An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans—Results of the KarMeN Study
Abstract
1. Introduction
2. Results
2.1. Basic Characteristics of Study Participants
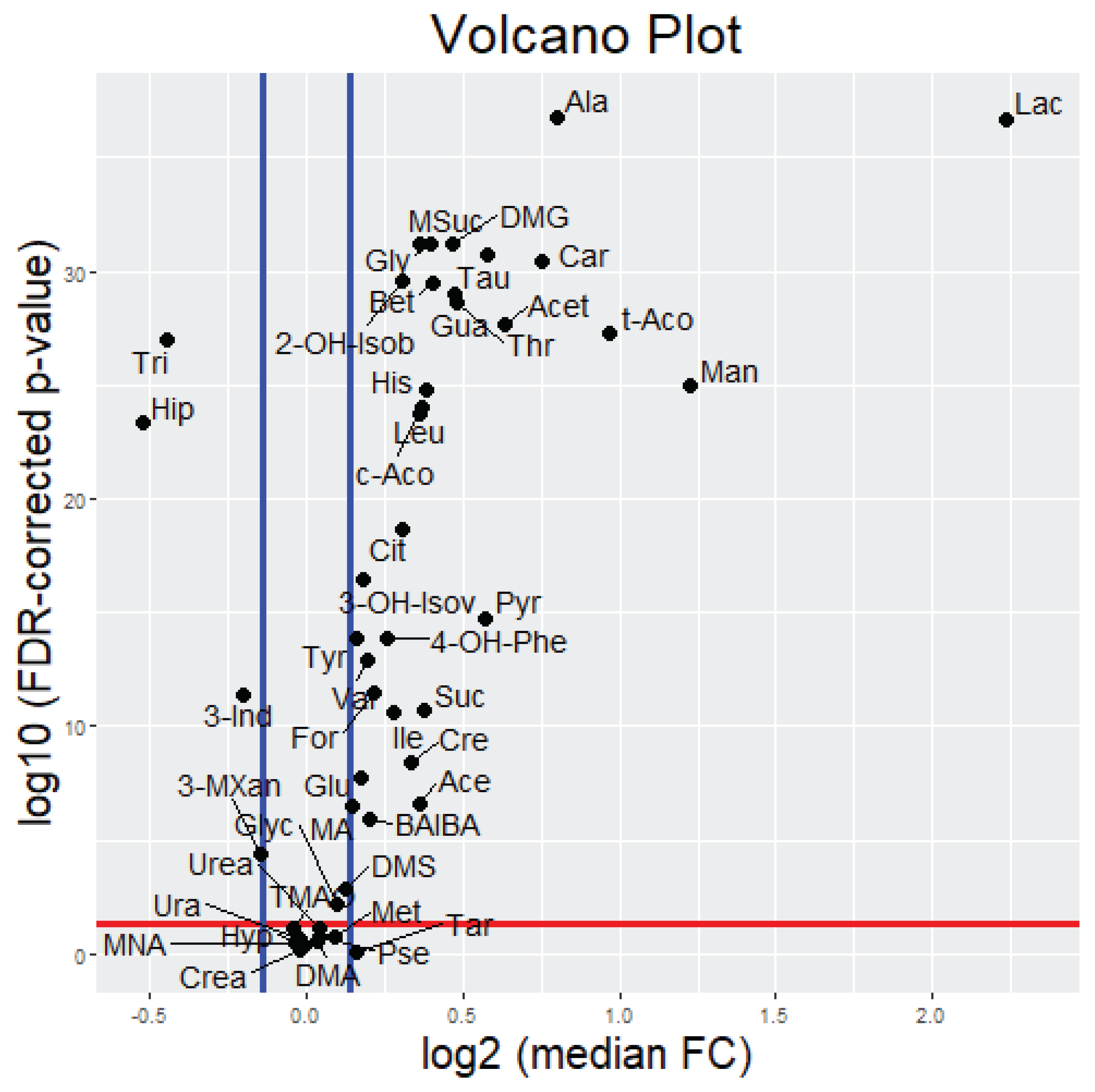
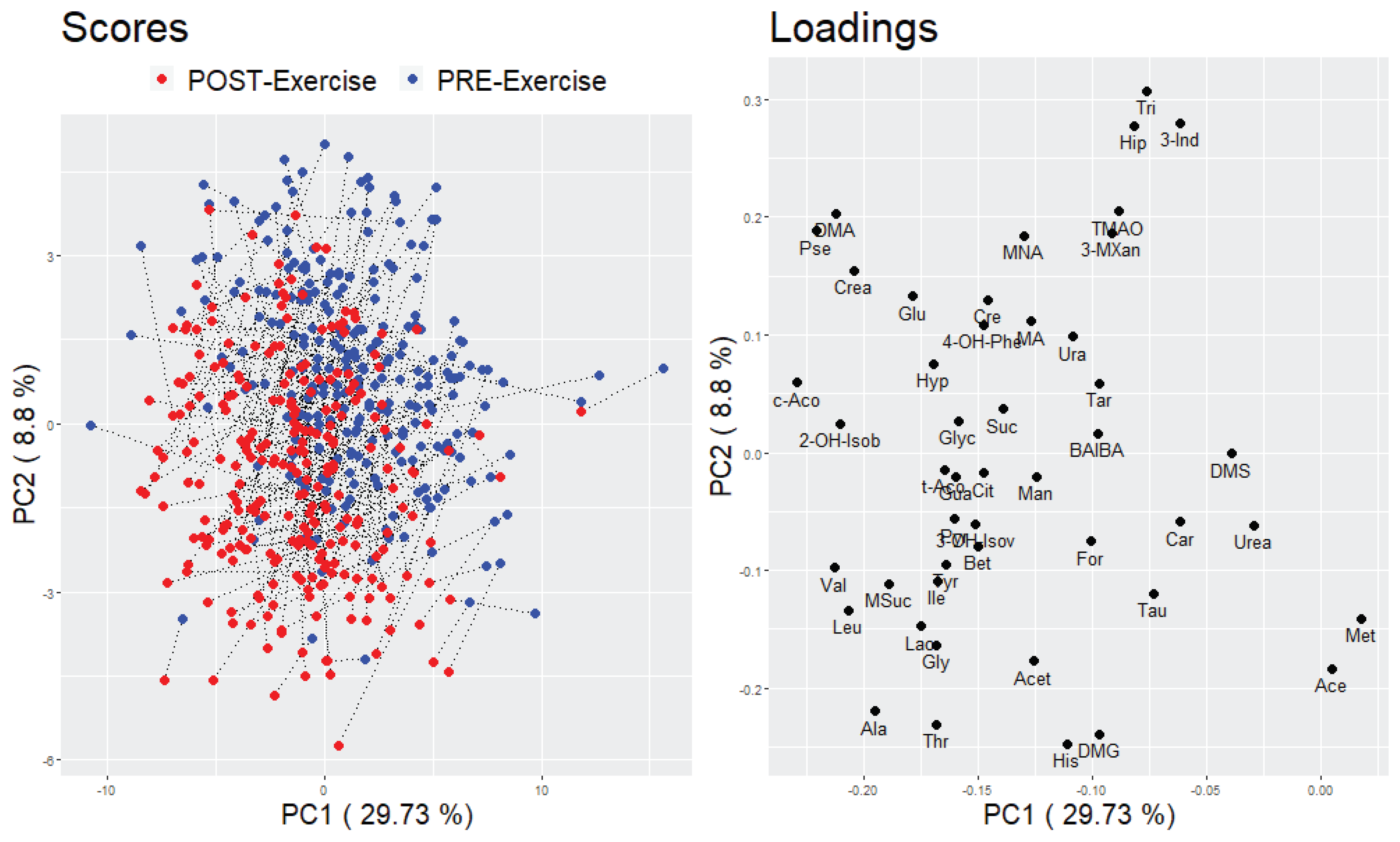
2.2. Alterations of Urinary Metabolites in Response to a Standardized Exercise Test
2.2.1. Uni- and Bivariate Analysis
2.2.2. Multivariate Analysis
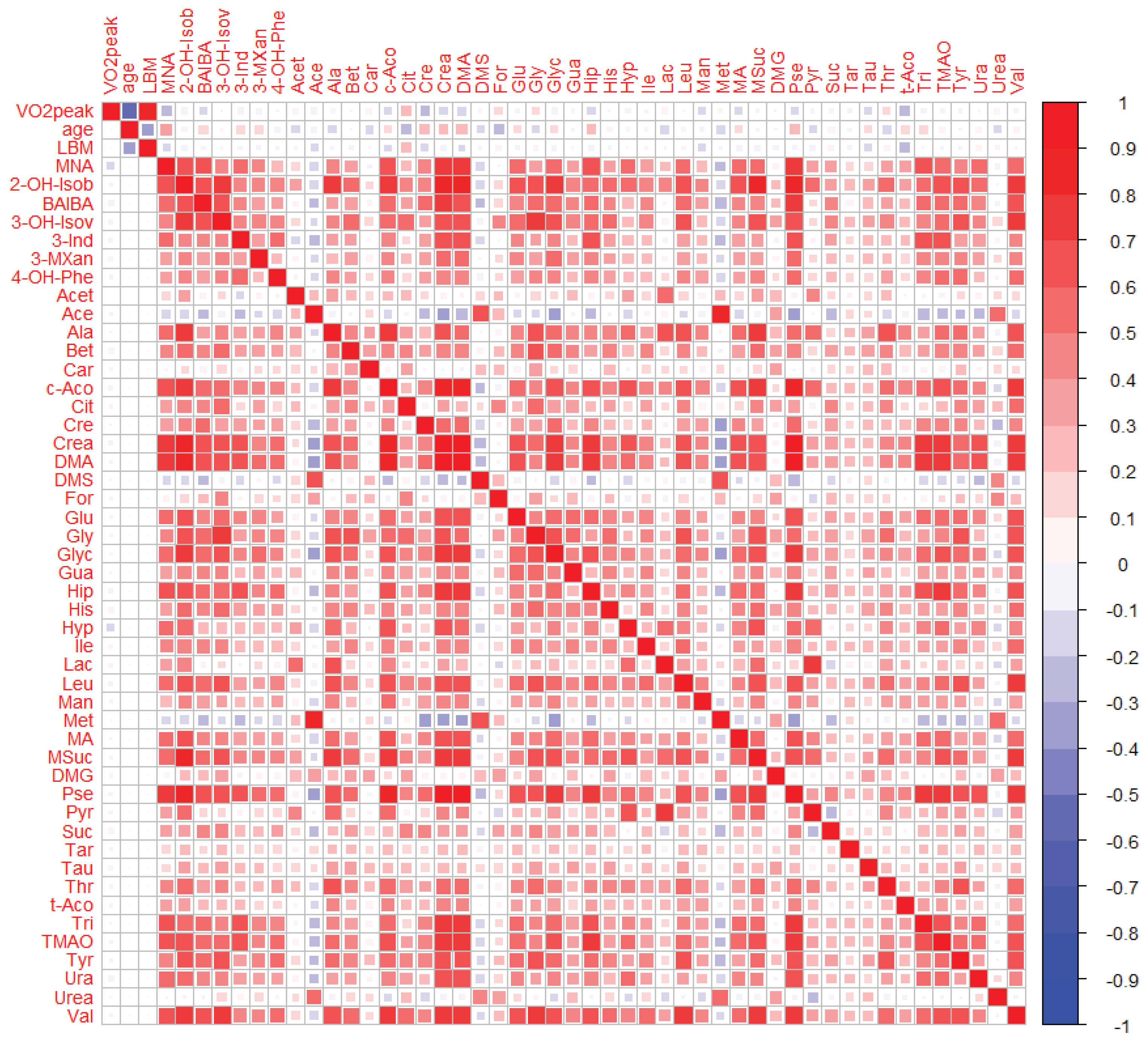
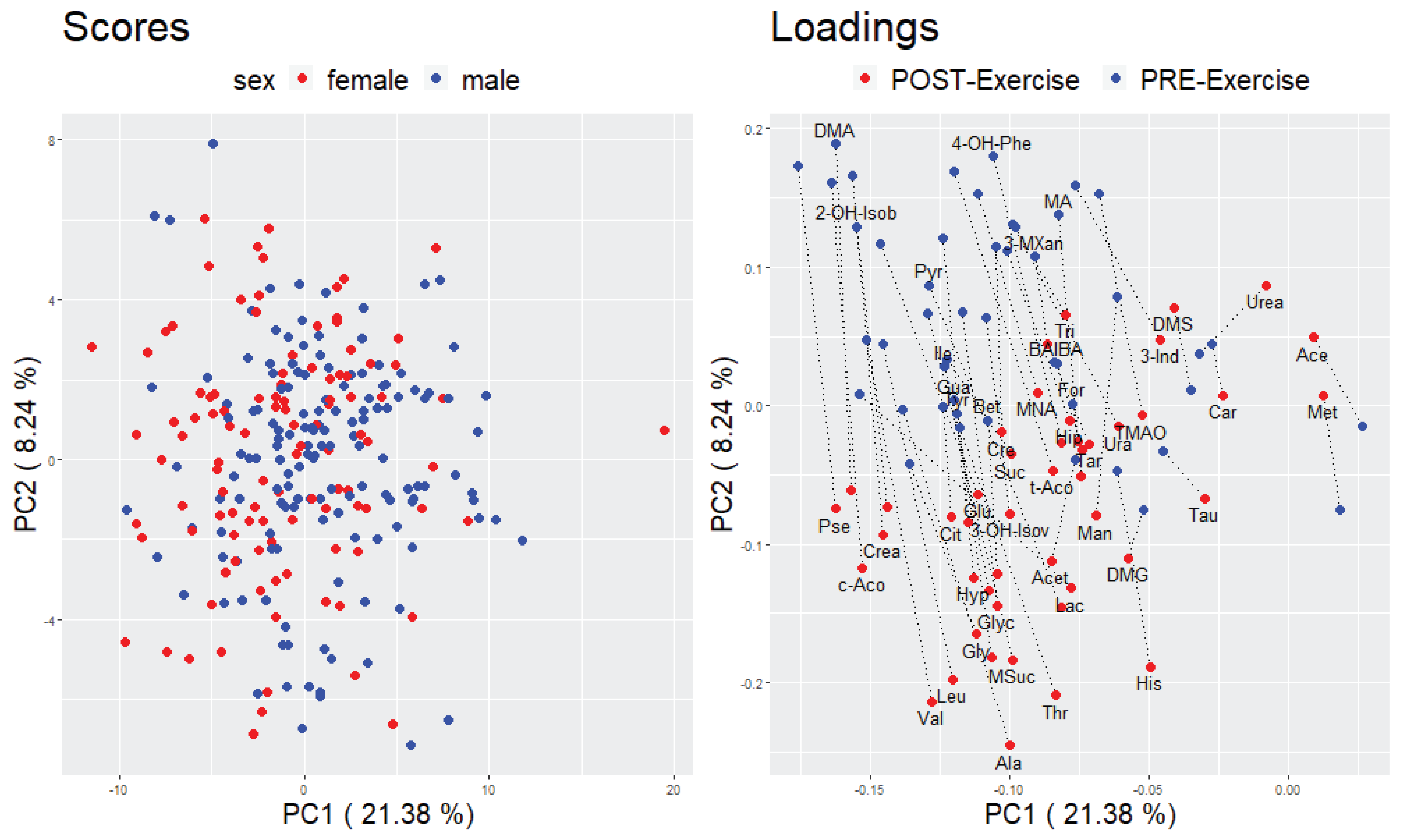
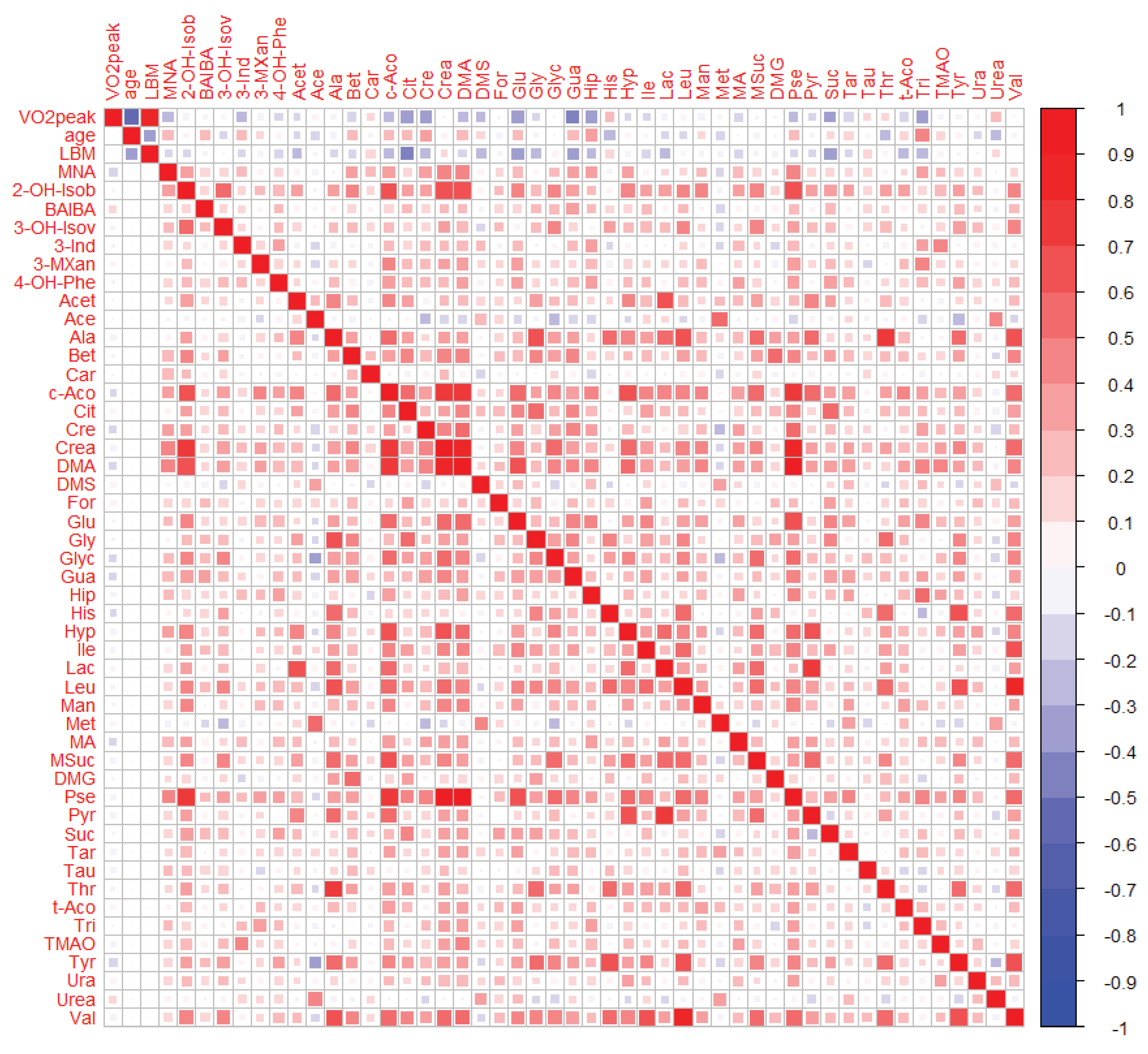
2.3. Relationship between the Cardiorespiratory Fitness Status and Urinary Metabolites
2.3.1. Bivariate Analyses
2.3.2. Multivariate Analyses
3. Discussion
3.1. Post-Exercise Alterations in Urinary Metabolites Are Partly Reflective of Energy Metabolism
3.2. Urinary Metabolites at Rest and after Exercise Are Not Substantially Related to Physical Fitness
3.3. Strengths and Limitations
4. Materials and Methods
4.1. Subjects and Study Design
4.2. Exercise Examination Day and Urine Sample Collection
4.3. Urine Sample Preparation
4.4. 1H-NMR Analysis
4.5. Data Handling and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BIC | Bayesian information criterion |
| CI | confidence interval |
| CRF | cardiorespiratory fitness |
| DXA | dual-energy X-ray absorptiometry |
| FC | fold change |
| FCs | fold changes |
| FDR | false discovery rat |
| HRmax | maximum heart rate |
| KarMeN | Karlsruhe Metabolomics and Nutrition |
| LBM | lean body mass |
| NMR | nuclear magnetic resonance |
| NOESY | nuclear overhauser enhancement spectroscopy |
| p | p-value of context-dependent test |
| PC | principal component |
| PCA | principal component analysis |
| PCs | principal components |
| PIAT | power at individual anaerobic threshold |
| Pmax | maximal power |
| QC | quality control |
| r | Pearson correlation coefficient |
| R2 | coefficient of determination |
| R2 (adjusted) | adjusted coefficient of determination |
| SD | standard deviation |
| TCA | tricarboxylic acid |
| TSP | trimethylsilylpropanoic acid |
| VO2 | oxygen uptake |
| VO2max | maximal oxygen uptake |
| VO2peak | peak oxygen uptake |
Appendix A


References
- Harber, M.P.; Kaminsky, L.A.; Arena, R.; Blair, S.N.; Franklin, B.A.; Myers, J.; Ross, R. Impact of Cardiorespiratory Fitness on All-Cause and Disease-Specific Mortality: Advances Since 2009. Prog. Cardiovasc. Dis. 2017, 60, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Blair, S.N.; Kohl, H.W.; Barlow, C.E.; Paffenbarger, R.S.; Gibbons, L.W.; Macera, C.A. Changes in physical fitness and all-cause mortality: A prospective study of healthy and unhealthy men. Jama 1995, 273, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Löllgen, H.; Leyk, D. Exercise testing in sports medicine. Dtsch. Ärzteblatt Int. 2018, 115, 409. [Google Scholar] [CrossRef]
- Day, J.R.; Rossiter, H.B.; Coats, E.M.; Skasick, A.; Whipp, B.J. The maximally attainable Vo2 during exercise in humans: The peak vs. maximum issue. J. Appl. Physiol. 2003, 95, 1901–1907. [Google Scholar] [CrossRef]
- Hill, A.V.; Long, C.; Lupton, H. Muscular exercise, lactic acid and the supply and utilisation of oxygen.—Parts VII–VIII. Proc. R. Soc. Lond. Ser. BContain. Pap. A Biol. Character 1924, 97, 155–176. [Google Scholar] [CrossRef]
- Laukkanen, J.A.; Laaksonen, D.; Lakka, T.A.; Savonen, K.; Rauramaa, R.; Mäkikallio, T.; Kurl, S. Determinants of cardiorespiratory fitness in men aged 42 to 60 years with and without cardiovascular disease. Am. J. Cardiol. 2009, 103, 1598–1604. [Google Scholar] [CrossRef]
- Zeiher, J.; Ombrellaro, K.J.; Perumal, N.; Keil, T.; Mensink, G.B.; Finger, J.D. Correlates and Determinants of Cardiorespiratory Fitness in Adults: A Systematic Review. Sports Med. -Open 2019, 5, 39. [Google Scholar] [CrossRef]
- Al-Mallah, M.H.; Juraschek, S.P.; Whelton, S.; Dardari, Z.A.; Ehrman, J.K.; Michos, E.D.; Blumenthal, R.S.; Nasir, K.; Qureshi, W.T.; Brawner, C.A. Sex differences in cardiorespiratory fitness and all-cause mortality: The Henry Ford ExercIse Testing (FIT) Project. Mayo Clin. Proc. 2016, 91, 755–762. [Google Scholar] [CrossRef]
- Wong, S.Y.S.; Chan, F.W.K.; Lee, C.K.; Li, M.; Yeung, F.; Lum, C.C.M.; Woo, J.; Choy, D.T. Maximum oxygen uptake and body composition of healthy Hong Kong Chinese adult men and women aged 20–64 years. J. Sports Sci. 2008, 26, 295–302. [Google Scholar] [CrossRef]
- Bouchard, C.; Boulay, M.R.; Simoneau, J.-A.; Lortie, G.; Pérusse, L. Heredity and Trainability of Aerobic and Anaerobic Performances an Update. Sports Med. 1988, 5, 69–73. [Google Scholar] [CrossRef]
- McKinney, J.; Lithwick, D.J.; Morrison, B.N.; Nazzari, H.; Isserow, S.H.; Heilbron, B.; Krahn, A.D. The health benefits of physical activity and cardiorespiratory fitness. Br. Columbia Med. J. 2016, 58, 131–137. [Google Scholar]
- Gabriel, B.M.; Zierath, J.R. The Limits of Exercise Physiology: From Performance to Health. Cell Metab. 2017, 25, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Zierath, J.R.; Wallberg-Henriksson, H. Looking Ahead Perspective: Where Will the Future of Exercise Biology Take Us? Cell Metab. 2015, 22, 25–30. [Google Scholar] [CrossRef]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for laboratory diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef]
- Daskalaki, E.; Easton, C.; Watson, D.G. The Application of Metabolomic Profiling to the Effects of Physical Activity. Curr. Metab. 2014, 2, 233–263. [Google Scholar] [CrossRef]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef]
- Heaney, L.M.; Deighton, K.; Suzuki, T. Non-targeted metabolomics in sport and exercise science. J. Sports Sci. 2017, 37, 959–967. [Google Scholar] [CrossRef]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra37. [Google Scholar] [CrossRef]
- Pohjanen, E.; Thysell, E.; Johnson, P.; Eklund, C.; Silfver, A.; Carlsson, I.-B.; Lundgren, K.; Moritz, T.; Svensson, M.B.; Antti, H. A Multivariate Screening Strategy for Investigating Metabolic Effects of Strenuous Physical Exercise in Human Serum. J. Proteome Res. 2007, 6, 2113–2120. [Google Scholar] [CrossRef]
- Enea, C.; Seguin, F.; Petitpas-Mulliez, J.; Boildieu, N.; Boisseau, N.; Delpech, N.; Diaz, V.; Eugène, M.; Dugué, B. 1H NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal. Bioanal. Chem. 2010, 396, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Mougios, V.; Gika, H.G.; Mikros, E.; Theodoridis, G.A. 1H NMR-Based Metabonomic Investigation of the Effect of Two Different Exercise Sessions on the Metabolic Fingerprint of Human Urine. J. Proteome Res. 2010, 9, 6405–6416. [Google Scholar] [CrossRef] [PubMed]
- Netzer, M.; Weinberger, K.M.; Handler, M.; Seger, M.; Fang, X.; Kugler, K.G.; Graber, A.; Baumgartner, C. Profiling the human response to physical exercise: A computational strategy for the identification and kinetic analysis of metabolic biomarkers. J. Clin. Bioinform. 2011, 1, 34. [Google Scholar] [CrossRef] [PubMed]
- Breit, M.; Netzer, M.; Weinberger, K.M.; Baumgartner, C. Modeling and Classification of Kinetic Patterns of Dynamic Metabolic Biomarkers in Physical Activity. PLoS Comp. Biol. 2015, 11, e1004454. [Google Scholar] [CrossRef] [PubMed]
- Muhsen Ali, A.; Burleigh, M.; Daskalaki, E.; Zhang, T.; Easton, C.; Watson, D.G. Metabolomic Profiling of Submaximal Exercise at a Standardised Relative Intensity in Healthy Adults. Metabolites 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Edgett, B.A.; Burrows, H.W.; Castro, C.; Griffin, J.L.; Schwertani, A.G.; Gurd, B.J.; Funk, C.D. Whole blood transcriptomics and urinary metabolomics to define adaptive biochemical pathways of high-intensity exercise in 50-60 year old masters athletes. PLoS ONE 2014, 9, e92031. [Google Scholar] [CrossRef]
- Pechlivanis, A.; Papaioannou, K.G.; Tsalis, G.; Saraslanidis, P.; Mougios, V.; Theodoridis, G.A. Monitoring the Response of the Human Urinary Metabolome to Brief Maximal Exercise by a Combination of RP-UPLC-MS and (1)H NMR Spectroscopy. J. Proteome Res. 2015, 14, 4610–4622. [Google Scholar] [CrossRef]
- Siopi, A.; Deda, O.; Manou, V.; Kosmidis, I.; Komninou, D.; Raikos, N.; Theodoridis, G.A.; Mougios, V. Comparison of the Serum Metabolic Fingerprint of Different Exercise Modes in Men with and without Metabolic Syndrome. Metabolites 2019, 9, 116. [Google Scholar] [CrossRef]
- Siopi, A.; Deda, O.; Manou, V.; Kellis, S.; Kosmidis, I.; Komninou, D.; Raikos, N.; Christoulas, K.; Theodoridis, G.A.; Mougios, V. Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome. Metabolites 2017, 7, 5. [Google Scholar] [CrossRef]
- Chorell, E.; Svensson, M.B.; Moritz, T.; Antti, H. Physical fitness level is reflected by alterations in the human plasma metabolome. Mol. Biosyst. 2012, 8, 1187–1196. [Google Scholar] [CrossRef]
- Morris, C.; Grada, C.O.; Ryan, M.; Roche, H.M.; De Vito, G.; Gibney, M.J.; Gibney, E.R.; Brennan, L. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol. Nutr. Food Res. 2013, 57, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.S.; Gao, F.; Tan, R.S.; Zhong, L.; Leng, S.; Zhao, X.; Fridianto, K.T.; Ching, J.; Lee, S.Y.; Keng, B.M.H.; et al. Metabolomic correlates of aerobic capacity among elderly adults. Clin. Cardiol. 2018, 41, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, M.S.; Price, L.L.; Logvinenko, T.; Hatzis, C.; Padukone, N.; Reo, N.V.; Phillips, E.M.; Kirn, D.; Mills, J.; Fielding, R.A. Identification of serum analytes and metabolites associated with aerobic capacity. Eur. J. Appl. Physiol. 2013, 113, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Wientzek, A.; Bachlechner, U.; Jacobs, S.; Drogan, D.; Prehn, C.; Adamski, J.; Krumsiek, J.; Schulze, M.B.; Pischon, T.; et al. Linking diet, physical activity, cardiorespiratory fitness and obesity to serum metabolite networks: Findings from a population-based study. Int. J. Obes. 2014, 38, 1388–1396. [Google Scholar] [CrossRef]
- Bye, A.; Vettukattil, R.; Aspenes, S.T.; Giskeodegard, G.F.; Gribbestad, I.S.; Wisloff, U.; Bathen, T.F. Serum levels of choline-containing compounds are associated with aerobic fitness level: The HUNT-study. PLoS ONE 2012, 7, e42330. [Google Scholar] [CrossRef]
- Mercuro, G.; Saiu, F.; Deidda, M.; Mercuro, S.; Vitale, C.; Rosano, G.M. Impairment of physical exercise capacity in healthy postmenopausal women. Am. Heart J. 2006, 151, 923–927. [Google Scholar] [CrossRef]
- Rist, M.J.; Roth, A.; Frommherz, L.; Weinert, C.H.; Krüger, R.; Merz, B.; Bunzel, D.; Mack, C.; Egert, B.; Bub, A.; et al. Metabolite patterns predicting sex and age in participants of the Karlsruhe Metabolomics and Nutrition (KarMeN) study. PLoS ONE 2017, 12, e0183228. [Google Scholar] [CrossRef]
- Korostishevsky, M.; Steves, C.J.; Malkin, I.; Spector, T.; Williams, F.M.K.; Livshits, G. Genomics and metabolomics of muscular mass in a community-based sample of UK females. Eur. J. Hum. Genet. 2016, 24, 277–283. [Google Scholar] [CrossRef]
- Jourdan, C.; Petersen, A.-K.; Gieger, C.; Döring, A.; Illig, T.; Wang-Sattler, R.; Meisinger, C.; Peters, A.; Adamski, J.; Prehn, C.; et al. Body Fat Free Mass Is Associated with the Serum Metabolite Profile in a Population-Based Study. PLoS ONE 2012, 7, e40009. [Google Scholar] [CrossRef]
- Stretch, C.; Eastman, T.; Mandal, R.; Eisner, R.; Wishart, D.S.; Mourtzakis, M.; Prado, C.M.M.; Damaraju, S.; Ball, R.O.; Greiner, R.; et al. Prediction of Skeletal Muscle and Fat Mass in Patients with Advanced Cancer Using a Metabolomic Approach. J. Nutr. 2011, 142, 14–21. [Google Scholar] [CrossRef]
- Armbruster, M.; Rist, M.; Seifert, S.; Frommherz, L.; Weinert, C.; Mack, C.; Roth, A.; Merz, B.; Bunzel, D.; Kruger, R.; et al. Metabolite profiles evaluated, according to sex, do not predict resting energy expenditure and lean body mass in healthy non-obese subjects. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, Y. Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev. Proteom. 2015, 12, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, R.; Nascimento, E.M.; Urso, R.P.; Damasceno, M.; Lima-Silva, A.E. Energy system contributions during incremental exercise test. J. Sports Sci. Med. 2013, 12, 454. [Google Scholar] [PubMed]
- Adeva-Andany, M.; López-Ojén, M.; Funcasta-Calderón, R.; Ameneiros-Rodríguez, E.; Donapetry-García, C.; Vila-Altesor, M.; Rodríguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among skeletal muscle metabolic energy systems during intense exercise. J. Nutr. Metab. 2010, 2010, 1–13. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Felig, P.; Wahren, J. Amino acid metabolism in exercising man. J. Clin. Investig. 1971, 50, 2703–2714. [Google Scholar] [CrossRef]
- Knowles, S.E.; Jarrett, I.G.; Filsell, O.H.; Ballard, F.J. Production and utilization of acetate in mammals. Biochem. J. 1974, 142, 401–411. [Google Scholar] [CrossRef]
- Liu, X.; Cooper, D.E.; Cluntun, A.A.; Warmoes, M.O.; Zhao, S.; Reid, M.A.; Liu, J.; Lund, P.J.; Lopes, M.; Garcia, B.A. Acetate production from glucose and coupling to mitochondrial metabolism in mammals. Cell 2018, 175, 502–513. [Google Scholar] [CrossRef]
- Bassenge, E.; Sommer, O.; Schwemmer, M.; Bünger, R. Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. Am. J. Physiol. -Heart Circ. Physiol. 2000, 279, H2431–H2438. [Google Scholar] [CrossRef] [PubMed]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A Regulator of Cellular Redox Homeostasis and Skeletal Muscle Function. Mol. Nutr. Food Res. 2018, 63, e1800569. [Google Scholar] [CrossRef] [PubMed]
- Cuisinier, C.; De Welle, J.M.; Verbeeck, R.K.; Poortmans, J.R.; Ward, R.; Sturbois, X.; Francaux, M. Role of taurine in osmoregulation during endurance exercise. Eur. J. Appl. Physiol. 2002, 87, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Kristensen, D.; Holm, J.B.; Mortensen, O.H. Physiological role of taurine–from organism to organelle. Acta Physiol. 2015, 213, 191–212. [Google Scholar] [CrossRef]
- Duran, M.; Walther, F.; Bruinvis, L.; Wadman, S. The urinary excretion of ethylmalonic acid: What level requires further attention? Biochem. Med. 1983, 29, 171–175. [Google Scholar] [CrossRef]
- Dercksen, M.; Koekemoer, G.; Duran, M.; Wanders, R.J.; Mienie, L.J.; Reinecke, C.J. Organic acid profile of isovaleric acidemia: A comprehensive metabolomics approach. Metabolomics 2013, 9, 765–777. [Google Scholar] [CrossRef]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.L.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E539–E552. [Google Scholar] [CrossRef]
- Zafeiridis, A.; Chatziioannou, A.C.; Sarivasiliou, H.; Kyparos, A.; Nikolaidis, M.G.; Vrabas, I.S.; Pechlivanis, A.; Zoumpoulakis, P.; Baskakis, C.; Dipla, K.; et al. Global Metabolic Stress of Isoeffort Continuous and High Intensity Interval Aerobic Exercise: A Comparative (1)H NMR Metabonomic Study. J. Proteome Res. 2016, 15, 4452–4463. [Google Scholar] [CrossRef]
- Stephens, F.B. Does skeletal muscle carnitine availability influence fuel selection during exercise? Proc. Nutr. Soc. 2018, 77, 11–19. [Google Scholar] [CrossRef]
- Arenas, J.; Ricoy, J.; Encinas, A.; Pola, P.; D’iddio, S.; Zeviani, M.; DiDonato, S.; Corsi, M. Carnitine in muscle, serum, and urine of nonprofessional athletes: Effects of physical exercise, training, and L-carnitine administration. Muscle Nerve:Off. J. Am. Assoc. Electrodiagn. Med. 1991, 14, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.; Reddan, W.; Sanjak, M.; Hodach, R. Carnitine metabolism during prolonged exercise and recovery in humans. J. Appl. Physiol. 1986, 61, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Light, A.R.; Hoppel, C.L.; Campbell, C.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Hughen, R.W.; Keim, N.L.; et al. Acylcarnitines as markers of exercise-associated fuel partitioning, xenometabolism, and potential signals to muscle afferent neurons. Exp. Physiol. 2017, 102, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Zhao, X.; Weigert, C.; Simon, P.; Fehrenbach, E.; Fritsche, J.; Machann, J.; Schick, F.; Wang, J.; Hoene, M.; et al. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS ONE 2010, 5, e11519. [Google Scholar] [CrossRef]
- Hiatt, W.R.; Regensteiner, J.G.; Wolfel, E.E.; Ruff, L.; Brass, E.P. Carnitine and acylcarnitine metabolism during exercise in humans. Dependence on skeletal muscle metabolic state. J. Clin. Investig. 1989, 84, 1167–1173. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Z.; Zhong, S.; Li, R.; Xia, H.; Jie, Z.; Wen, B.; Chen, X.; Yan, W.; Fan, Y. Integrated metabolomics and metagenomics analysis of plasma and urine identified microbial metabolites associated with coronary heart disease. Sci. Rep. 2016, 6, 22525. [Google Scholar] [CrossRef]
- Wisselink, H.; Weusthuis, R.; Eggink, G.; Hugenholtz, J.; Grobben, G. Mannitol production by lactic acid bacteria: A review. Int. DairyJ. 2002, 12, 151–161. [Google Scholar] [CrossRef]
- Slupsky, C.M.; Rankin, K.N.; Wagner, J.; Fu, H.; Chang, D.; Weljie, A.M.; Saude, E.J.; Lix, B.; Adamko, D.J.; Shah, S. Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal. Chem. 2007, 79, 6995–7004. [Google Scholar] [CrossRef]
- Yuhara, K.; Yonehara, H.; Hattori, T.; Kobayashi, K.; Kirimura, K. Enzymatic characterization and gene identification of aconitate isomerase, an enzyme involved in assimilation of trans-aconitic acid, from Pseudomonas sp. WU-0701. FEBS J. 2015, 282, 4257–4267. [Google Scholar] [CrossRef]
- Ueland, P.M.; Holm, P.I.; Hustad, S. Betaine: A key modulator of one-carbon metabolism and homocysteine status. Clin. Chem. Lab. Med. 2005, 43, 1069–1075. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of ketone bodies during exercise and training: Physiological basis for exogenous supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Amberg, A.; Rosner, E.; Dekant, W. Biotransformation and kinetics of excretion of methyl-tert-butyl ether in rats and humans. Toxicol. Sci. 1999, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yap, I.K.; Brown, I.J.; Chan, Q.; Wijeyesekera, A.; Garcia-Perez, I.; Bictash, M.; Loo, R.L.; Chadeau-Hyam, M.; Ebbels, T.; De Iorio, M.; et al. Metabolome-wide association study identifies multiple biomarkers that discriminate north and south Chinese populations at differing risks of cardiovascular disease: INTERMAP study. J Proteome Res 2010, 9, 6647–6654. [Google Scholar] [CrossRef]
- Irwin, C.; Mienie, L.J.; Wevers, R.A.; Mason, S.; Westerhuis, J.A.; van Reenen, M.; Reinecke, C.J. GC–MS-based urinary organic acid profiling reveals multiple dysregulated metabolic pathways following experimental acute alcohol consumption. Sci. Rep. 2018, 8, 5775. [Google Scholar] [CrossRef]
- Behr, C.; Kamp, H.; Fabian, E.; Krennrich, G.; Mellert, W.; Peter, E.; Strauss, V.; Walk, T.; Rietjens, I.M.C.M.; van Ravenzwaay, B. Gut microbiome-related metabolic changes in plasma of antibiotic-treated rats. Arch. Toxicol. 2017, 91, 3439–3454. [Google Scholar] [CrossRef]
- Lees, H.J.; Swann, J.R.; Wilson, I.D.; Nicholson, J.K.; Holmes, E. Hippurate: The natural history of a mammalian–microbial cometabolite. J. Proteome Res. 2013, 12, 1527–1546. [Google Scholar] [CrossRef]
- Geraets, L.; Moonen, H.J.; Wouters, E.F.; Bast, A.; Hageman, G.J. Caffeine metabolites are inhibitors of the nuclear enzyme poly(ADP-ribose)polymerase-1 at physiological concentrations. Biochem. Pharm. 2006, 72, 902–910. [Google Scholar] [CrossRef]
- Madrid-Gambin, F.; Garcia-Aloy, M.; Vázquez-Fresno, R.; Vegas-Lozano, E.; de Villa Jubany, M.C.R.; Misawa, K.; Hase, T.; Shimotoyodome, A.; Andres-Lacueva, C. Impact of chlorogenic acids from coffee on urine metabolome in healthy human subjects. Food Res. Int. 2016, 89, 1064–1070. [Google Scholar] [CrossRef]
- Zieliński, J.; Kusy, K. Pathways of Purine Metabolism: Effects of Exercise and Training in Competitive Athletes. Trends in Sport Sciences. 2015, 3, 103–112. [Google Scholar]
- Sahlin, K.; Tonkonogi, M.; Söderlund, K. Plasma hypoxanthine and ammonia in humans during prolonged exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; Fonseca, L.M. How sample size influences research outcomes. Dent. Press J. Orthod. 2014, 19, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Kriebel, A.; Dorr, C.; Bandt, S.; Rist, M.; Roth, A.; Hummel, E.; Kulling, S.; Hoffmann, I.; Watzl, B. The Karlsruhe Metabolomics and Nutrition (KarMeN) Study: Protocol and Methods of a Cross-Sectional Study to Characterize the Metabolome of Healthy Men and Women. Jmir Res. Protoc. 2016, 5, e146. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Hashim, Y.-Y.; Wingfield, M.; Culliton, M.; McAuliffe, F.; Gibney, M.; Brennan, L. Effects of menstrual cycle phase on metabolomic profiles in premenopausal women. Hum. Reprod. 2010, 25, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Biniaminov, N.; Bandt, S.; Roth, A.; Haertel, S.; Neumann, R.; Bub, A. Irisin, physical activity and fitness status in healthy humans: No association under resting conditions in a cross-sectional study. PLoS ONE 2018, 13, e0189254. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Rist, M.J.; Muhle-Goll, C.; Gorling, B.; Bub, A.; Heissler, S.; Watzl, B.; Luy, B. Influence of Freezing and Storage Procedure on Human Urine Samples in NMR-Based Metabolomics. Metabolites 2013, 3, 243–258. [Google Scholar] [CrossRef]
- Chetwynd, A.J.; Abdul-Sada, A.; Holt, S.G.; Hill, E.M. Use of a pre-analysis osmolality normalisation method to correct for variable urine concentrations and for improved metabolomic analyses. J. Chromatogr. 2016, 1431, 103–110. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. 2019. Available online: https://www.R-project.org (accessed on 5 May 2020).
- Mirai Solutions GmbH. XLConnect: Excel Connector for R. R package version 0.2-15. Available online: https://CRAN.R-project.org/package=XLConnect (accessed on 5 May 2020).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York. NY, USA, 2016. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.2.4. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 5 May 2020).
- Wei, T.; Simko, V. R package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 5 May 2020).
- Slowikowski, K. Ggrepel: Automatically Position Non-Overlapping Text Labels with ‘ggplot2’. R package version 0.8.0. Available online: https://CRAN.R-project.org/package=ggrepel (accessed on 5 May 2020).
| Characteristics of Participants | Total (n = 255) | Men (n = 148) | Women (n = 107) 1 |
|---|---|---|---|
| Age (years) | 46.1 ± 16.9 * | 42.8 ± 17.6 | 50.7 ± 14.7 |
| Body weight (kg) | 72.5 ± 11.6 * | 78.5 ± 10.0 | 64.2 ± 8.1 |
| Height (cm) | 174.6 ± 9.6 * | 180.1 ± 7.5 | 166.9 ± 6.5 |
| BMI (kg (m²)−1) | 23.7 ± 2.8 * | 24.2 ± 2.7 | 23.1 ± 2.9 |
| LBM (kg) | 50.6 ± 10.4 * | 58.0 ± 6.6 | 40.3 ± 3.8 |
| Fat mass (%) | 27.9 ± 8.7 * | 23.2 ± 6.5 | 34.5 ± 6.7 |
| Hemoglobin (g dL−1) 2 | 14.4 ± 1.1 * | 15.0 ± 0.9 | 13.5 ± 0.8 |
| BP systolic (mmHg) | 123.6 ± 15.4 * | 127.2 ± 13.6 | 118.6 ± 16.4 |
| BP diasystolic (mmHg) | 83.1 ± 10.5 | 83.9 ± 10.2 | 82.1 ± 10.9 |
| HRrest (bpm) | 62.4 ± 9.4 * | 59.9 ± 9.3 | 65.8 ± 8.6 |
| VO2peak, absolute (L min−1) | 2.83 ± 1.00 * | 3.46 ± 0.81 | 1.96 ± 0.42 |
| VO2peak, relative (mL kg−1 min−1) | 38.8 ± 11.6 * | 44.5 ± 10.7 | 30.9 ± 7.4 |
| PIAT (watt) | 144.5 ± 46.2 * | 170.5 ± 41.1 | 108.4 ± 22.6 |
| Pmax (watt) | 215.2 ± 69.1 * | 257.0 ± 56.0 | 157.5 ± 36.0 |
| HRmax (bpm) | 170.8 ± 16.8 * | 174.1 ± 16.1 | 166.2 ± 16.8 |
| Nr. | Metabolite (Abbreviation) | Median Fold Change (25th, 75th Percentiles) | FDR-Corrected p-Value | |
|---|---|---|---|---|
| 1 | Lactate (Lac) | 4.70 | (1.64, 32.98) | <0.0001 |
| 2 | Mannitol (Man) | 2.34 | (1.30, 4.29) | <0.0001 |
| 3 | trans-Aconitate (t-Aco) | 1.96 | (1.05, 3.49) | <0.0001 |
| 4 | Alanine (Ala) | 1.74 | (1.38, 2.20) | <0.0001 |
| 5 | Carnitine (Car) | 1.68 | (1.24, 2.37) | <0.0001 |
| 6 | Acetate (Acet) | 1.55 | (1.14, 2.68) | <0.0001 |
| 7 | Taurine (Tau) | 1.49 | (1.19, 2.02) | <0.0001 |
| 8 | Pyruvate (Pyr) | 1.48 | (0.88, 3.57) | <0.0001 |
| 9 | Threonine (Thr) | 1.40 | (1.12, 1.86) | <0.0001 |
| 10 | Guanidoacetate (Gua) | 1.39 | (1.14, 1.71) | <0.0001 |
| 11 | N,N-Dimethylglycine (DMG) | 1.38 | (1.13, 1.68) | <0.0001 |
| 12 | Betaine (Bet) | 1.32 | (1.12, 1.58) | <0.0001 |
| 13 | Glycine (Gly) | 1.32 | (1.13, 1.63) | <0.0001 |
| 14 | Histidine (His) | 1.30 | (1.07, 1.62) | <0.0001 |
| 15 | Succinate (Suc) | 1.30 | (0.92, 1.76) | <0.0001 |
| 16 | cis-Aconitate (c-Aco) | 1.29 | (1.08, 1.74) | <0.0001 |
| 17 | Methylsuccinate (MSuc) | 1.28 | (1.12, 1.54) | <0.0001 |
| 18 | Leucine (Leu) | 1.28 | (1.05, 1.71) | <0.0001 |
| 19 | Acetone (Ace) | 1.28 | (0.85, 1.99) | <0.0001 |
| 20 | Creatine (Cre) | 1.26 | (0.85, 1.91) | <0.0001 |
| 21 | Citrate (Cit) | 1.24 | (1.05, 1.48) | <0.0001 |
| 22 | 2-Hydroxyisobutyrate (2-OH-Isob) | 1.24 | (1.07, 1.43) | <0.0001 |
| 23 | Isoleucine (Ile) | 1.21 | (0.94, 1.49) | <0.0001 |
| 24 | 4-Hydroxyphenylacetate (4-OH-Phe) | 1.19 | (0.97, 1.69) | <0.0001 |
| 25 | Formate (For) | 1.16 | (0.96, 1.36) | <0.0001 |
| 26 | 3-Aminoisobutyrate (BAIBA) | 1.15 | (0.94, 1.40) | <0.0001 |
| 27 | Valine (Val) | 1.14 | (0.97, 1.39) | <0.0001 |
| 28 | 3-Hydroxyisovalerate (3-OH-Isov) | 1.13 | (1.02, 1.28) | <0.0001 |
| 29 | Gluconate (Glu) | 1.13 | (0.92, 1.44) | <0.0001 |
| 30 | Tyrosine (Tyr) | 1.12 | (0.97, 1.41) | <0.0001 |
| 31 | Tartrate (Tar) | 1.12 | (0.74, 1.51) | 0.8643 |
| 32 | Methylamine (MA) | 1.10 | (0.93, 1.41) | <0.0001 |
| 33 | Dimethylsulfone (DMS) | 1.09 | (0.87, 1.43) | 0.0013 |
| 34 | Glycolate (Glyc) | 1.07 | (0.86, 1.27) | 0.0066 |
| 35 | Methanol (Met) | 1.07 | (0.71, 1.72) | 0.1777 |
| 36 | Urea (Urea) | 1.03 | (0.87, 1.20) | 0.0715 |
| 37 | Pseudouridine (Pse) | 1.03 | (0.88, 1.20) | 0.1605 |
| 38 | Dimethylamine (DMA) | 1.02 | (0.86, 1.21) | 0.2783 |
| 39 | Hypoxanthine (Hyp) | 1.00 | (0.67, 1.65) | 0.4068 |
| 40 | Uracil (Ura) | 0.98 | (0.79, 1.22) | 0.2208 |
| 41 | Creatinine (Crea) | 0.98 | (0.84, 1.18) | 0.7036 |
| 42 | 1-Methylnicotinamide (MNA) | 0.97 | (0.78, 1.24) | 0.3221 |
| 43 | Trimethylamine N-oxide (TMAO) | 0.97 | (0.79, 1.19) | 0.0778 |
| 44 | 3-Methylxanthine (3-MXan) | 0.90 | (0.70, 1.16) | <0.0001 |
| 45 | 3-Indoxylsulfate (3-Ind) | 0.87 | (0.68, 1.04) | <0.0001 |
| 46 | Trigonelline (Tri) | 0.73 | (0.63, 0.89) | <0.0001 |
| 47 | Hippurate (Hip) | 0.70 | (0.54, 0.91) | <0.0001 |
| Model | R2 | R2 (Adjusted) | BIC | Std.-Beta | 95% CI (Lower) | 95% CI (Upper) |
|---|---|---|---|---|---|---|
| Pre | 0.176 | 0.153 | 300.9 | |||
| cis-Aconitate | −0.441 | −0.291 | −0.106 | |||
| 3-Aminoisobutyrate | 0.232 | 0.047 | 0.163 | |||
| trans-Aconitate | 0.295 | 0.054 | 0.212 | |||
| Tyrosine | −0.213 | −0.157 | −0.038 | |||
| Guanidoacetate | −0.208 | −0.165 | −0.038 | |||
| Uracil | 0.206 | 0.033 | 0.156 | |||
| Lactate | 0.230 | 0.041 | 0.218 | |||
| Post | 0.081 | 0.070 | 306.6 | |||
| Tyrosine | −0.197 | −0.145 | −0.034 | |||
| 3-Aminoisobutyrate | 0.182 | 0.028 | 0.141 | |||
| 1-Methylnicotinamide | −0.149 | −0.126 | −0.013 | |||
| Fold changes | 0.000 | 0.000 | 311.4 | |||
| (intercept-only model) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kistner, S.; Rist, M.J.; Döring, M.; Dörr, C.; Neumann, R.; Härtel, S.; Bub, A. An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans—Results of the KarMeN Study. Metabolites 2020, 10, 212. https://doi.org/10.3390/metabo10050212
Kistner S, Rist MJ, Döring M, Dörr C, Neumann R, Härtel S, Bub A. An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans—Results of the KarMeN Study. Metabolites. 2020; 10(5):212. https://doi.org/10.3390/metabo10050212
Chicago/Turabian StyleKistner, Sina, Manuela J. Rist, Maik Döring, Claudia Dörr, Rainer Neumann, Sascha Härtel, and Achim Bub. 2020. "An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans—Results of the KarMeN Study" Metabolites 10, no. 5: 212. https://doi.org/10.3390/metabo10050212
APA StyleKistner, S., Rist, M. J., Döring, M., Dörr, C., Neumann, R., Härtel, S., & Bub, A. (2020). An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans—Results of the KarMeN Study. Metabolites, 10(5), 212. https://doi.org/10.3390/metabo10050212





