholar] [CrossRef]
- Glenn, D.J.; Cardema, M.C.; Ni, W.; Zhang, Y.; Yeghiazarians, Y.; Grapov, D.; Fiehn, O.; Gardner, D.G. Cardiac steatosis potentiates angiotensin II effects in the heart. Am. J. Physiol. Circ. Physiol. 2015, 308, H339–H350. [Google Scholar] [CrossRef]
- Butler, T.J.; Ashford, D.; Seymour, A.M. Western diet increases cardiac ceramide content in healthy and hypertrophied hearts. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 991–998. [Google Scholar] [CrossRef]
- Pakiet, A.; Jakubiak, A.; Mierzejewska, P.; Zwara, A.; Liakh, I.; Sledzinski, T.; Mika, A. The Effect of a High-Fat Diet on the Fatty Acid Composition in the Hearts of Mice. Nutrients 2020, 12, 824. [Google Scholar] [CrossRef]
- Naoe, S.; Tsugawa, H.; Takahashi, M.; Ikeda, K.; Arita, M. Characterization of Lipid Profiles after Dietary Intake of Polyunsaturated Fatty Acids Using Integrated Untargeted and Targeted Lipidomics. Metabolites 2019, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- McCombie, G.; Medina-Gomez, G.; Lelliott, C.J.; Vidal-Puig, A.; Griffin, J.L. Metabolomic and Lipidomic Analysis of the Heart of Peroxisome Proliferator-Activated Receptor-γ Coactivator 1-β Knock Out Mice on a High Fat Diet. Metabolites 2012, 2, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Nwabuo, C.C.; Duncan, M.; Xanthakis, V.; Peterson, L.R.; Mitchell, G.F.; McManus, D.; Cheng, S.; Vasan, R.S. Association of Circulating Ceramides With Cardiac Structure and Function in the Community: The Framingham Heart Study. J. Am. Heart Assoc. 2019, 8, e013050. [Google Scholar] [CrossRef]
- Casquel De Tomasi, L.; Salomé Campos, D.H.; Grippa Sant’Ana, P.; Okoshi, K.; Padovani, C.R.; Masahiro Murata, G.; Nguyen, S.; Kolwicz, S.C.; Cicogna, A.C. Pathological hypertrophy and cardiac dysfunction are linked to aberrant endogenous unsaturated fatty acid metabolism. PLoS ONE 2018, 13, e0193553. [Google Scholar] [CrossRef]
- Lopez, E.F.; Kabarowski, J.H.; Ingle, K.A.; Kain, V.; Barnes, S.; Crossman, D.K.; Lindsey, M.L.; Halade, G.V. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am. J. Physiol. Hear. Circ. Physiol. 2015, 308, H269–H280. [Google Scholar] [CrossRef]
- Marín-Royo, G.; Ortega-Hernández, A.; Martínez-Martínez, E.; Jurado-López, R.; Luaces, M.; Islas, F.; Gómez-Garre, D.; Delgado-Valero, B.; Lagunas, E.; Ramchandani, B.; et al. The Impact of Cardiac Lipotoxicity on Cardiac Function and Mirnas Signature in Obese and Non-Obese Rats with Myocardial Infarction. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010, 11, 31–39. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Abendschein, D.R.; Kelley, J.G.; Gross, R.W. Diabetes-induced changes in specific lipid molecular species in rat myocardium. Biochem. J. 2000, 352, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Han, X.; Mancuso, D.J.; Abendschein, D.R.; Gross, R.W. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: Identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry 2005, 44, 5234–5245. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, J.; Cheng, H.; Yang, K.; Abendschein, D.R.; Gross, R.W. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 2005, 44, 16684–16694. [Google Scholar] [CrossRef]
- DeMarco, V.G.; Ford, D.A.; Henriksen, E.J.; Aroor, A.R.; Johnson, M.S.; Habibi, J.; Ma, L.; Yang, M.; Albert, C.J.; Lally, J.W.; et al. Obesity-related alterations in cardiac lipid profile and nondipping blood pressure pattern during transition to diastolic dysfunction in male db/db mice. Endocrinology 2013, 154, 159–171. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, R.; Liang, Y.; Shi, J.; Li, J.; Shang, F.; Mao, X.; Sun, J. Changes of myocardial lipidomics profiling in a rat model of diabetic cardiomyopathy using UPLC/Q-TOF/MS analysis. Diabetol. Metab. Syndr. 2017, 9, 56. [Google Scholar] [CrossRef]
- Gradman, A.H.; Alfayoumi, F. From Left Ventricular Hypertrophy to Congestive Heart Failure: Management of Hypertensive Heart Disease. Prog. Cardiovasc. Dis. 2006, 48, 326–341. [Google Scholar] [CrossRef]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the heart: A new therapeutic target? Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Dealmeida, A.C.; Van Oort, R.J.; Wehrens, X.H. Transverse Aortic Constriction in Mice. J. Vis. Exp. 2010, 1729. [Google Scholar] [CrossRef]
- Goldenberg, J.R.; Carley, A.N.; Ji, R.; Zhang, X.; Fasano, M.; Schulze, P.C.; Lewandowski, E.D. Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking. Circulation 2019, 139, 2765–2777. [Google Scholar] [CrossRef]
- Roche, C.M.; Blanch, H.W.; Clark, D.S.; Louise Glass, N. Physiological role of acyl coenzyme a synthetase homologs in lipid metabolism in Neurospora crassa. Eukaryot. Cell 2013, 12, 1244–1257. [Google Scholar] [CrossRef] [PubMed]
- Dadson, K.; Hauck, L.; Billia, F. Molecular mechanisms in cardiomyopathy. Clin. Sci. 2017, 131, 1375–1392. [Google Scholar] [CrossRef]
- McNally, E.M.; Mestroni, L. Dilated cardiomyopathy: Genetic determinants and mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Sparagna, G.C.; Chicco, A.J.; Murphy, R.C.; Bristow, M.R.; Johnson, C.A.; Rees, M.L.; Maxey, M.L.; McCune, S.A.; Moore, R.L. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J. Lipid Res. 2007, 48, 1559–1570. [Google Scholar] [CrossRef]
- Le, C.H.; Mulligan, C.M.; Routh, M.A.; Bouma, G.J.; Frye, M.A.; Jeckel, K.M.; Sparagna, G.C.; Lynch, J.M.; Moore, R.L.; McCune, S.A.; et al. Delta-6-desaturase links polyunsaturated fatty acid metabolism with phospholipid remodeling and disease progression in heart failure. Circ. Hear. Fail. 2014, 7, 172–183. [Google Scholar] [CrossRef]
- Chatfield, K.C.; Sparagna, G.C.; Sucharov, C.C.; Miyamoto, S.D.; Grudis, J.E.; Sobus, R.D.; Hijmans, J.; Stauffer, B.L. Dysregulation of cardiolipin biosynthesis in pediatric heart failure. J. Mol. Cell. Cardiol. 2014, 74, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kremer, L.C.M.; Van Dalen, E.C.; Offringa, M.; Voûte, P.A. Frequency and risk factors of anthracycline-induced clinical heart failure in children: A systematic review. Ann. Oncol. 2002, 13, 503–512. [Google Scholar] [CrossRef]
- Pinder, M.C.; Duan, Z.; Goodwin, J.S.; Hortobagyi, G.N.; Giordano, S.H. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J. Clin. Oncol. 2007, 25, 3808–3815. [Google Scholar] [CrossRef]
- Moulin, M.; Solgadi, A.; Veksler, V.; Garnier, A.; Ventura-Clapier, R.; Chaminade, P. Sex-specific cardiac cardiolipin remodelling after doxorubicin treatment. Biol. Sex. Differ. 2015, 6, 20. [Google Scholar] [CrossRef]
- Bielawska, A.E.; Shapiro, J.P.; Jiang, L.; Melkonyan, H.S.; Piot, C.; Wolfe, C.L.; Tomei, L.D.; Hannun, Y.A.; Umansky, S.R. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 1997, 151, 1257–1263. [Google Scholar]
- Delpy, E.; Hatem, S.N.; Andrieu, N.; De Vaumas, C.; Henaff, M.; Rücker-Martin, C.; Jaffrézou, J.P.; Laurent, G.; Levade, T.; Mercadier, J.J. Doxorubicin induces slow ceramide accumulation and late apoptosis in cultured adult rat ventricular myocytes. Cardiovasc. Res. 1999, 43, 398–407. [Google Scholar] [CrossRef]
- Tohyama, J.; Oya, Y.; Ezoe, T.; Vanier, M.T.; Nakayasu, H.; Fujita, N.; Suzuki, K. Ceramide accumulation is associated with increased apoptotic cell death in cultured fibroblasts of sphingolipid activator protein-deficient mouse but not in fibroblasts of patients with Farber disease. J. Inherit. Metab. Dis. 1999, 22, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Walls, S.M.; Cammarato, A.; Chatfield, D.A.; Ocorr, K.; Harris, G.L.; Correspondence, R.B. Ceramide-Protein Interactions Modulate Ceramide-Associated Lipotoxic Cardiomyopathy. Cell Rep. 2018, 22, 2702–2715. [Google Scholar] [CrossRef]
- Zhang, L.; Ussher, J.R.; Oka, T.; Cadete, V.J.J.; Wagg, C.; Lopaschuk, G.D. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc. Res. 2011, 89, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.B.; Newgard, C.B.; et al. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Myers, J. Exercise and Cardiovascular Health Jonathan Myers. Health Care (Don. Mills). 2003, 107, 1–4. [Google Scholar]
- Tham, Y.K.; Bernardo, B.C.; Huynh, K.; Giles, C.; Meikle, P.J.; Mcmullen Correspondence, J.R. Lipidomic Profiles of the Heart and Circulation in Response to Exercise versus Cardiac Pathology: A Resource of Potential Biomarkers and Drug Targets. Cell Rep. 2018, 24, 2757–2772. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Maridakis, V.; O’Neill, E.A.; Hubbard, B.K.; Strack, A.; Beals, C.; Herman, G.A.; Wong, P. The effects of simvastatin treatment on plasma lipid-related biomarkers in men with dyslipidaemia. Biomarkers 2011, 16, 321–333. [Google Scholar] [CrossRef]
- Meikle, P.J.; Wong, G.; Tan, R.; Giral, P.; Robillard, P.; Orsoni, A.; Hounslow, N.; Magliano, D.J.; Shaw, J.E.; Curran, J.E.; et al. Statin action favors normalization of the plasma lipidome in the atherogenic mixed dyslipidemia of MetS: Potential relevance to statin-associated dysglycemia. J. Lipid Res. 2015, 56, 2381–2392. [Google Scholar] [CrossRef]
- Bergheanu, S.C.; Reijmers, T.; Zwinderman, A.H.; Bobeldijk, I.; Ramaker, R.; Liem, A.H.; van der Greef, J.; Hankemeier, T.; Jukema, J.W. Lipidomic approach to evaluate rosuvastatin and atorvastatin at various dosages: Investigating differential effects among statins. Curr. Med. Res. Opin. 2008, 24, 2477–2487. [Google Scholar] [CrossRef]
- Orsoni, A.; Thérond, P.; Tan, R.; Giral, P.; Robillard, P.; Kontush, A.; Meikle, P.J.; Chapman, M.J. Statin action enriches HDL3 in polyunsaturated phospholipids and plasmalogens and reduces LDL-derived phospholipid hydroperoxides in atherogenic mixed dyslipidemia. J. Lipid Res. 2016, 57, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Yetukuri, L.; Huopaniemi, I.; Koivuniemi, A.; Maranghi, M.; Hiukka, A.; Nygren, H.; Kaski, S.; Taskinen, M.R.; Vattulainen, I.; Jauhiainen, M.; et al. High density lipoprotein structural changes and drug response in lipidomic profiles following the long-term fenofibrate therapy in the FIELD substudy. PLoS ONE 2011, 6, e23589. [Google Scholar] [CrossRef]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. The FIELD study investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [PubMed]
- Dolinsky, V.W.; Jones, K.E.; Sidhu, R.S.; Haykowsky, M.; Czubryt, M.P.; Gordon, T.; Dyck, J.R.B. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J. Physiol. 2012, 590, 2783–2799. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Cole, L.K.; Sparagna, G.C.; Hatch, G.M. Cardiac mitochondrial energy metabolism in heart failure: Role of cardiolipin and sirtuins. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 1544–1554. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, S.; Chen, Z.; Zhang, R.; Tian, L.; Cheng, L.; Shang, F.; Sun, J. Berberine Could Ameliorate Cardiac Dysfunction via Interfering Myocardial Lipidomic Profiles in the Rat Model of Diabetic Cardiomyopathy. Front. Physiol. 2018, 9, 1042. [Google Scholar] [CrossRef]
- Gao, R.Y.; Mukhopadhyay, P.; Mohanraj, R.; Wang, H.; Horváth, B.; Yin, S.; Pacher, P. Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol. Med. Rep. 2011, 4, 151–155. [Google Scholar]
- Qi, M.Y.; Feng, Y.; Dai, D.Z.; Li, N.; Cheng, Y.S.; Dai, Y. CPU86017, a berberine derivative, attenuates cardiac failure through normalizing calcium leakage and downregulated phospholamban and exerting antioxidant activity. Acta Pharmacol. Sin. 2010, 31, 165–174. [Google Scholar] [CrossRef]
- Steinhubl, S.R. Why Have Antioxidants Failed in Clinical Trials? Am. J. Cardiol. 2008, 101, S14–S19. [Google Scholar] [CrossRef]
- Britton, R.G.; Kovoor, C.; Brown, K. Direct molecular targets of resveratrol: Identifying key interactions to unlock complex mechanisms. Ann. N. Y. Acad. Sci. 2015, 1348, 124–133. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Smejkal, K.; Malaník, M.; et al. Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef] [PubMed]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Ebrahimi, B.; Zhang, X.; Zhu, X.Y.; Woollard, J.R.; He, Q.; Textor, S.C.; Lerman, A.; Lerman, L.O. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc. Res. 2014, 103, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Shao, P.L.; Wallace, C.G.; Chua, S.; Sung, P.H.; Ko, S.F.; Chai, H.T.; Chung, S.Y.; Chen, K.H.; Lu, H.I.; et al. Combined therapy with SS31 and mitochondria mitigates myocardial ischemia-reperfusion injury in rats. Int. J. Mol. Sci. 2018, 19, 2782. [Google Scholar] [CrossRef]
- Cho, J.; Won, K.; Wu, D.; Soong, Y.; Liu, S.; Szeto, H.H.; Hong, M.K. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron. Artery Dis. 2007, 18, 215–220. [Google Scholar] [CrossRef]
- Dai, D.F.; Chen, T.; Szeto, H.; Nieves-Cintrón, M.; Kutyavin, V.; Santana, L.F.; Rabinovitch, P.S. Mitochondrial targeted antioxidant peptide ameliorates hypertensive cardiomyopathy. J. Am. Coll. Cardiol. 2011, 58, 73–82. [Google Scholar] [CrossRef]
- Min, K.; Kwon, O.-S.; Smuder, A.J.; Wiggs, M.P.; Sollanek, K.J.; Christou, D.D.; Yoo, J.-K.; Hwang, M.-H.; Szeto, H.H.; Kavazis, A.N.; et al. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. J. Physiol. 2015, 593, 2017–2036. [Google Scholar] [CrossRef]
- Chatfield, K.C.; Sparagna, G.C.; Chau, S.; Phillips, E.K.; Ambardekar, A.V.; Aftab, M.; Mitchell, M.B.; Sucharov, C.C.; Miyamoto, S.D.; Stauffer, B.L.; et al. Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Trans. Sci. 2019, 4, 147–157. [Google Scholar] [CrossRef]
| Lipid Class | Examples of General Structure | |
|---|---|---|
| Fatty Acyl Lipids | Fatty Acids (FA) | 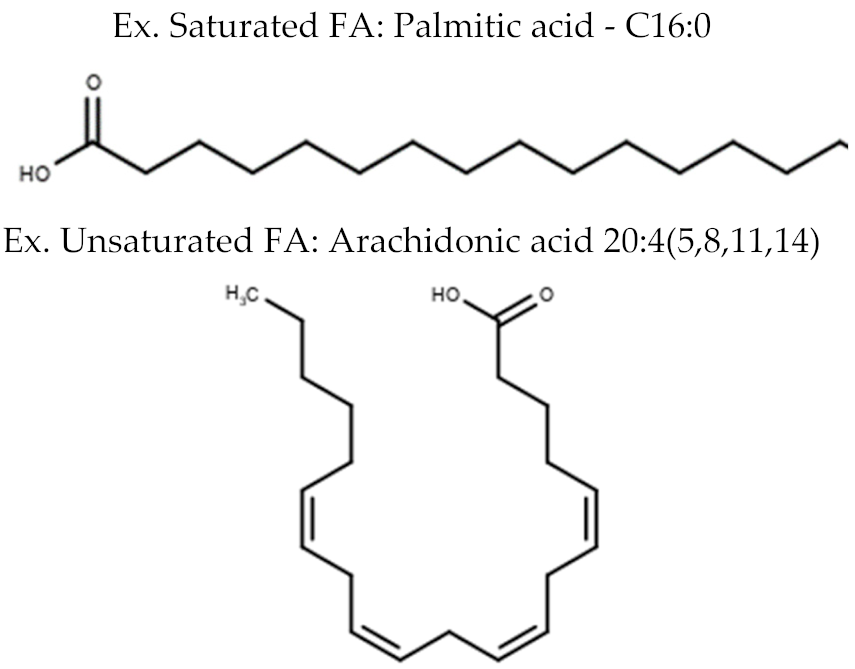 |
| Glycerolipids | Diacylglycerol (DG) Triacylglycerol (TG) |  |
| Glycero-phospholipids | Phosphatidylcholine (PC) Phosphatidylethanolamine (PE) Phosphatidylserine (PS) Phosphatidylinositol (PI) Phosphatidylglycerol (PG) Cardiolipin (CL) | 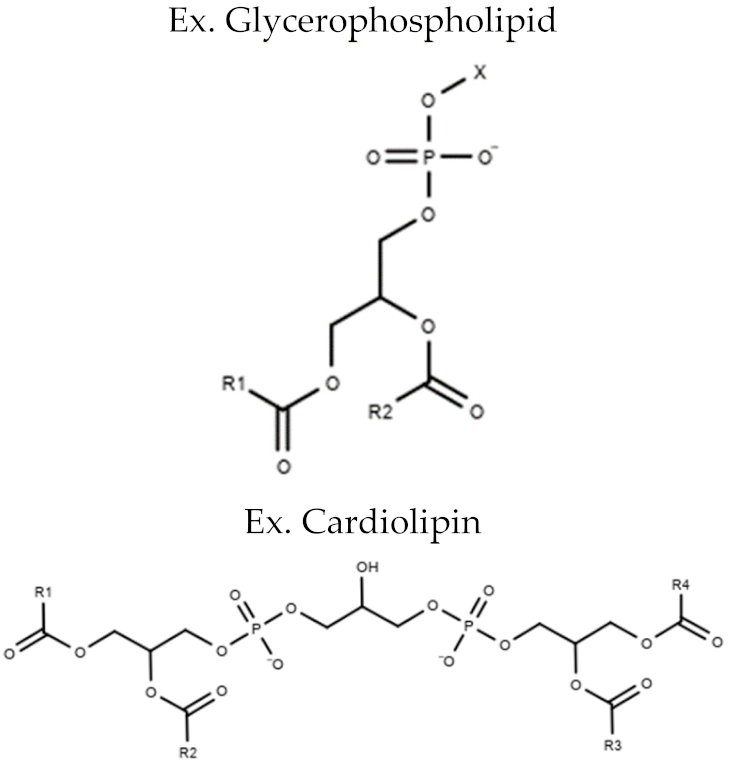 |
| Sphingolipids | Sphingosine Sphingomyelin (SM) Ceramide (CER) |  |
| Sterol Lipids | Cholesterol Cholesterol Ester (CE) | 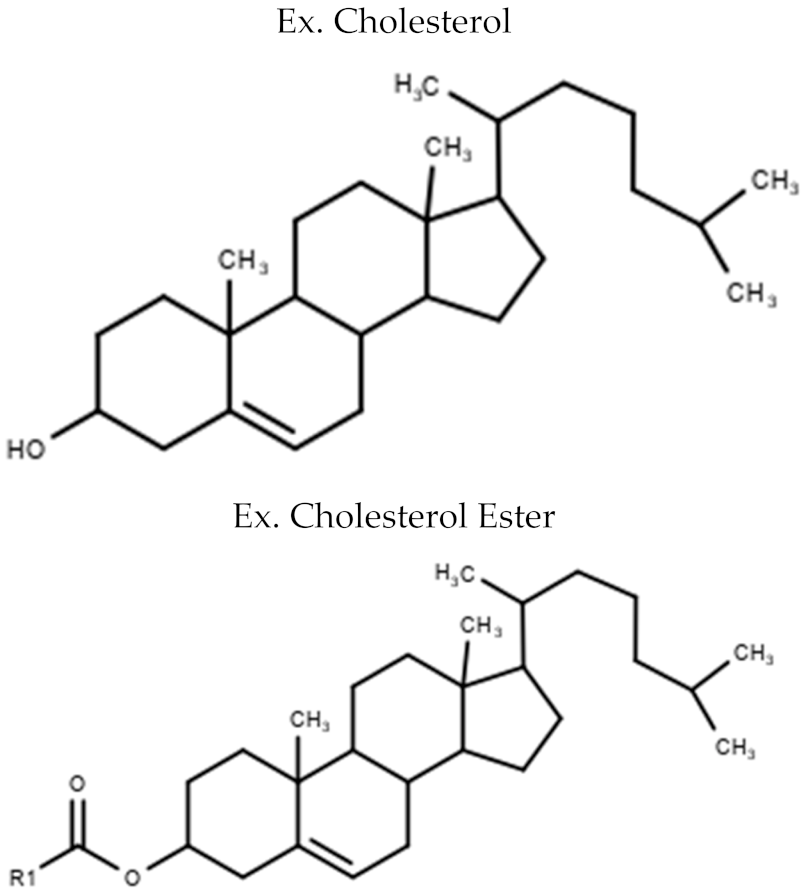 |
| CVD Model | Animal/Cell Species | N Number | Other | Lipid Species | Mass Spectrometry (MS) Technology | Reference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycolipids | Phospholipids | Sphingolipids | |||||||||||||||
| TG | DG | PC | PE | PI | PS | PG | LysoPL | OxPL | CL | CER | SM | ||||||
| MI and IR Models | |||||||||||||||||
| Starvation/Ischemic | H9c2 | 3 | FFA↕ | ↕ | ↑ | ↕ | ↕ | ↕ | ↕ | ↓ | HPLC-MS/MS | [54] | |||||
| LAD CA Ligation | Rat | 8 | FFA/AC↑ | ↑ | ↑ | - | ↑ | ↑ | ↑ | ↑ | ↑ | UPLC-QTOF-MS | [55] | ||||
| Ischemic CM | Patient (Serum/Tissue) | 15–64 | ↕ | LC-MS | [56] | ||||||||||||
| LAD CA Ligation | Mice (Serum/Tissue) | 4–20 | ↕ | LC-MS | [56] | ||||||||||||
| LAD CA Ligation | Rat | 5 | ↕ | ↑ | MALDI-MS | [57] | |||||||||||
| LAD CA Ligation | Mice | 6 | UFA/SFA↑ | ↑ | ↑ | MALDI-MSI and LC-MS/MS | [58] | ||||||||||
| IR Injury (15 min) | Rat | 6 | ↓ | ↓ | ESI-MS/MS | [59] | |||||||||||
| LAD CA Ligation | LOX-/- Mice | 37–49 | AC ↕ | ↕PL | ↕ | ↑ | LC-MS/MS | [60] | |||||||||
| Obesity Models | |||||||||||||||||
| HF Diet or HFHS Diet | Rat | 6 | ↑ | LC-MS | [63] | ||||||||||||
| HF Diet | Mice | 10 | ↑ | ↑ | ↕PL | ↑ | ↑ | GC-MS | [64] | ||||||||
| PUFA Diet | Mice | 5 | TC↓/FA↕ | ↑ | ↕ | GC-MS | [65] | ||||||||||
| HF Diet | PGC1β-/-Mice | 5–10 | ↑ | ↕ | ↕ | GC-MS LC-MS | [68] | ||||||||||
| Cardiac Steatosis | DGAT1 Mice | 6–9 | ↑ | ↕ | UPLC-QTOF-MS | [62] | |||||||||||
| Mixed Models | |||||||||||||||||
| SVAS HUFA | Mice | 11–14 | UFA↓/↑SA | ↕ | ↕ | GC-MS | [70] | ||||||||||
| HF Diet/Aging/ LAD CA Ligation | Mice (plasma) | 3–8 | AA ↑ | LC-MS/MS | [71] | ||||||||||||
| HF Diet/LAD CA Ligation | Rat | 8–10 | ↑ | ↓MI | ↓MI | ↕ MI | ↕ | ↕ | UPLC-QTOF-MS | [72] | |||||||
| Diabetic CM Models | |||||||||||||||||
| Streptozotocin Injection | Rat | 6 | ↑ | - | ↓ | ↑ | ↑ | ↑ | - | - | ESI-MS | [76] | |||||
| Streptozotocin Injection | Rat | 4–6 | AC↑ | ESI-MS | [77] | ||||||||||||
| Streptozotocin Injection | Mice | 7 | ↑ | ↓ | ↓ | ESI-MS | [78] | ||||||||||
| Genetic | LepRdb/db Mice | 5–6 | FFA↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ESI-MS | [79] | |||||
| HF Diet and Streptozotocin | Rat | 11–12 | ↕ | ↑ | UPLC-QTOF-MS | [80] | |||||||||||
| Hypertrophy Models | |||||||||||||||||
| TAC | ACL1 Mice | 3–17 | ↓ | ↕ | ESI-MS/MS | [84] | |||||||||||
| Dilated CM Models (SHHF Rats as Validation) | |||||||||||||||||
| IDCM | Patient (Serum) | 8–11 | ↓ | ↓ | ↓ | UPLC-MS | [10] | ||||||||||
| SHHF/TAB | Rat | 4 | ↕ | LC-ESI-MS | [88] | ||||||||||||
| IDCM | Patient (LV Tissue) | 10–11 | ↕ | LC-ESI-MS | [88] | ||||||||||||
| SSHF | Rat | 4–10 | ↕AA | LC-ESI-MS | [89] | ||||||||||||
| IDCM | Human (LV Tissue) | 8 | ↕AA | LC-ESI-MS | [89] | ||||||||||||
| IDCM | Pediatric (LV Tissue) | 20–44 | ↓ | LC-ESI-MS | [90] | ||||||||||||
| DOX (2mg/kg Weekly 7X) | Rat | 4 | ↕ | ↕ | ↕ | ↕ | LC-MS/MS | [93] | |||||||||
| DOX/HFHS Diet (15 mg/kg CD) | Rat | 6 | ↑ | LC-MS | [63] | ||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyk, M.M.; Dolinsky, V.W. The Cardiac Lipidome in Models of Cardiovascular Disease. Metabolites 2020, 10, 254. https://doi.org/10.3390/metabo10060254
Tomczyk MM, Dolinsky VW. The Cardiac Lipidome in Models of Cardiovascular Disease. Metabolites. 2020; 10(6):254. https://doi.org/10.3390/metabo10060254
Chicago/Turabian StyleTomczyk, Mateusz M., and Vernon W. Dolinsky. 2020. "The Cardiac Lipidome in Models of Cardiovascular Disease" Metabolites 10, no. 6: 254. https://doi.org/10.3390/metabo10060254
APA StyleTomczyk, M. M., & Dolinsky, V. W. (2020). The Cardiac Lipidome in Models of Cardiovascular Disease. Metabolites, 10(6), 254. https://doi.org/10.3390/metabo10060254






