Design, Synthesis, and Biological Evaluation of 1,2,3-Triazole-linked triazino[5,6-b]indole-benzene sulfonamide Conjugates as Potent Carbonic Anhydrase I, II, IX, and XIII Inhibitors
Abstract
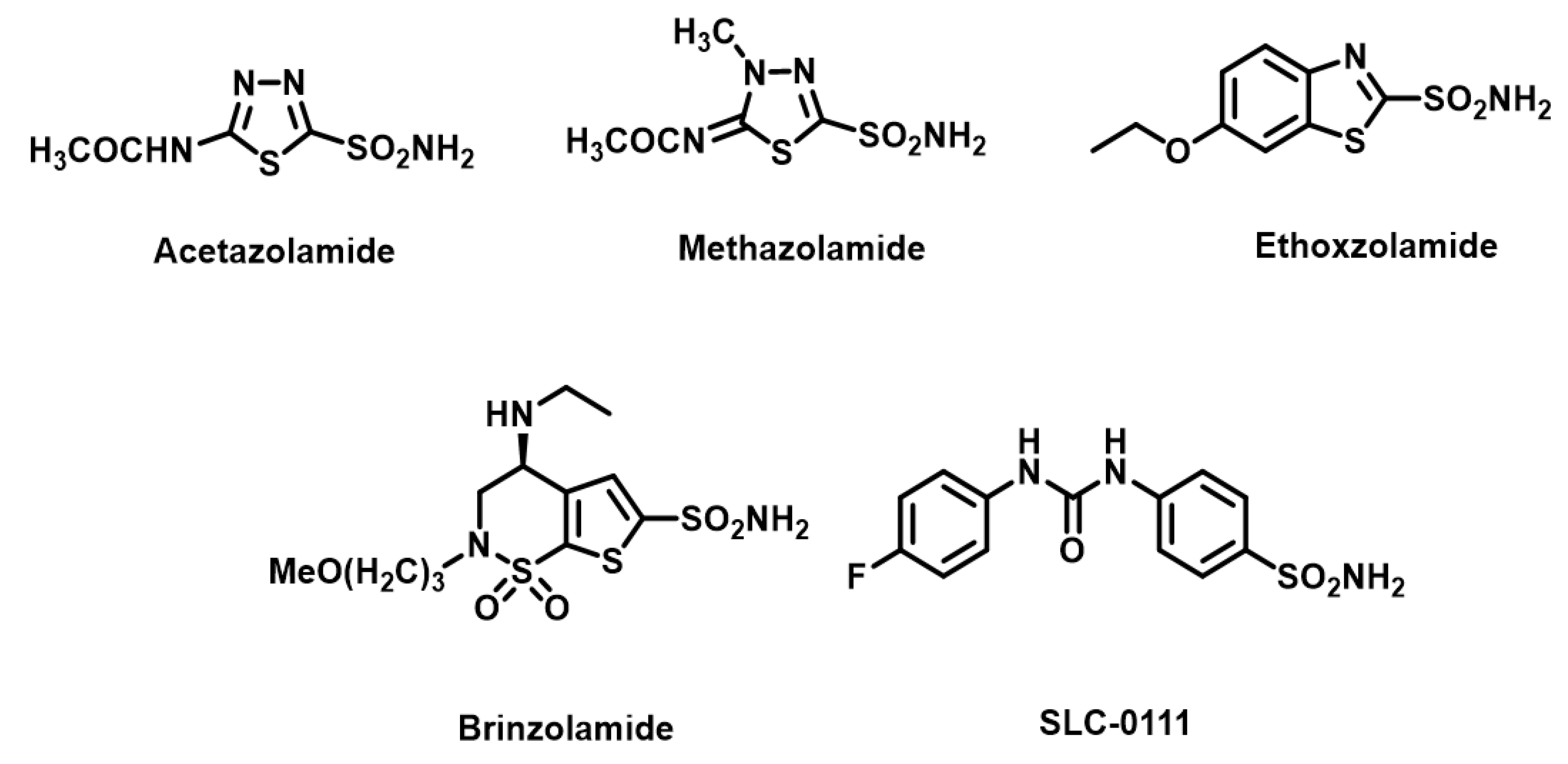
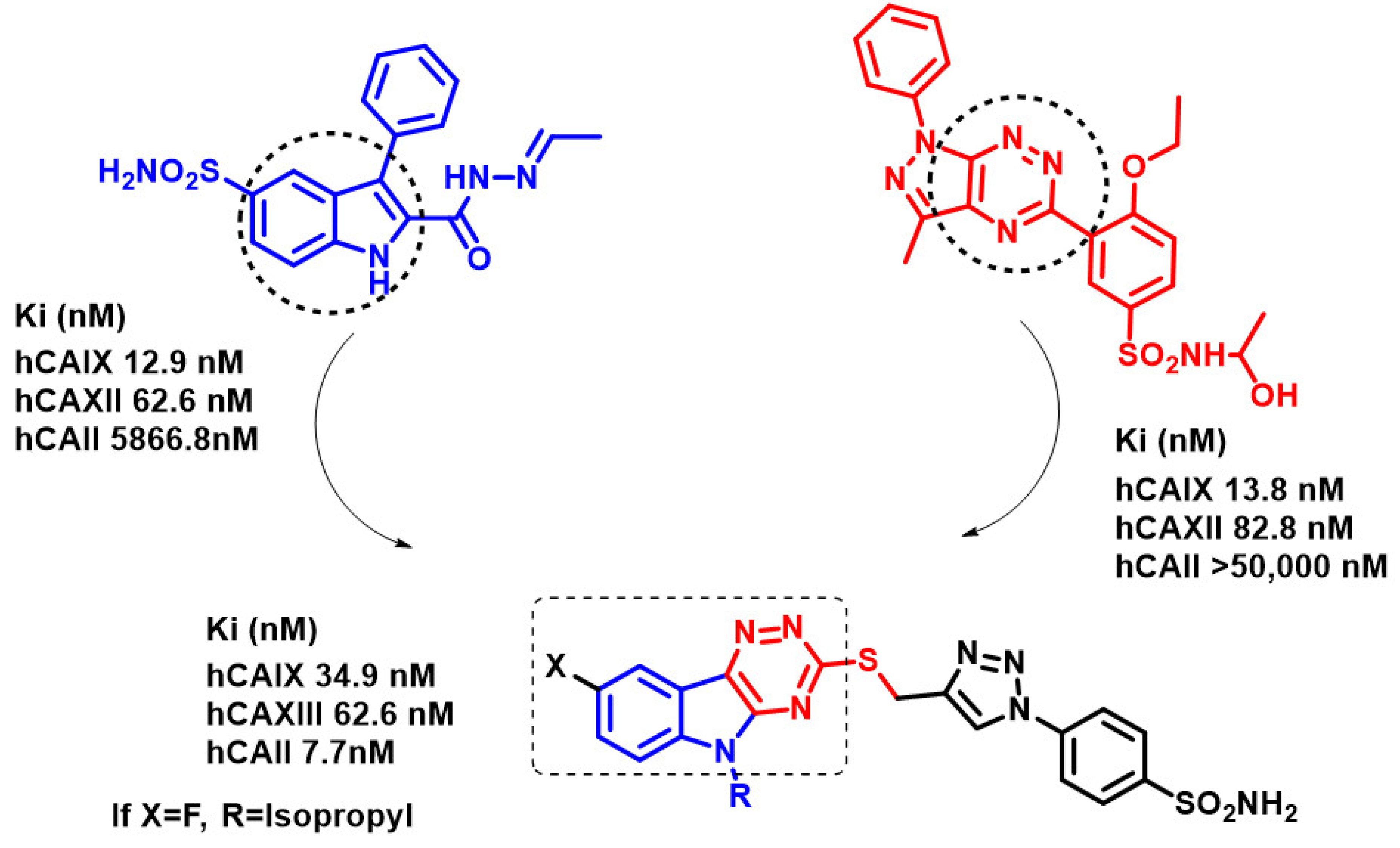
1. Introduction
2. Results and Discussion
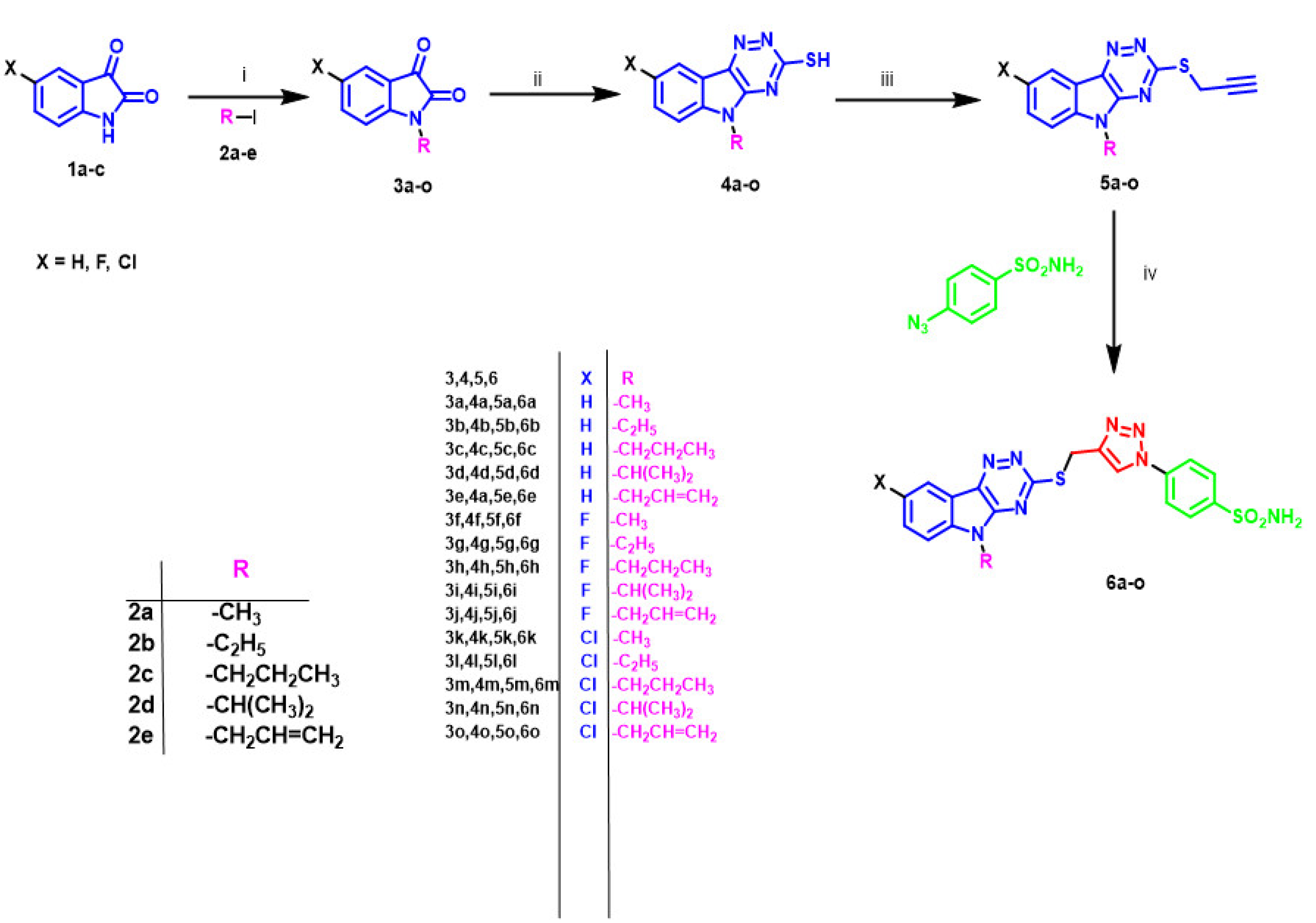
2.1. Synthesis of the Target Molecules
2.2. Carbonic Anhydrase Inhibition
- I
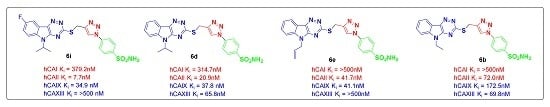
- Tthe cytosolic hCA II isoform was strongly inhibited by all the synthesized compounds 6a–o, in a low to medium nanomolar range (Kis = 7.7 nM to 0.2527 µM). The best activity against hCA II was shown by compound 6i (Ki = 7.7 nM), possessing a fluoro group attached at the 5th position of the indole ring and an isopropyl group anchored to the nitrogen of indole. It was almost twofold more active than the standard AAZ (Ki = 12.1 nM). Compounds 6d–6g, were found to have potent activity at the nanomolar concentration against hCA II, with Ki ranging from 20.9 to 63.9 nM. Compounds 6k–6o, containing a chloro group at the 5th position of indole, showed lower activity in the range of 61.7 to 252.7 nM, compared to compounds containing a fluoro group and unsubstituted indole.
- II
- The transmembrane hCA IX isoform, which is expressed exclusively in tumors, was also strongly inhibited by the synthesized compounds in the medium nanomolar range (Kis = 34.9 nM to 0.3246 µM). Compounds 6d, 6e, 6f, and 6i showed equipotent nanomolar activity with AAZ, with Kis ranging from 34.9 nM to 41.3 nM. Among these compounds, 6i showed the best activity (Ki = 34.9 nM) against hCA IX isoform.
- III
- The cytosolic hCA I and hCA XIII isoforms were inhibited by all synthesized compounds in the high nanomolar range (Kis > 500 nM). However, compounds 6b and 6d showed moderate activity with Kis of 69.8 nM and 65.8 nM respectively against hCA XIII isoform.
3. Conclusions
4. Experimental Section
4.1. General Experimental Conditions
4.1.1. Synthesis of N-Alkylated isatins (3a–o)
4.1.2. Synthesis of N-Alkylated triazino[5,6-b]indolethioether derivatives (4a–o)
4.1.3. Synthesis of N-Alkyl-3-prop-2-yn-1-ylthio)-5H-[1,2,4]triazino[5,6b]indole derivatives (5a–o)
4.1.4. Synthesis of 4-(4-(N-Alkyl-5H-[1,2,4]triazino[5,6b]indol-3-yl)thio)methyl)- 1H-1,2,3-triazol-1-yl)benzenesulfonamide derivatives (6a–o)
4.2. CA Inhibition
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alterio, V.; Di Fiore, A.; Ambrosio, K.D.; Supuran, C.T.; de Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases and metabolism. Metabolites 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.L.; Clement, R.; Kosta, A.; Maberly, S.C.; Gontero, B. A new wide spread sub class of carbonic anhydrase in marine phytoplankton. ISME J. 2019, 13, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Lock, F.E.; McDonald, P.C.; Lou, Y.; Serrano, I.; Chafe, S.C.; Ostlund, C.; Aparicio, S.; Winum, J.Y.; Supuran, C.T.; Dedhar, S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 2013, 32, 5210–5219. [Google Scholar] [CrossRef]
- Bayram, E.; Senturk, M.; Kufrevioglu, O.I.; Supuran, C.T. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg. Med. Chem. 2008, 16, 9101–9105. [Google Scholar] [CrossRef]
- Bozdag, M.; Pinard, M.; Carta, F.; Masini, E.; Scozzafava, A.; McKenna, R.; Supuran, C.T. A Class of 4-Sulfamoylphenyl-ω-aminoalkyl Ethers with Effective Carbonic Anhydrase Inhibitory Action and Antiglaucoma Effects. J. Med. Chem. 2014, 57, 9673–9686. [Google Scholar] [CrossRef]
- Chegaev, K.; Lazzarato, L.; Tamboli, Y.; Boschi, D.; Blangetti, M.; Scozzafava, A.; Carta, F.; Masini, E.; Fruttero, R.; Supuran, C.T.; et al. Furazan and furoxan sulfonamides are strong α-carbonic anhydrase inhibitors and potential anti-glaucoma agents. Bioorg. Med. Chem. 2014, 22, 3913–3921. [Google Scholar]
- De Luca, L.; Ferro, S.; Damiano, F.M.; Supuran, C.T.; Vullo, D.; Chimirri, A.; Gitto, R. Structure-based screening for the discovery of new carbonic anhydrase XII inhibitors, Eur. J. Med. Chem. 2014, 71, 105–111. [Google Scholar] [CrossRef]
- Bruno, E.; Buemi, M.R.; De Luca, L.; Ferro, S.; Monforte, A.M.; Supuran, C.T.; Vullo, D.; De Sarro, G.; Russo, E.; Gitto, R. In Vivo Evaluation of Selective Carbonic Anhydrase Inhibitors as Potential Anticonvulsant Agents. ChemMedChem 2016, 11, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Menabuoni, L.; Mincione, F.; Supuran, C.T. Carbonic anhydrase inhibitors: A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long-lasting, Topical intraocular pressure-lowering properties. J. Med. Chem. 2002, 45, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Tars, K.; Vullo, D.; Kazaks, A.; Leitans, J.; Lends, A.; Grandane, A.; Zalubovskis, A.; Scozzafava, A.; Supuran., C.T. Sulfocoumarins (1,2-Benzoxathiine-2,2-dioxides): A Class of Potent and Isoform-Selective Inhibitors of Tumor-Associated Carbonic Anhydrases. J. Med. Chem. 2013, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Scozzafava, A.; Supuran, C.T. 7,8-Disubstituted-but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor associated carbonic anhydrase IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg. Med. Chem. Lett. 2010, 20, 7255–7258. [Google Scholar] [CrossRef]
- Innocenti, A.; Sarıkaya, S.B.O.; Gulcin, I.; Supuran, C.T. Carbonic anhydrase inhibitors, Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg. Med. Chem. 2010, 18, 2159–2164. [Google Scholar] [CrossRef]
- Pinard, M.A.; Mahon, B.; McKenna, R. Probing the surface of human carbonicanhydrase for clues towards the design of isoform specific inhibitors. Biomed. Res. Int. 2015, 2015, 453543. [Google Scholar] [CrossRef]
- Aggarwal, M.; Kondeti, B.; McKenna, R. Insights towards sulfonamide drug specificity in alpha-carbonic anhydrases. Bioorg. Med. Chem. 2013, 21, 1526–1533. [Google Scholar] [CrossRef]
- Kgokong, J.L.; Smith, P.P.; Matsabisa, G.M. 1,2,4-Triazino-[5,6b]indole derivatives: Effects of trifluoromethyl group on in-vitro antimalarial activity. Bioorg. Med. Chem. 2005, 13, 2935–2942. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, K.; Ullah, H. Triazinoindole analogs as potent inhibitors of α-glucosidase: Synthesis, biological evaluation and molecular docking studies. Bioorg. Med. Chem. 2015, 58, 81–87. [Google Scholar] [CrossRef]
- Shelke, S.M.; Bhosale, S.H. Synthesis, antidepressant evaluation and QSAR studies of novel 2-(5H-[1,2,4]triazino[5,6b]indol3-ylthio)-N-(substituted phenyl)acetamides. Bioorg. Med. Chem. Lett. 2010, 20, 4661–4664. [Google Scholar] [CrossRef]
- Monge, A.; Palop, J.A.; Ramierz, C.; Font, M.; Fernandez, A.E. New 5H-1,2,4-triazino[5,6b]indole and aminoindole derivatives. Synthesis and studies as inhibitors of blood platelet aggregation, anti-hypertensive agents and thromboxane synthetase inhibitors. Eur. J. Med. Chem. 1991, 26, 179–188. [Google Scholar] [CrossRef]
- Aswar, U.M.; Kalshetti, P.P.; Shelke, S.M.; Bhosale, S.H.; Bodhankar, S.L. Effect of newly synthesized 1,2,4-triazino[5,6b]indole-3-thione derivatives on olfactory bulbectomy induced depression in rats. Asian. J. Trop. Biomed. 2012, 2, 992–998. [Google Scholar] [CrossRef]
- Tomchin, A.B.; Uryupov, O.Y.; Zhukova, T.I.; Kuznetsova, T.A. Thiourea and thiosemicarbazide derivatives: Structure, transformations, and pharmacological activity. Part III. Antihypoxic and anti-inflammatory activity of 1,2,4-triazino[5,6b]indole derivatives. Pharma. Chem. J. 1997, 31, 632–637. [Google Scholar] [CrossRef]
- Demir-Yazici, K.; Bua, S.; Supuran, C.T. Indole-Based Hydrazones Containing A Sulfonamide Moiety as Selective Inhibitors of Tumor-Associated Human Carbonic Anhydrase Isoforms IX and XII. Int. J. Mol. Sci. 2019, 20, 2354. [Google Scholar] [CrossRef] [PubMed]
- Peerzada, M.N.; Khan, P.; Ahmad, K. Synthesis, characterization and biological evaluation of tertiary sulfonamide derivatives of pyridyl-indole based heteroaryl chalcone as potential carbonic anhydrase IX inhibitors and anticancer agents. Eur. J. Med. Chem. 2018, 155, 13–23. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Nocentini, A.; Hassan, G.S.; Supuran, C.T. Tumor-associated carbonic anhydrase isoform IX and XII inhibitory properties of certain isatin-bearing sulfonamides endowed with in-vitro antitumor activity towards colon cancer. Bioorg. Med. Chem. 2018, 81, 425–432. [Google Scholar] [CrossRef]
- Mojzych, M.; Ceruso, M.; Supuran. C.T. New pyrazolo[4,3e][1.2.4]triazino sulfonamides as carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2015, 23, 3674–3680. [Google Scholar] [CrossRef]
- Matysiak, J.; Skrzypek, A.; Tarasiuk, P. QSAR study of pyrazolo[4,3e][1,2,4]triazino sulfonamide against tumor-associated human carbonic anhydrase isoforms IX and XII. Comp. Boil. Chem. 2017, 71, 57–62. [Google Scholar] [CrossRef]
- Nocentini, A.; Ferraroni, M.; Carta, F.; Supuran, C.T. Benzene sulfonamides incorporating flexible Triazole Moieties Are Highly Effective Carbonic anhydrase Inhibitors: Synthesis and Kinetic, Crystallographic, Computational and Intraocular Pressure Lowering Investigations. J. Med. Chem. 2016, 59, 10692–10704. [Google Scholar] [CrossRef]
- Huy, X.N.; Green, K.D.; Gajadeera, C.S. Potent 1,2,4-Triazino[5,6b]indole-3-thioether inhibitors of Kanamycin Resistance Enzyme Eis from Mycobacterium tuberculosis. ACS Infect. Dis. 2018, 4, 1030–10440. [Google Scholar]
- Patel, D.V.; Patel, N.R.; Kanhed, A.M. Novel Multitarget Directed Triazinoindole Derivatives as Anti-Alzheimer Agents. ACS. Chem. Neurosci. 2019, 10, 3635–3661. [Google Scholar] [CrossRef] [PubMed]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stopflowkinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- Supuran, C.T.; Barboiu, M.; Luca, C.; Pop, E.; Brewster, M.E.; Dinculescu, A. Carbonic anhydrase activators. Part 14. Synthesis of mono- and bis- pyridinium salt derivatives of 2-amino-5-(2-aminoethyl)- and 2-amino-5-(3-aminopropyl)-1,3,4-thiadiazole, and their interaction with isozyme II. Eur. J. Med. Chem. 1996, 31, 597–606. [Google Scholar] [CrossRef]
- Öztürk Sarıkaya, S.B.; Gülçin, I.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids Chem. Biol. Drug Des. 2010, 75, 515–520. [Google Scholar] [CrossRef]
- Carta, F.; Aggarwal, M.; Maresca, A.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Dithiocarbamates: A new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun. 2012, 48, 1868–1870. [Google Scholar] [CrossRef]
- Boztaş, M.; Çetinkaya, Y.; Topal, M.; Gülçin, İ.; Menzek, A.; Şahin, E.; Tanc, M.; Supuran, C.T. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxybromophenol derivatives incorporating cyclopropane moieties. J. Med. Chem. 2015, 58, 640–650. [Google Scholar] [CrossRef]
- De Simone, G.; Supuran, C.T. (In)organic anions as carbonic anhydrase inhibitors. J. Inorg. Biochem. 2012, 111, 117–129. [Google Scholar] [CrossRef]
- Innocenti, A.; Gülçin, I.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I–XV. Bioorg. Med. Chem. Lett. 2010, 20, 5050–5053. [Google Scholar] [CrossRef]
| Disease | Isoform Target |
|---|---|
| Glaucoma | CA II, CA IV, CA XII |
| Cancer | CA IX, CA XII |
| Epilepsy | CA VII |
| Antineuropathic pain | CA VII |
| Obesity | CA VA |
| Organisms | CA Class | Acronym | Kcat (s−1) | kcat/KM (M−1 × s−1) | KI (Acetazolamide) (nM) |
|---|---|---|---|---|---|
| Homo sapiens | α | hCA I | 2.0 × 105 | 5.0 × 107 | 250 |
| α | hCA II | 1.4 × 106 | 1.5 × 108 | 12.1 | |
| α | hCA IX a | 3.8 × 105 | 5.5 × 107 | 25.8 | |
| α | hCA_XIII | 1.5 × 105 | 1.1 × 107 | 17.0 |
| KI (nM) | |||||
|---|---|---|---|---|---|
| Compound | Structure | hCA I | hCA II | hCA IX | hCA XIII |
| 6a | 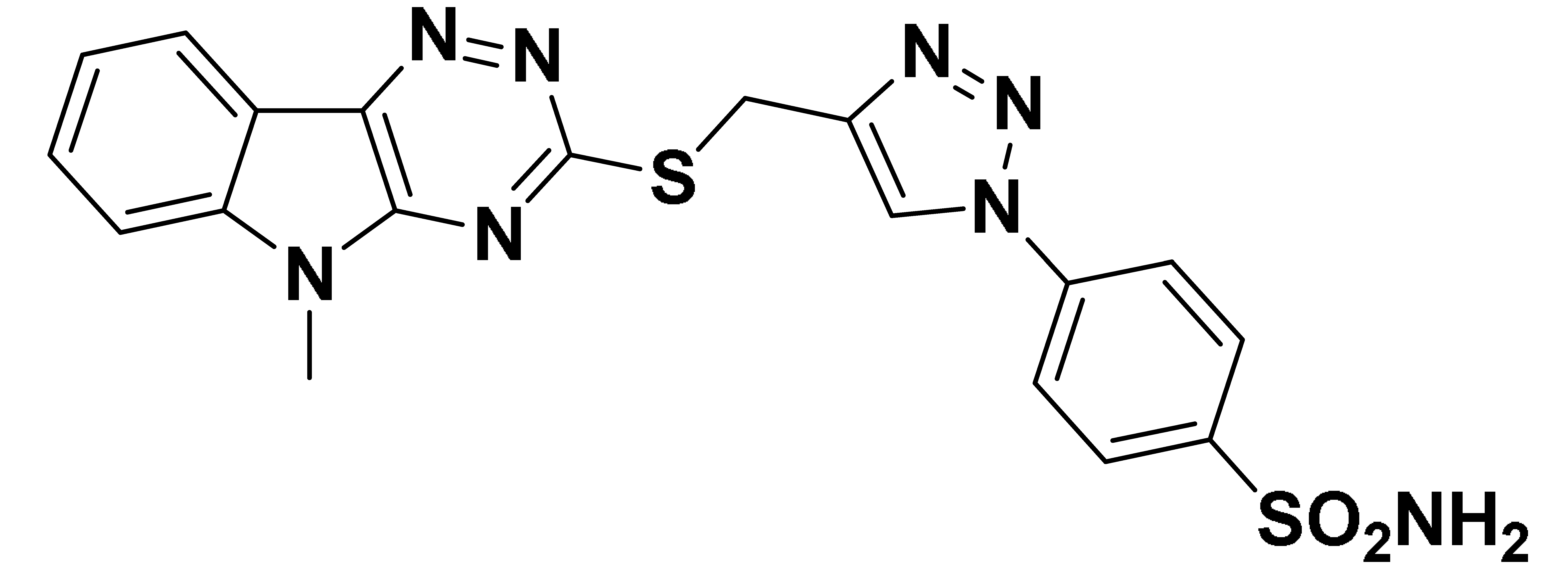 | 910.1 | 65.5 | 285.6 | 77.8 |
| 6b | 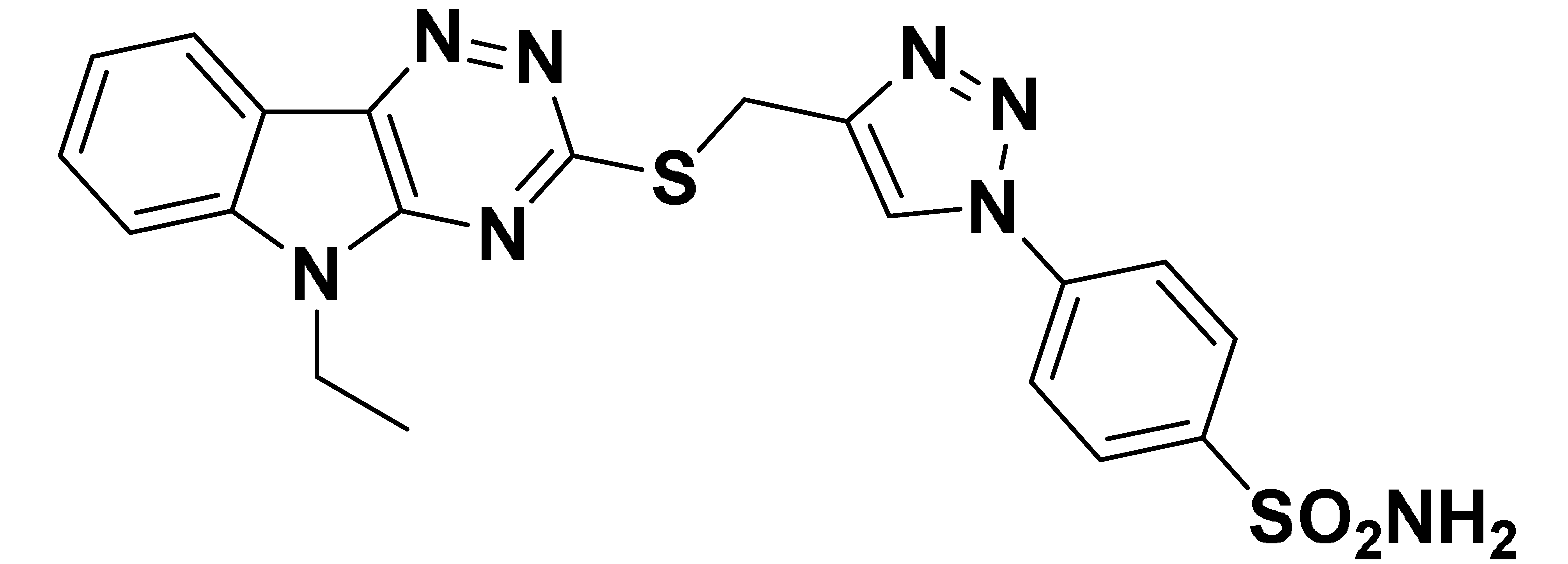 | 642.2 | 72.0 | 172.5 | 69.8 |
| 6c | 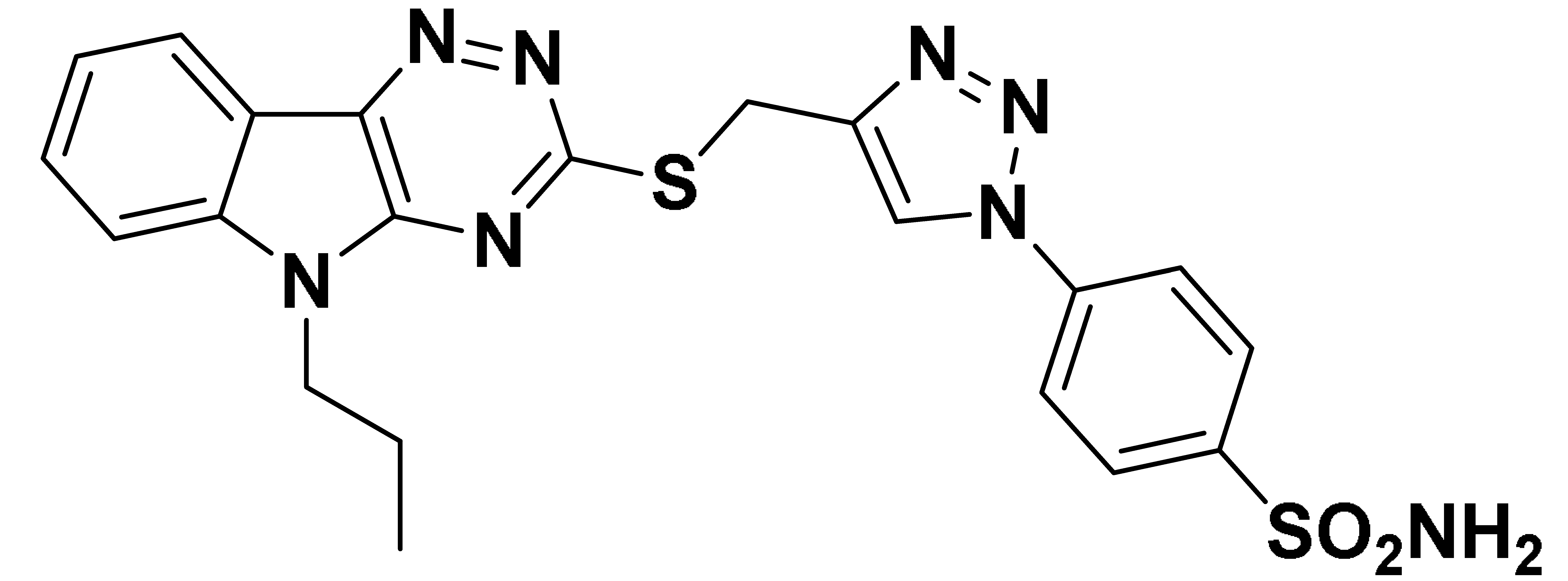 | 3960 | 88.7 | 219.4 | 364.8 |
| 6d | 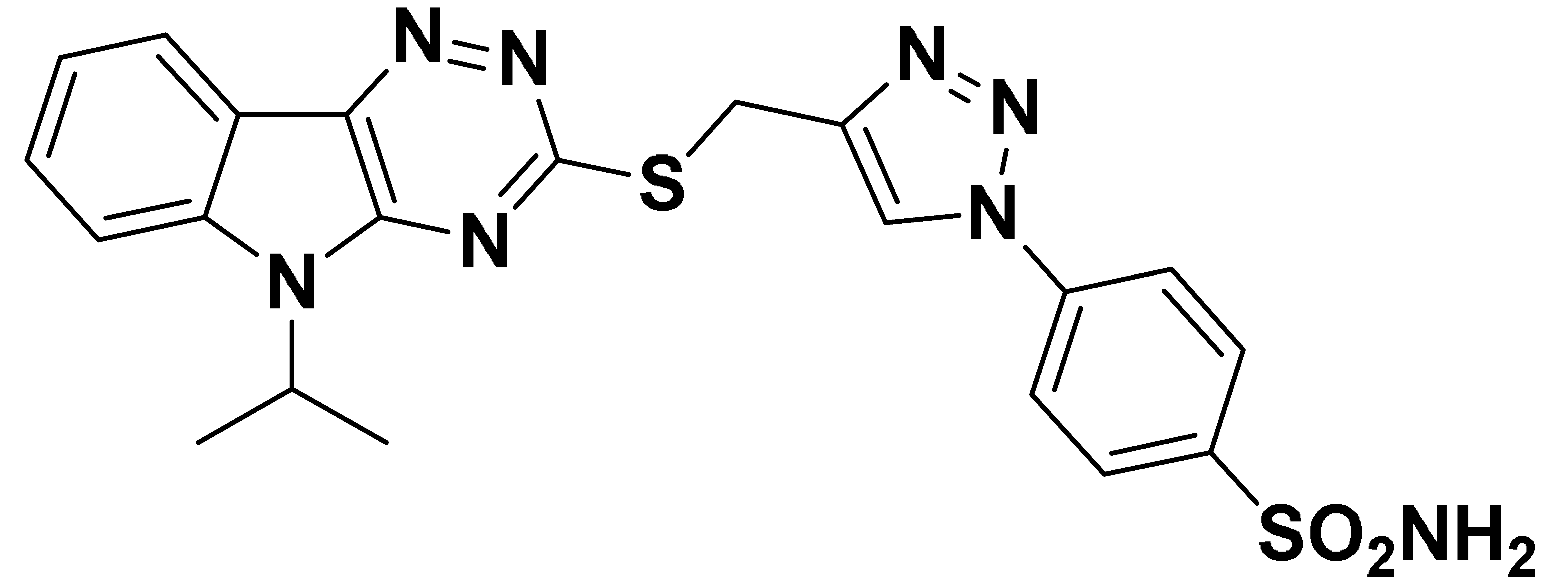 | 314.7 | 20.9 | 37.8 | 65.8 |
| 6e | 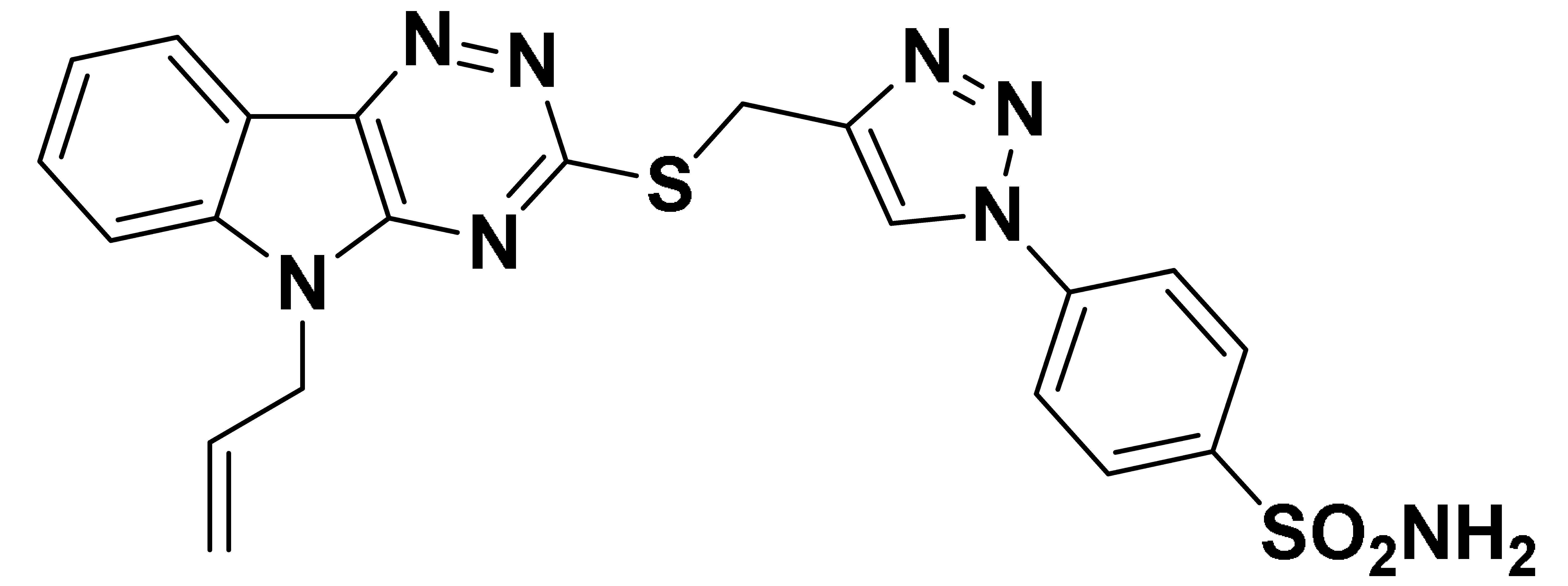 | 535.8 | 41.7 | 41.1 | 626.7 |
| 6f | 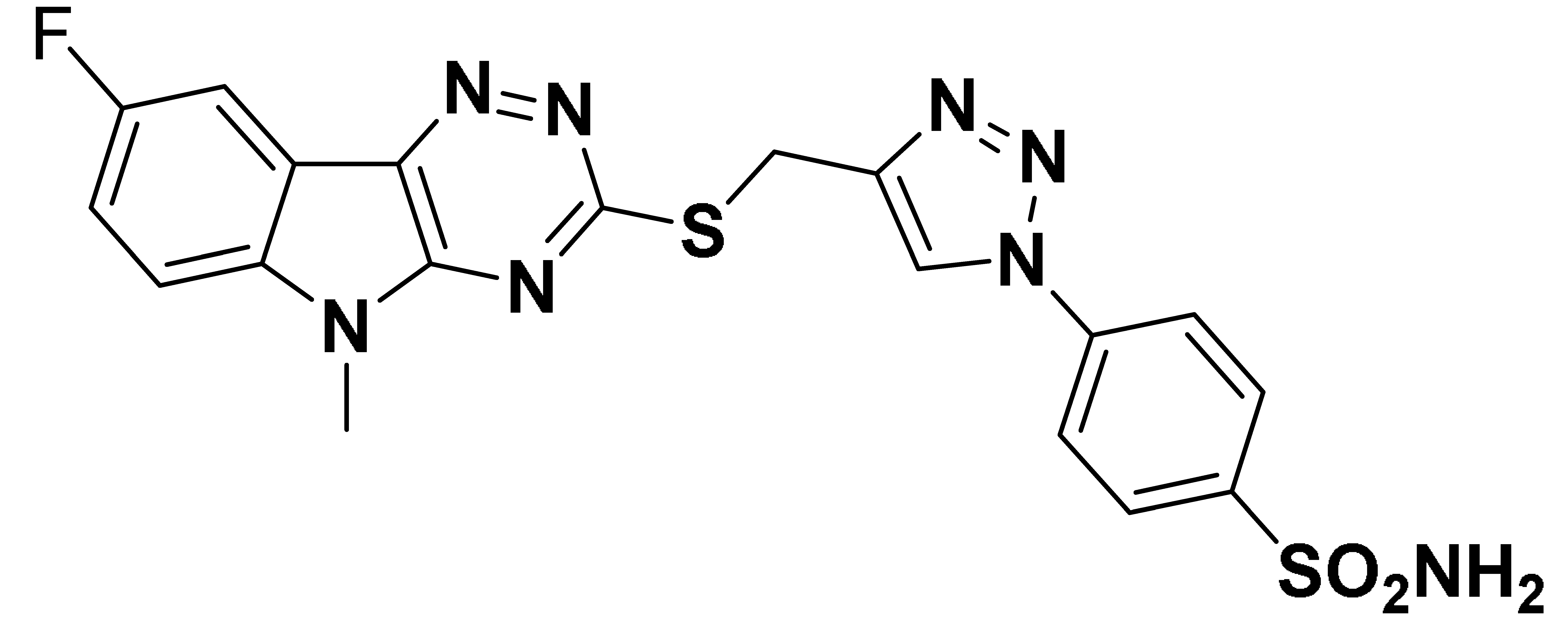 | 766.2 | 59.6 | 41.3 | 834.8 |
| 6g | 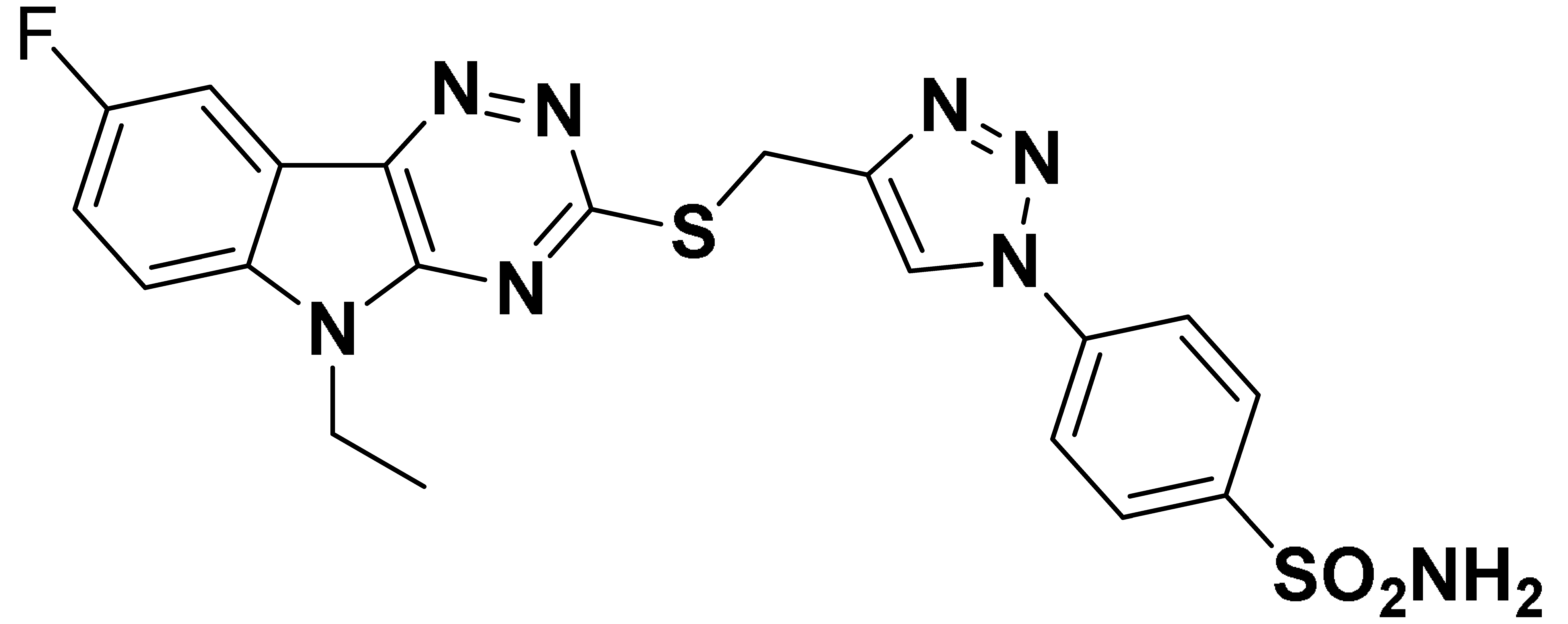 | 698.5 | 63.9 | 193.1 | 675.0 |
| 6h | 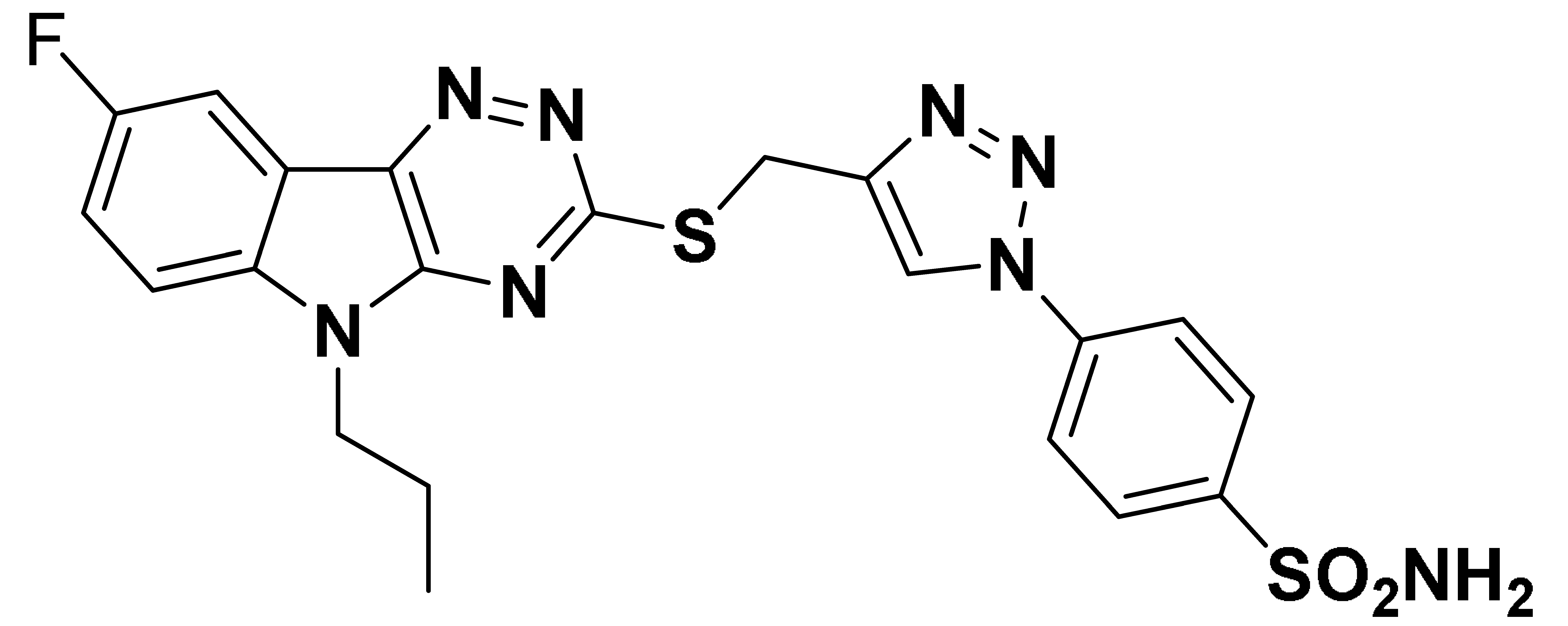 | 764.2 | 682.7 | 118.6 | 1815 |
| 6i | 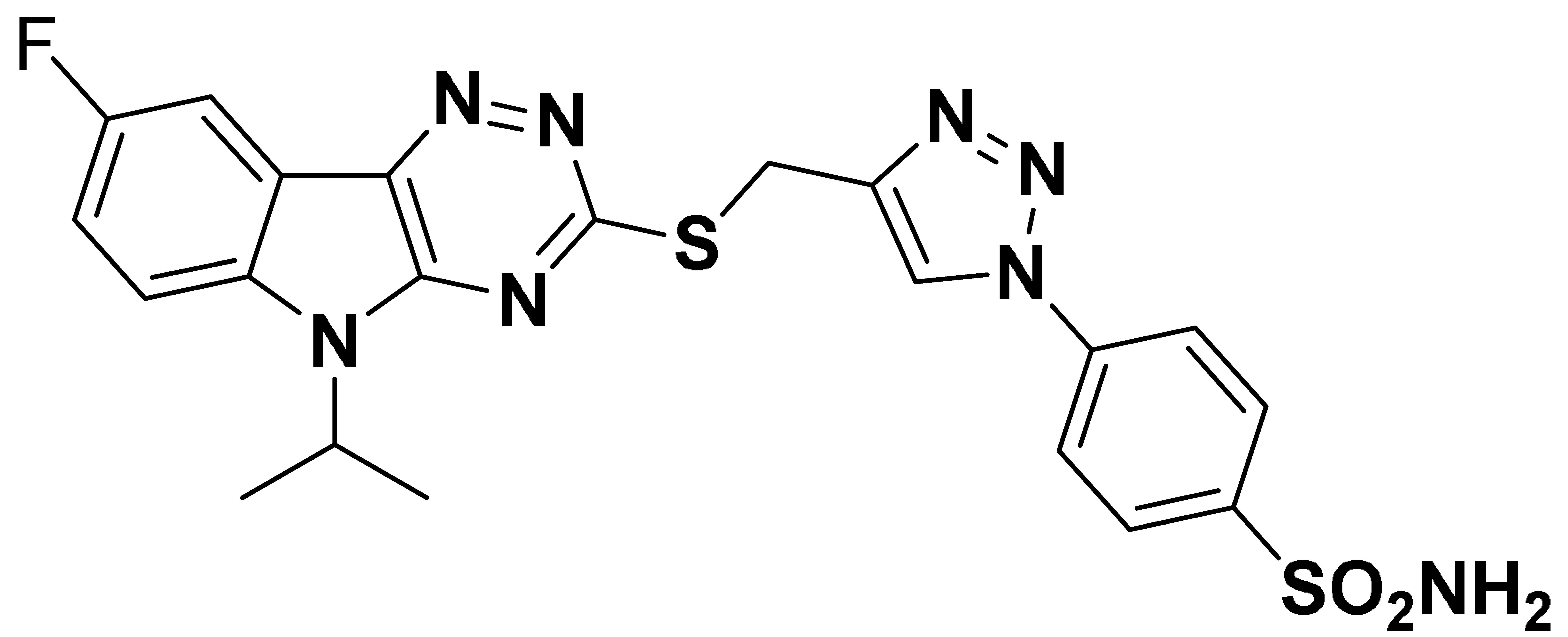 | 379.2 | 7.7 | 34.9 | 736.2 |
| 6j | 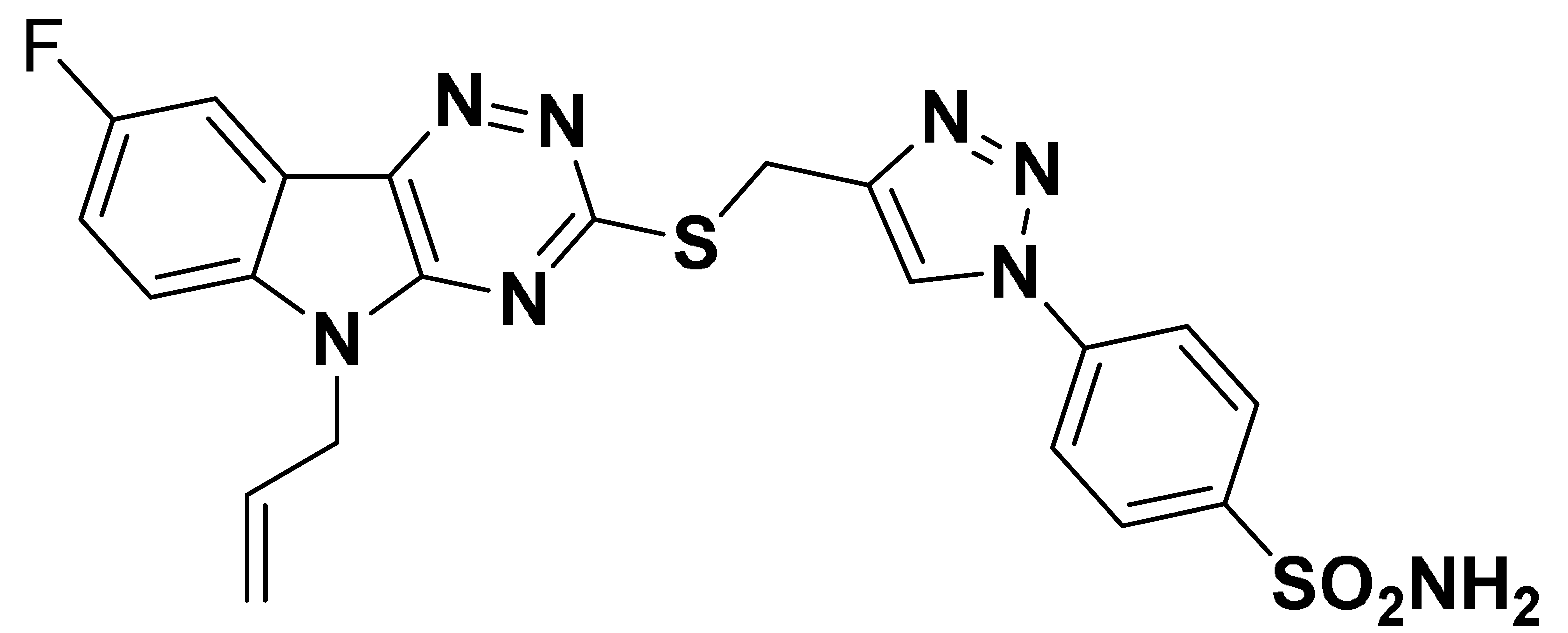 | 4592 | 73.7 | 401.7 | 793.6 |
| 6k | 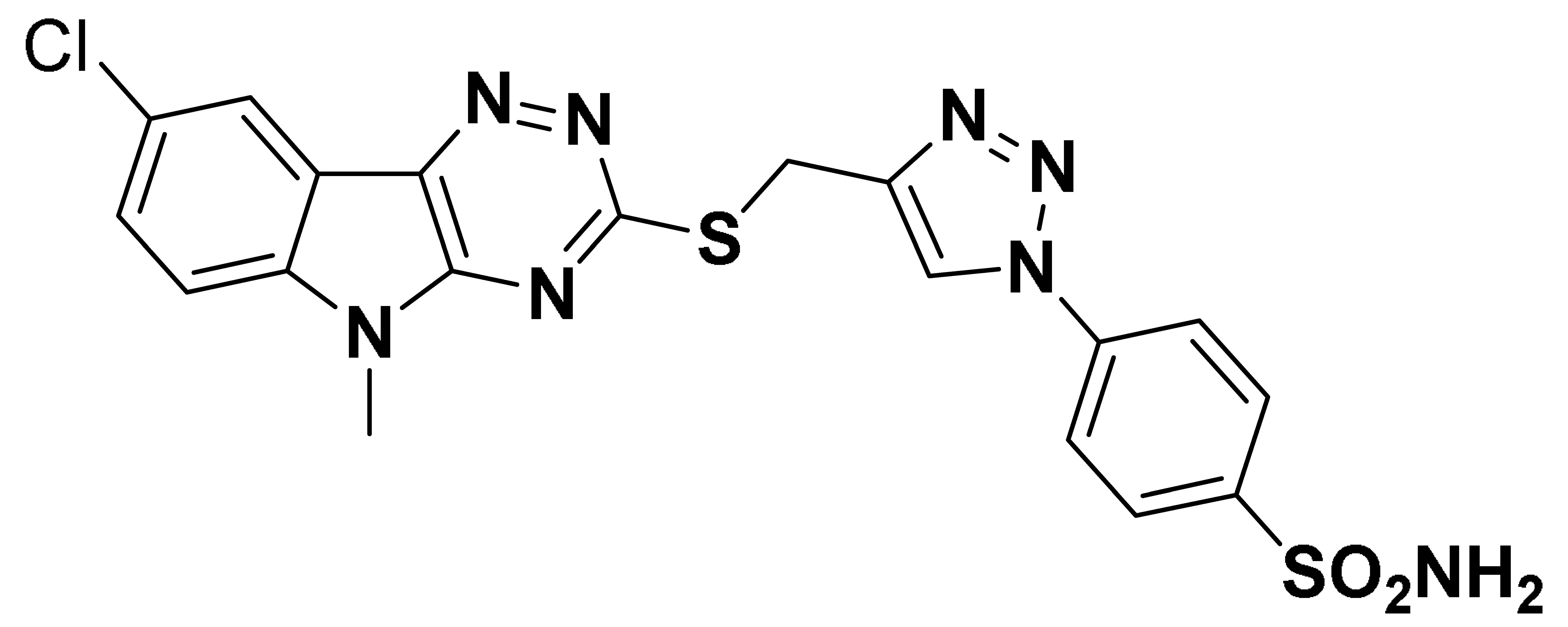 | 5140 | 252.7 | 330.7 | 867.0 |
| 6l | 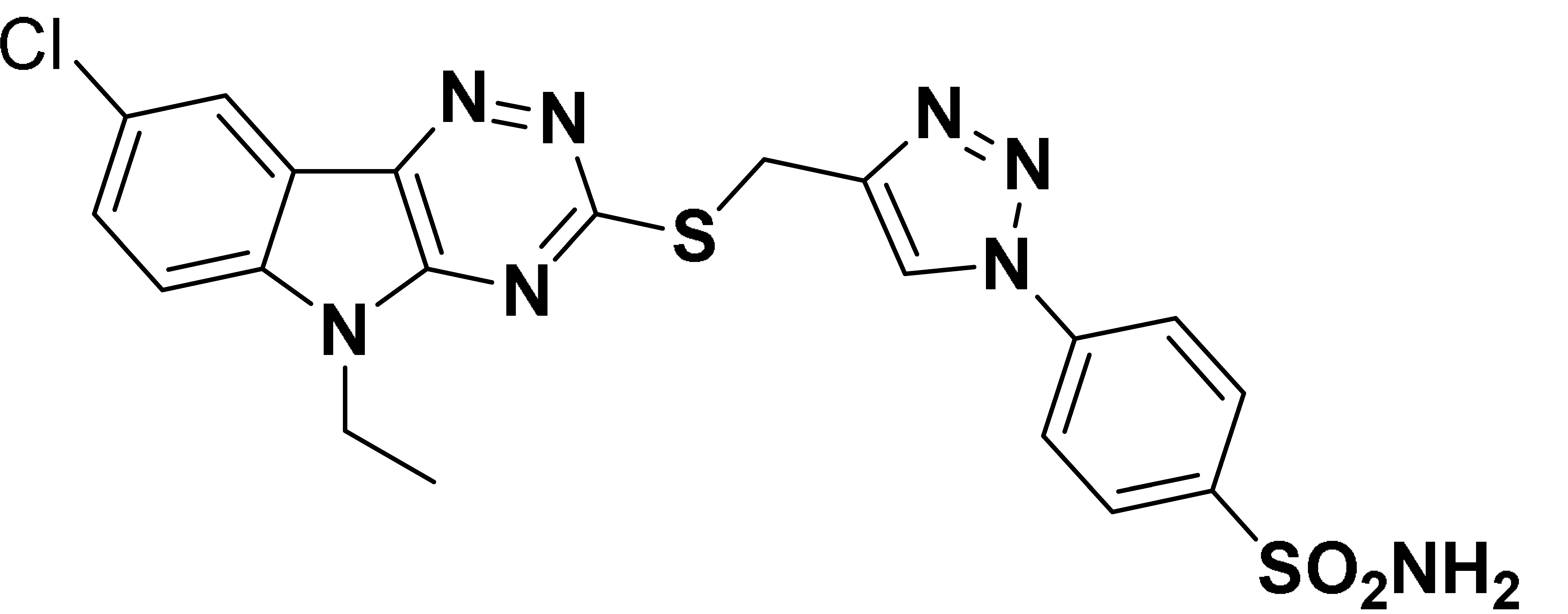 | 2837 | 184.6 | 150.4 | 3980 |
| 6m | 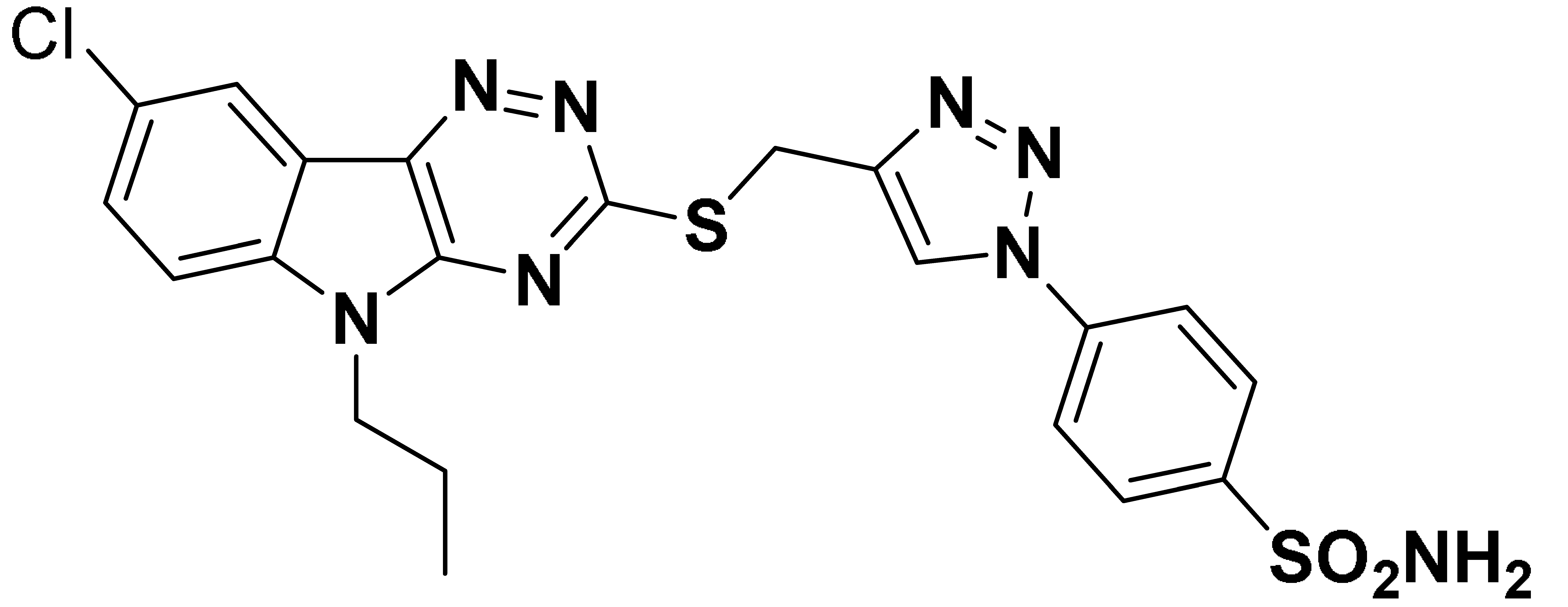 | 6513 | 61.7 | 204.5 | 823.8 |
| 6n | 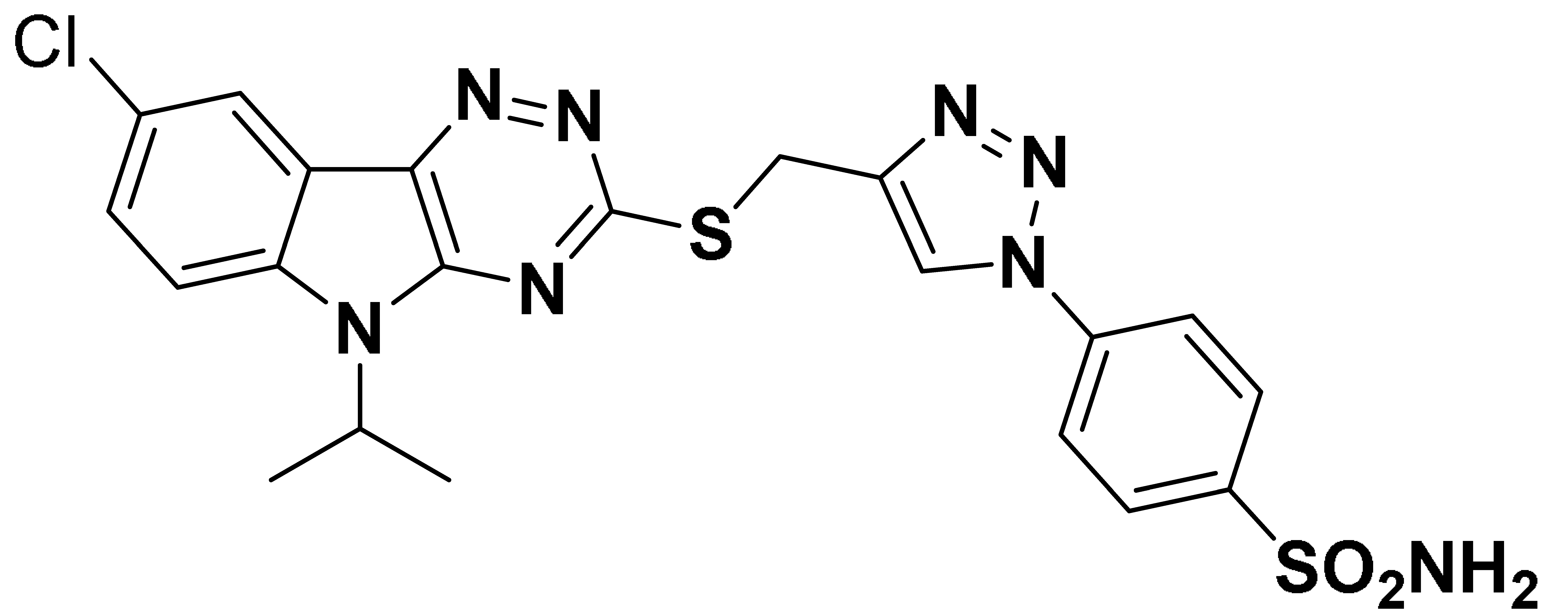 | 571.9 | 179.2 | 320.8 | 91.3 |
| 6o | 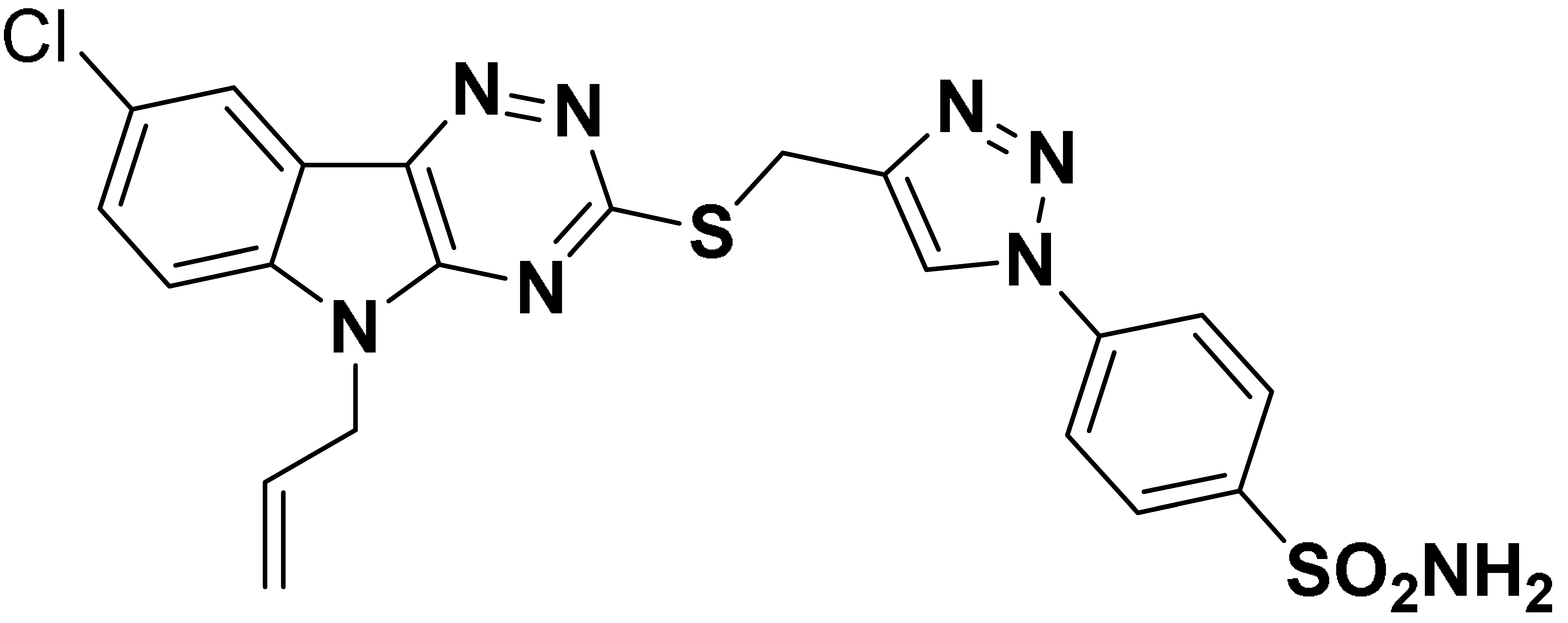 | 694.4 | 97.1 | 324.6 | 2300 |
| AAZ | 250.0 | 12.1 | 25.8 | 17.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinchilli, K.K.; Angeli, A.; Thacker, P.S.; Korra, L.N.; Biswas, R.; Arifuddin, M.; Supuran, C.T. Design, Synthesis, and Biological Evaluation of 1,2,3-Triazole-linked triazino[5,6-b]indole-benzene sulfonamide Conjugates as Potent Carbonic Anhydrase I, II, IX, and XIII Inhibitors. Metabolites 2020, 10, 200. https://doi.org/10.3390/metabo10050200
Chinchilli KK, Angeli A, Thacker PS, Korra LN, Biswas R, Arifuddin M, Supuran CT. Design, Synthesis, and Biological Evaluation of 1,2,3-Triazole-linked triazino[5,6-b]indole-benzene sulfonamide Conjugates as Potent Carbonic Anhydrase I, II, IX, and XIII Inhibitors. Metabolites. 2020; 10(5):200. https://doi.org/10.3390/metabo10050200
Chicago/Turabian StyleChinchilli, Krishna Kartheek, Andrea Angeli, Pavitra S. Thacker, Laxman Naik Korra, Rashmita Biswas, Mohammed Arifuddin, and Claudiu T. Supuran. 2020. "Design, Synthesis, and Biological Evaluation of 1,2,3-Triazole-linked triazino[5,6-b]indole-benzene sulfonamide Conjugates as Potent Carbonic Anhydrase I, II, IX, and XIII Inhibitors" Metabolites 10, no. 5: 200. https://doi.org/10.3390/metabo10050200
APA StyleChinchilli, K. K., Angeli, A., Thacker, P. S., Korra, L. N., Biswas, R., Arifuddin, M., & Supuran, C. T. (2020). Design, Synthesis, and Biological Evaluation of 1,2,3-Triazole-linked triazino[5,6-b]indole-benzene sulfonamide Conjugates as Potent Carbonic Anhydrase I, II, IX, and XIII Inhibitors. Metabolites, 10(5), 200. https://doi.org/10.3390/metabo10050200