Restoration of Physiological Levels of Uric Acid and Ascorbic Acid Reroutes the Metabolism of Stored Red Blood Cells
Abstract
1. Introduction
2. Results
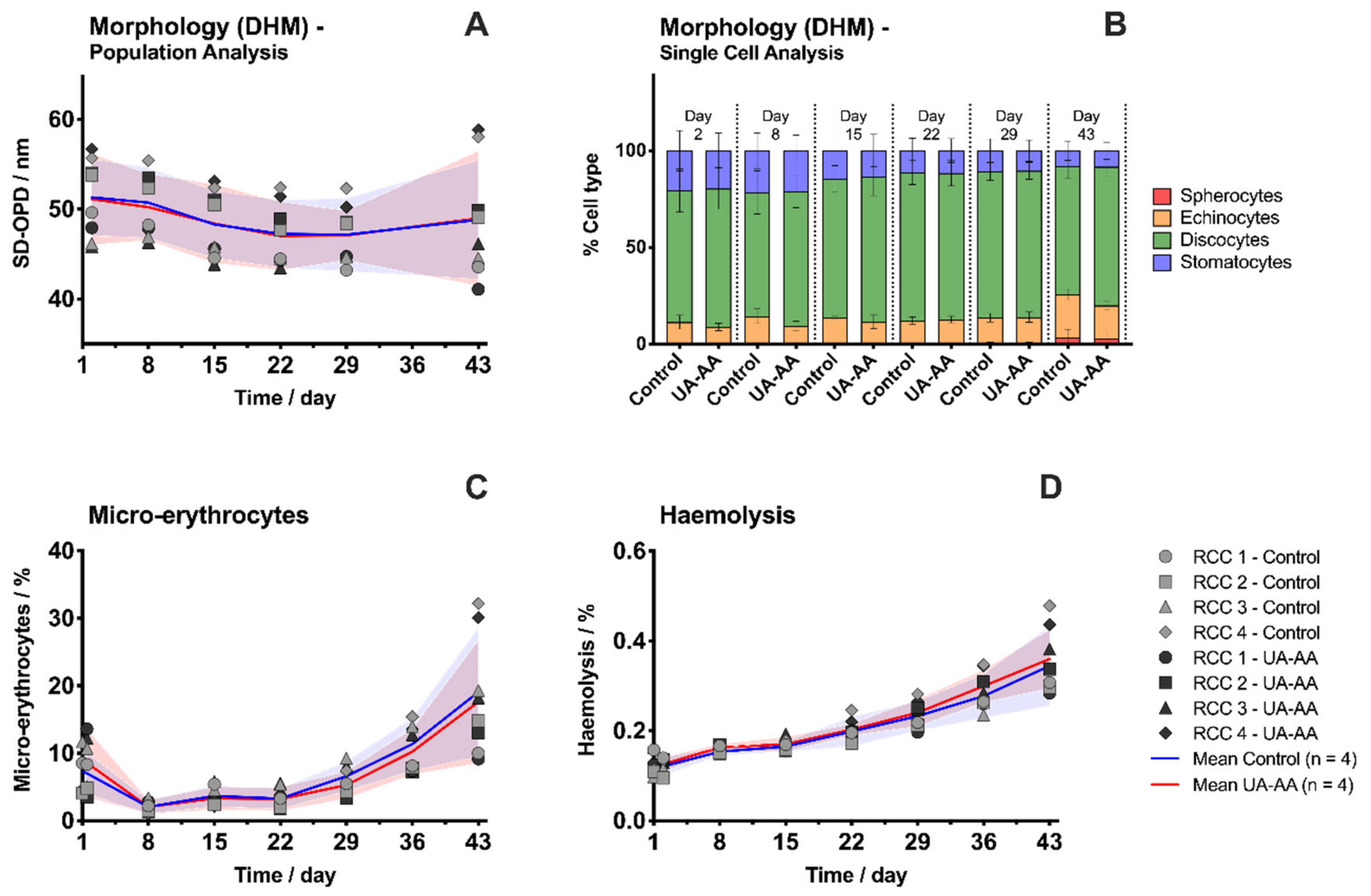
2.1. Evolution of Red Blood Cell Parameters
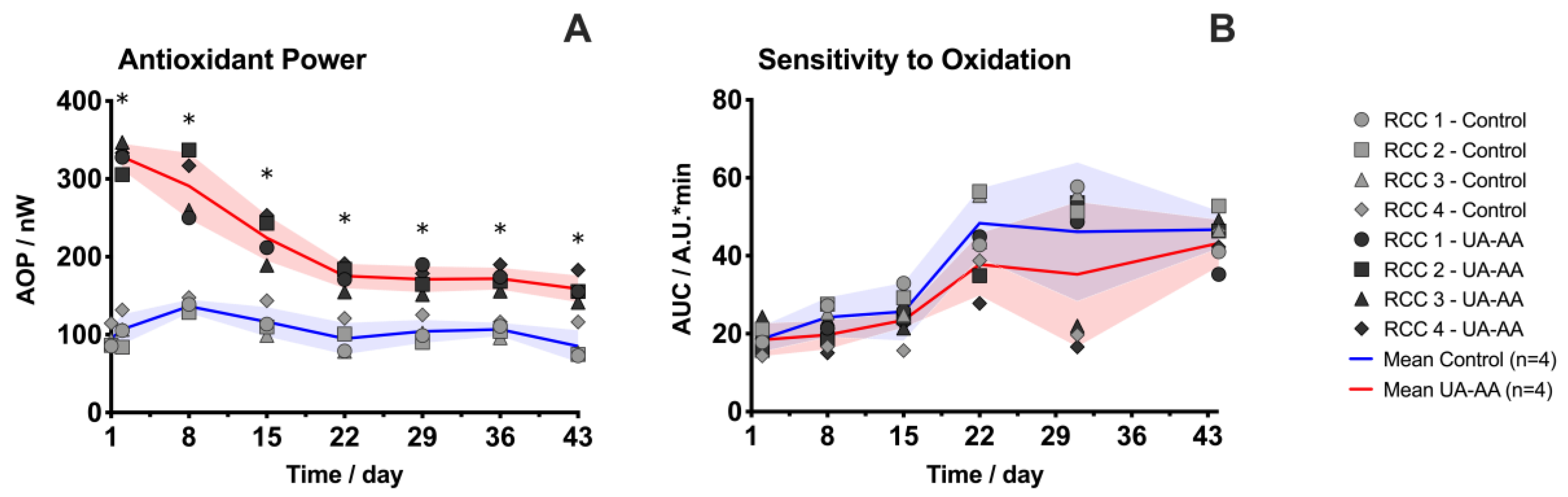
2.2. Increase of the Antioxidant Properties
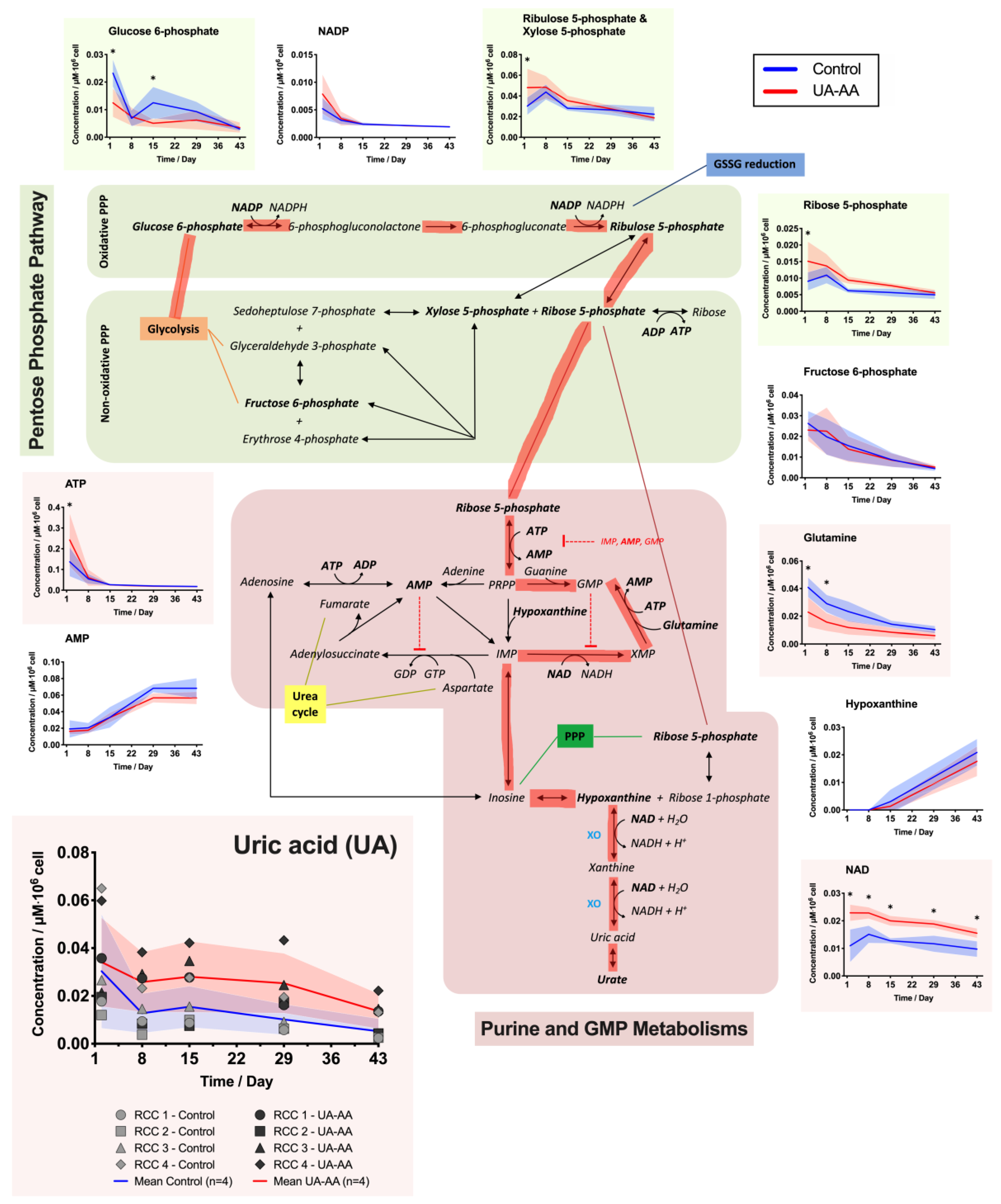
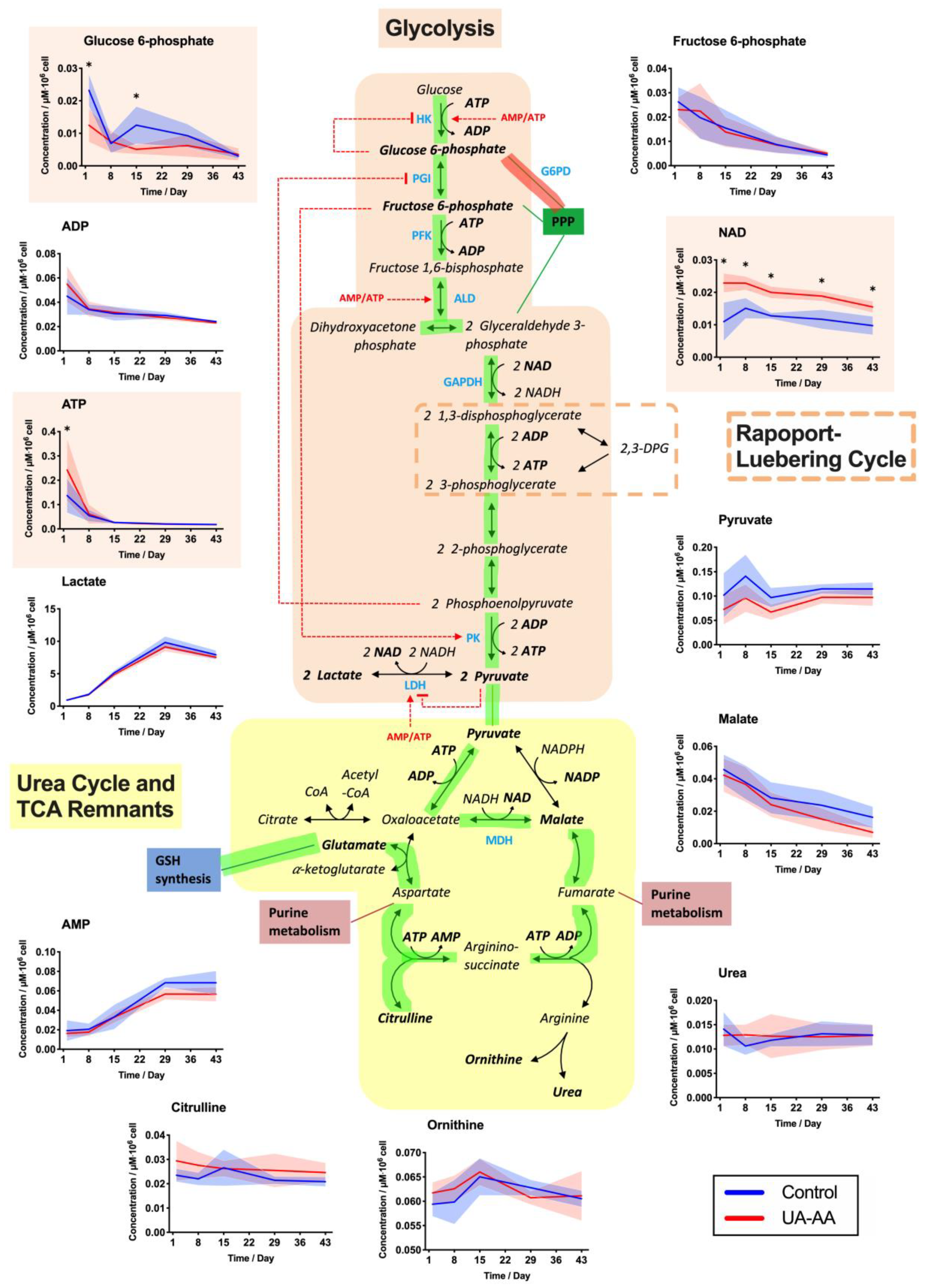
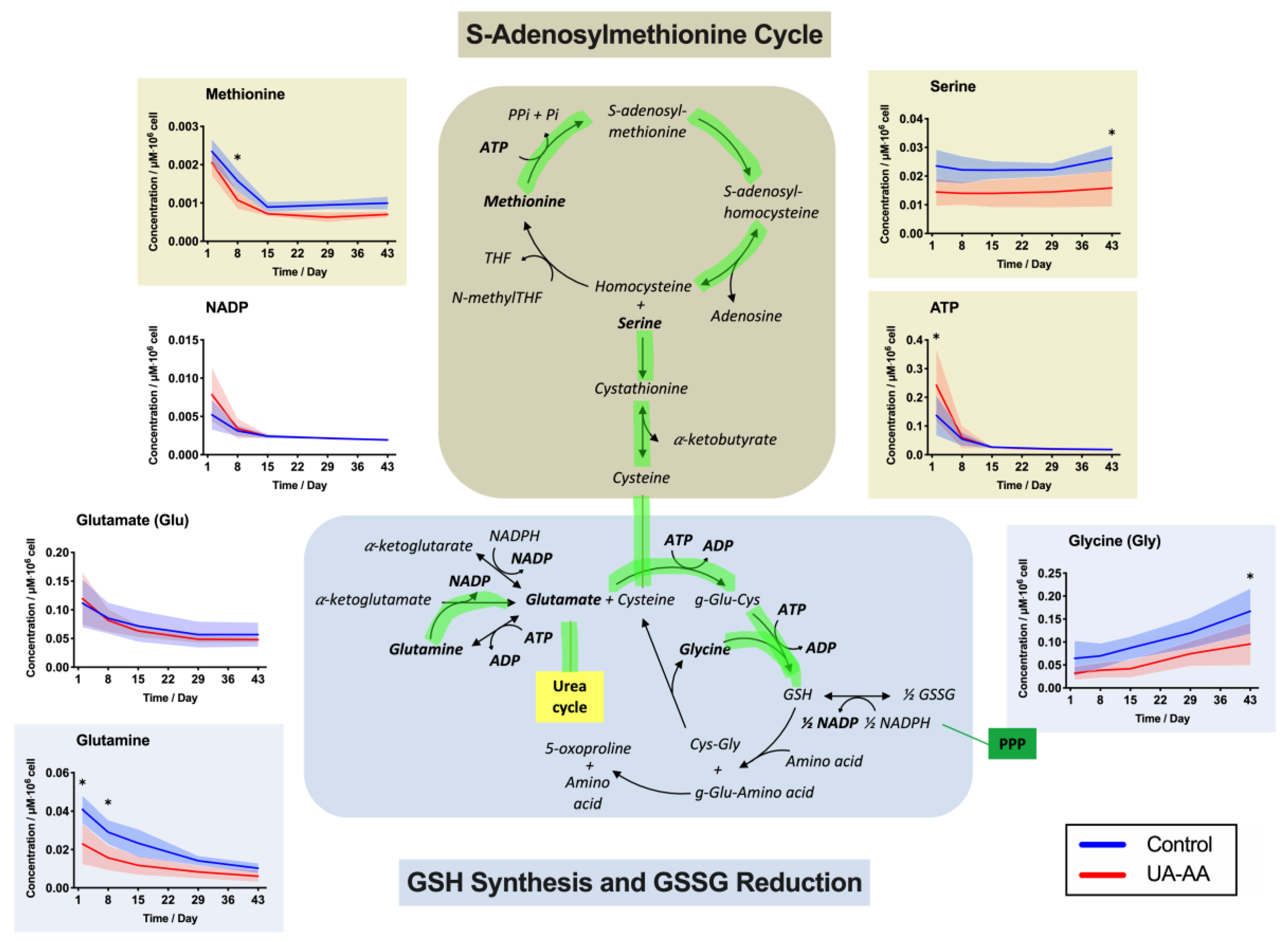
2.3. Rerouting of the Metabolism
3. Discussion
3.1. Tackle the Effect of Plasma Dilution
3.2. Metabolism Rerouting
3.3. Oxidative Stress
4. Materials and Methods
4.1. Preparation of Red Blood Cell Concentrates and Treatment
4.2. Follow-Up during Storage
4.3. Morphology Analysis Using Digital Holographic Microscopy
4.4. Storage-Induced Micro-Erythrcocytes Quantification
4.5. Electrochemical Antioxidant Power Measurement
4.6. Evaluation of Red Blood Cell Sensitivity to Oxidation
4.7. Quantification of Extracellular-Glucose and Lactate, and Intracellular-2,3-DPG and ATP Levels
4.8. Metabolomic Analysis
4.9. Data Presentation and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
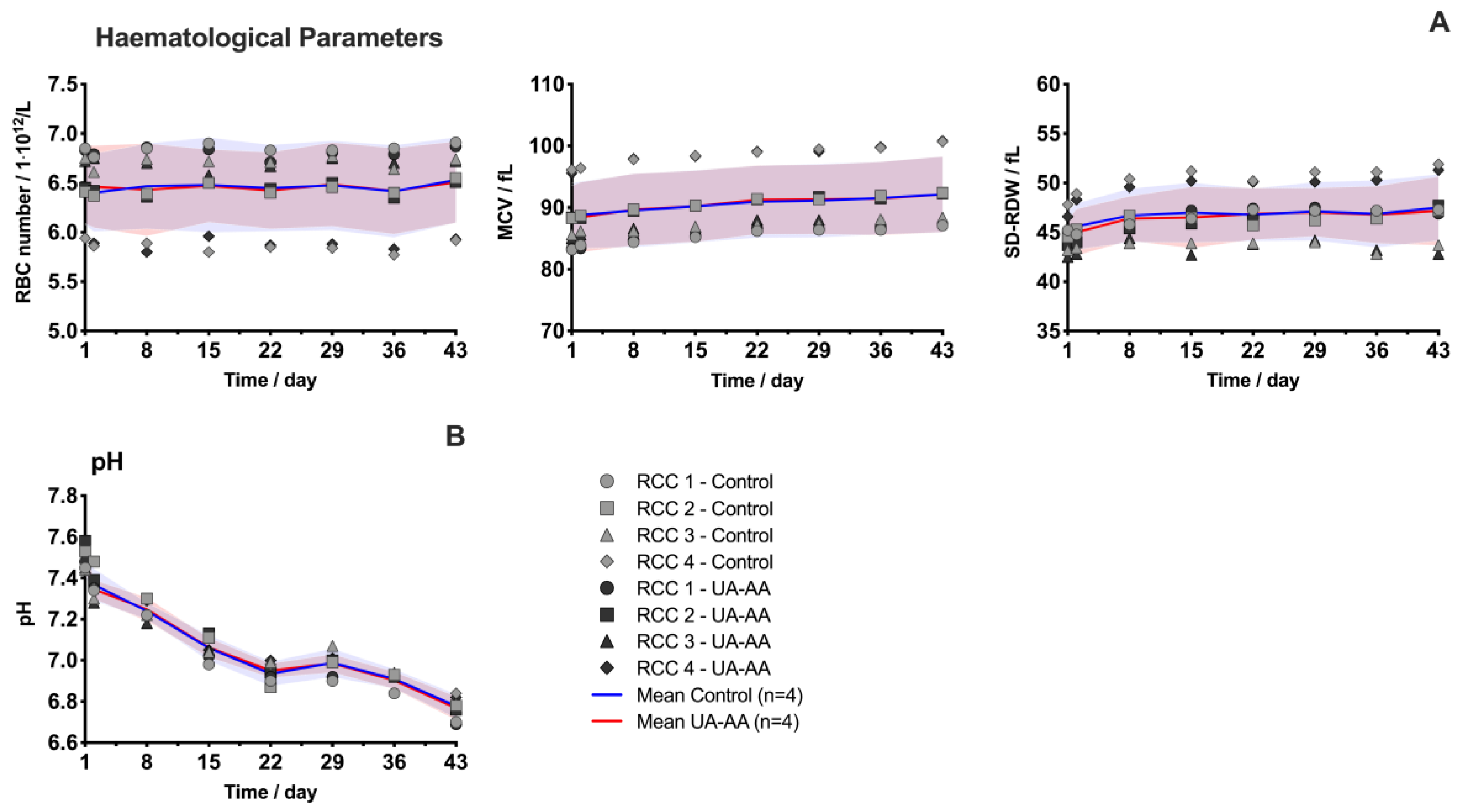
Appendix B
| Sample | Sex | Age/Year | Blood Group |
|---|---|---|---|
| RCC 1 | Male | 52 | A + |
| RCC 2 | Male | 54 | A + |
| RCC 3 | Male | 27 | B + |
| RCC 4 | Male | 61 | A + |
Appendix C
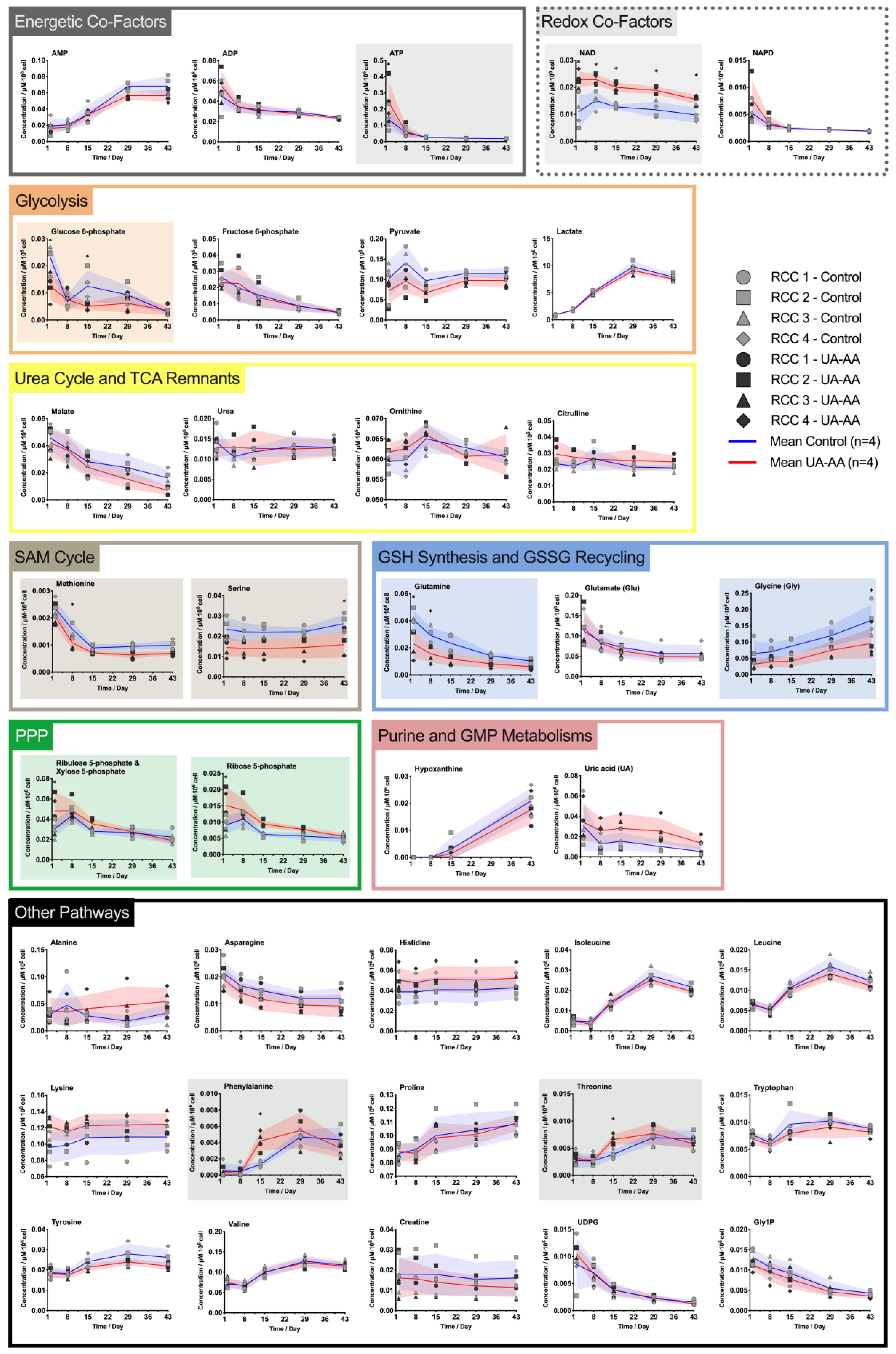
References
- Grimshaw, K.; Sahler, J.; Spinelli, S.L.; Phipps, R.P.; Blumberg, N. New frontiers in transfusion biology: Identification and significance of mediators of morbidity and mortality in stored red cell concentrates. Transfusion 2011, 51, 874–880. [Google Scholar] [CrossRef] [PubMed]
- García-Roa, M.; Del Carmen Vicente-Ayuso, M.; Bobes, A.M.; Pedraza, A.C.; González-Fernández, A.; Martín, M.P.; Sáez, I.; Seghatchian, J.; Gutiérrez, L. Red blood cell storage time and transfusion: Current practice, concerns and future perspectives. Blood Transfus. 2017, 15, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Bosman, G.J.C.G.M.; Werre, J.M.; Willekens, F.L.A.; Novotný, V.M.J. Erythrocyte ageing in vivo and in vitro: Structural aspects and implications for transfusion. Transfus. Med. 2008, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.O.; Spitalnik, S.L. Red blood cell components: Meeting the quantitative and qualitative transfusion needs. Presse Méd. 2016, 45, e281–e288. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Prudent, M.; D’Alessandro, A. Red blood cell storage lesion: Causes and potential clinical consequences. Blood Transfus. 2019, 17, 27–52. [Google Scholar] [CrossRef]
- Valeri, C.R.; Hirsch, N.M. Restoration in vivo of erythrocyte adenosine triphosphate, 2,3-diphosphoglycerate, potassium ion, and sodium ion concentrations following the transfusion of acid-citrate-dextrose-stored human red blood cells. J. Lab. Clin. Med. 1969, 73, 722–733. [Google Scholar] [CrossRef]
- Delobel, J.; Prudent, M.; Rubin, O.; Crettaz, D.; Tissot, J.-D.; Lion, N. Subcellular fractionation of stored red blood cells reveals a compartment-based protein carbonylation evolution. J. Proteom. 2012, 76, 181–193. [Google Scholar] [CrossRef]
- Delobel, J.; Prudent, M.; Tissot, J.-D.; Lion, N. Proteomics of the red blood cell carbonylome during blood banking of erythrocyte concentrates. Prot. Clin. Appl. 2016, 10, 257–266. [Google Scholar] [CrossRef]
- Roussel, C.; Dussiot, M.; Marin, M.; Morel, A.; Ndour, P.A.; Duez, J.; Le Van Kim, C.; Hermine, O.; Colin, Y.; Buffet, P.A.; et al. Spherocytic shift of red blood cells during storage provides a quantitative whole cell-based marker of the storage lesion. Transfusion 2017, 57, 1007–1018. [Google Scholar] [CrossRef]
- Reisz, J.A.; Nemkov, T.; Dzieciatkowska, M.; Culp-Hill, R.; Stefanoni, D.; Hill, R.C.; Yoshida, T.; Dunham, A.; Kanias, T.; Dumont, L.J.; et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion 2018, 58, 2978–2991. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Seghatchian, J.; Papassideri, I.S.; Antonelou, M.H. Unraveling the Gordian knot: Red blood cell storage lesion and transfusion outcomes. Blood Transfus. 2017. [Google Scholar] [CrossRef]
- Prudent, M.; Tissot, J.-D.; Lion, N. The 3-phase evolution of stored red blood cells and the clinical trials: An obvious relationship. Blood Transfus. 2017, 15, 188. [Google Scholar] [PubMed]
- Ng, M.S.Y.; David, M.; Middelburg, R.A.; Ng, A.S.Y.; Suen, J.Y.; Tung, J.-P.; Fraser, J.F. Transfusion of packed red blood cells at the end of shelf life is associated with increased risk of mortality – a pooled patient data analysis of 16 observational trials. Haematologica 2018, 103, 1542–1548. [Google Scholar] [CrossRef]
- Tissot, J.-D.; Bardyn, M.; Sonego, G.; Abonnenc, M.; Prudent, M. The storage lesions: From past to future. Transfus. Clin. Biol. 2017. [Google Scholar] [CrossRef]
- Gevi, F.; D’Alessandro, A.; Rinalducci, S.; Zolla, L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD–SAGM. J. Proteom. 2012, 76, 168–180. [Google Scholar] [CrossRef]
- Bordbar, A.; Johansson, P.I.; Paglia, G.; Harrison, S.J.; Wichuk, K.; Magnusdottir, M.; Valgeirsdottir, S.; Gybel-Brask, M.; Ostrowski, S.R.; Palsson, S.; et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfus. 2016, 56, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Acker, J.P. Determining the Volume of Additive Solution and Residual Plasma in Whole Blood Filtered and Buffy Coat Processed Red Cell Concentrates. Transfus. Med. Hemother 2016, 43, 133–136. [Google Scholar] [CrossRef]
- Bardyn, M.; Maye, S.; Lesch, A.; Delobel, J.; Tissot, J.-D.; Cortés-Salazar, F.; Tacchini, P.; Lion, N.; Girault, H.H.; Prudent, M. The antioxidant capacity of erythrocyte concentrates is increased during the first week of storage and correlated with the uric acid level. Vox Sang. 2017, 112, 638–647. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.F. Towards the physiological function of uric acid. Free Radic. Biol. Med. 1993, 14, 615–631. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Furger, P. Guidelines SURF-Med©: Scientific-Units-Recommendations-Formulas, 3. Aufl.-Update; Suter, T.M., Ed.; Editions D&F: Neuhausen, Switzerland, 2010; ISBN 978-3-905699-23-4. [Google Scholar]
- Tzounakas, V.L.; Georgatzakou, H.T.; Kriebardis, A.G.; Papageorgiou, E.G.; Stamoulis, K.E.; Foudoulaki-Paparizos, L.E.; Antonelou, M.H.; Papassideri, I.S. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion 2015, 55, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Tzounakas, V.L.; Karadimas, D.G.; Anastasiadi, A.T.; Georgatzakou, H.T.; Kazepidou, E.; Moschovas, D.; Velentzas, A.D.; Kriebardis, A.G.; Zafeiropoulos, N.E.; Avgeropoulos, A.; et al. Donor-specific individuality of red blood cell performance during storage is partly a function of serum uric acid levels. Transfusion 2018, 58, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sanford, K.; Fisher, B.J.; Fowler, E.; Fowler, A.A.; Natarajan, R. Attenuation of Red Blood Cell Storage Lesions with Vitamin, C. Antioxidants 2017, 6, 55. [Google Scholar] [CrossRef]
- Abuja, P.M. Ascorbate prevents prooxidant effects of urate in oxidation of human low density lipoprotein. FEBS Letters 1999, 446, 305–308. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Johnson, R.J. Uric Acid: The Oxidant-Antioxidant Paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef]
- Turner, R.; Brennan, S.O.; Ashby, L.V.; Dickerhof, N.; Hamzah, M.R.; Pearson, J.F.; Stamp, L.K.; Kettle, A.J. Conjugation of urate-derived electrophiles to proteins during normal metabolism and inflammation. J. Biol. Chem. 2018, 293, 19886–19898. [Google Scholar] [CrossRef]
- Roussel, C.; Morel, A.; Dussiot, M.; Marin, M.; Colard, M.; Fricot, A.; Martinez, A.; Chambrion, C.; Henry, B.; Volle, G.; et al. Storage-Induced Micro-Erythrocytes Are Rapidly Cleared from Recipient Circulation and Predict Transfusion Recovery. Blood 2019, 134, 717. [Google Scholar] [CrossRef]
- Bardyn, M.; Rappaz, B.; Jaferzadeh, K.; Crettaz, D.; Tissot, J.-D.; Moon, I.; Turcatti, G.; Lion, N.; Prudent, M. Red blood cells ageing markers: A multi-parametric analysis. Blood Transfus. 2017, 15, 239–248. [Google Scholar] [CrossRef]
- D’Alessandro, A.; D’Amici, G.M.; Vaglio, S.; Zolla, L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: From metabolism to proteomics. Haematologica 2012, 97, 107–115. [Google Scholar] [CrossRef]
- Qi, Z.; Roback, J.; Voit, E. Effects of Storage Time on Glycolysis in Donated Human Blood Units. Metabolites 2017, 7, 12. [Google Scholar] [CrossRef]
- Prudent, M.; Rochat, B.; Marvin, L.; Stauber, F.; Tissot, J.D.; Lion, N. Targeted Metabolomics of SAGM red blood cell storage. Clin. Lab. 2014, 60, S3. [Google Scholar]
- Reisz, J.A.; Wither, M.J.; Dzieciatkowska, M.; Nemkov, T.; Issaian, A.; Yoshida, T.; Dunham, A.J.; Hill, R.C.; Hansen, K.C.; D’Alessandro, A. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood 2016, 128, e32–e42. [Google Scholar] [CrossRef] [PubMed]
- D’alessandro, A.; Dzieciatkowska, M.; Nemkov, T.; Hansen, K.C. Red blood cell proteomics update: Is there more to discover? Blood Transfus. 2017, 15, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Blair, A.; D’alessandro, A.; Nemkov, T.; Dioguardi, M.; Silliman, C.C.; Dunham, A. Enhancing uniformity and overall quality of red cell concentrate with anaerobic storage. Blood Transfus. 2017, 15, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bardyn, M.; Tissot, J.-D.; Prudent, M. Oxidative stress and antioxidant defenses during blood processing and storage of erythrocyte concentrates. Transfus Clin. Biol. 2018, 25, 96–100. [Google Scholar] [CrossRef]
- Yoshida, T.; AuBuchon, J.P.; Tryzelaar, L.; Foster, K.Y.; Bitensky, M.W. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007, 92, 22–31. [Google Scholar] [CrossRef]
- Prudent, M.; Stauber, F.; Rapin, A.; Hallen, S.; Pham, N.; Abonnenc, M.; Marvin, L.; Rochat, B.; Tissot, J.-D.; Lion, N. Small-Scale Perfusion Bioreactor of Red Blood Cells for Dynamic Studies of Cellular Pathways: Proof-of-Concept. Front. Mol. Biosci. 2016, 3. [Google Scholar] [CrossRef][Green Version]
- D’Amici, G.M.; Rinalducci, S.; Zolla, L. Proteomic analysis of RBC membrane protein degradation during blood storage. J. Proteome Res. 2007, 6, 3242–3255. [Google Scholar] [CrossRef]
- Nemkov, T.; Sun, K.; Reisz, J.A.; Song, A.; Yoshida, T.; Dunham, A.; Wither, M.J.; Francis, R.O.; Roach, R.C.; Dzieciatkowska, M.; et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica 2018, 103, 361–372. [Google Scholar] [CrossRef]
- Han, V.; Serrano, K.; Devine, D.V. A comparative study of common techniques used to measure haemolysis in stored red cell concentrates. Vox Sang. 2010, 98, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Marquet, P.; Rappaz, B.; Magistretti, P.J.; Cuche, E.; Emery, Y.; Colomb, T.; Depeursinge, C. Digital holographic microscopy: A noninvasive contrast imaging technique allowing quantitative visualization of living cells with subwavelength axial accuracy. Opt. Lett. 2005, 30, 468. [Google Scholar] [CrossRef] [PubMed]
- Rappaz, B.; Breton, B.; Shaffer, E.; Turcatti, G. Digital Holographic Microscopy: A quantitative label-free microscopy technique for phenotypic screening. Comb. Chem. High Throughput Screen. 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Kühn, J.; Shaffer, E.; Mena, J.; Breton, B.; Parent, J.; Rappaz, B.; Chambon, M.; Emery, Y.; Magistretti, P.; Depeursinge, C.; et al. Label-Free Cytotoxicity Screening Assay by Digital Holographic Microscopy. Assay Drug Dev Technol. 2013, 11, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, P.; Lesch, A.; Neequaye, A.; Lagger, G.; Liu, J.; Cortés-Salazar, F.; Girault, H.H. Electrochemical Pseudo-Titration of Water-Soluble Antioxidants. Electroanalysis 2013, 25, 922–930. [Google Scholar] [CrossRef]
- Lesch, A.; Cortés-Salazar, F.; Prudent, M.; Delobel, J.; Rastgar, S.; Lion, N.; Tissot, J.-D.; Tacchini, P.; Girault, H.H. Large scale inkjet-printing of carbon nanotubes electrodes for antioxidant assays in blood bags. J. Electroanal. Chem. 2014, 717–718, 61–68. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef]
- Ghorbaniaghdam, A.; Chen, J.; Henry, O.; Jolicoeur, M. Analyzing clonal variation of monoclonal antibody-producing CHO cell lines using an in silico metabolomic platform. PLoS ONE 2014, 9, e90832. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.; Magon, N.J.; Maire, J.; Suarna, C.; Ayer, A.; Waldvogel, S.; Imhof, B.A.; Koury, M.J.; Stocker, R.; Schapira, M. Transition to 37 °C reveals importance of NADPH in mitigating oxidative stress in stored RBCs. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Kriebardis, A.G.; Georgatzakou, H.T.; Foudoulaki-Paparizos, L.E.; Dzieciatkowska, M.; Wither, M.J.; Nemkov, T.; Hansen, K.C.; Papassideri, I.S.; D’Alessandro, A.; et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic. Biol. Med. 2016, 96, 152–165. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardyn, M.; Chen, J.; Dussiot, M.; Crettaz, D.; Schmid, L.; Längst, E.; Amireault, P.; Tissot, J.-D.; Jolicoeur, M.; Prudent, M. Restoration of Physiological Levels of Uric Acid and Ascorbic Acid Reroutes the Metabolism of Stored Red Blood Cells. Metabolites 2020, 10, 226. https://doi.org/10.3390/metabo10060226
Bardyn M, Chen J, Dussiot M, Crettaz D, Schmid L, Längst E, Amireault P, Tissot J-D, Jolicoeur M, Prudent M. Restoration of Physiological Levels of Uric Acid and Ascorbic Acid Reroutes the Metabolism of Stored Red Blood Cells. Metabolites. 2020; 10(6):226. https://doi.org/10.3390/metabo10060226
Chicago/Turabian StyleBardyn, Manon, Jingkui Chen, Michaël Dussiot, David Crettaz, Lucas Schmid, Emmanuel Längst, Pascal Amireault, Jean-Daniel Tissot, Mario Jolicoeur, and Michel Prudent. 2020. "Restoration of Physiological Levels of Uric Acid and Ascorbic Acid Reroutes the Metabolism of Stored Red Blood Cells" Metabolites 10, no. 6: 226. https://doi.org/10.3390/metabo10060226
APA StyleBardyn, M., Chen, J., Dussiot, M., Crettaz, D., Schmid, L., Längst, E., Amireault, P., Tissot, J.-D., Jolicoeur, M., & Prudent, M. (2020). Restoration of Physiological Levels of Uric Acid and Ascorbic Acid Reroutes the Metabolism of Stored Red Blood Cells. Metabolites, 10(6), 226. https://doi.org/10.3390/metabo10060226






