GC-MS Based Metabolite Profiling to Monitor Ripening-Specific Metabolites in Pineapple (Ananas comosus)
Abstract
1. Introduction
2. Results
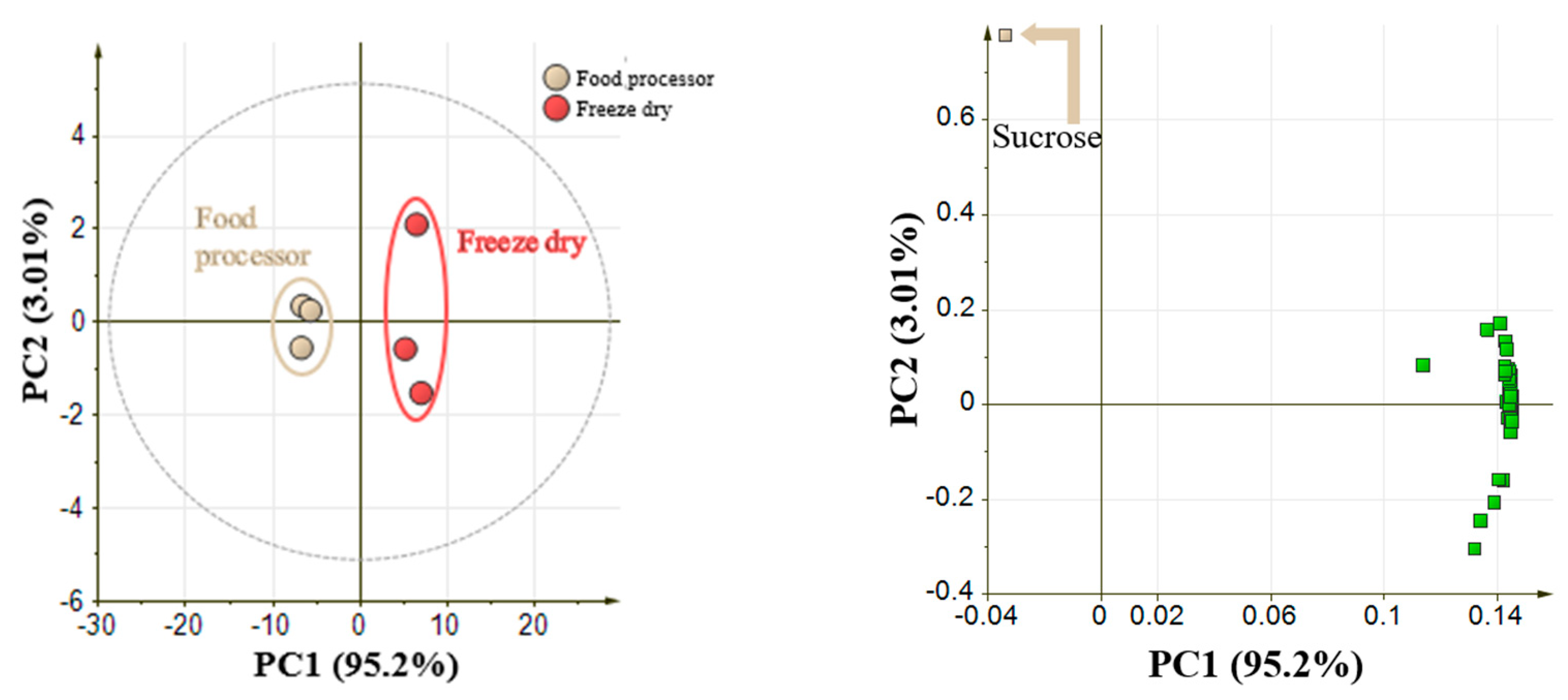
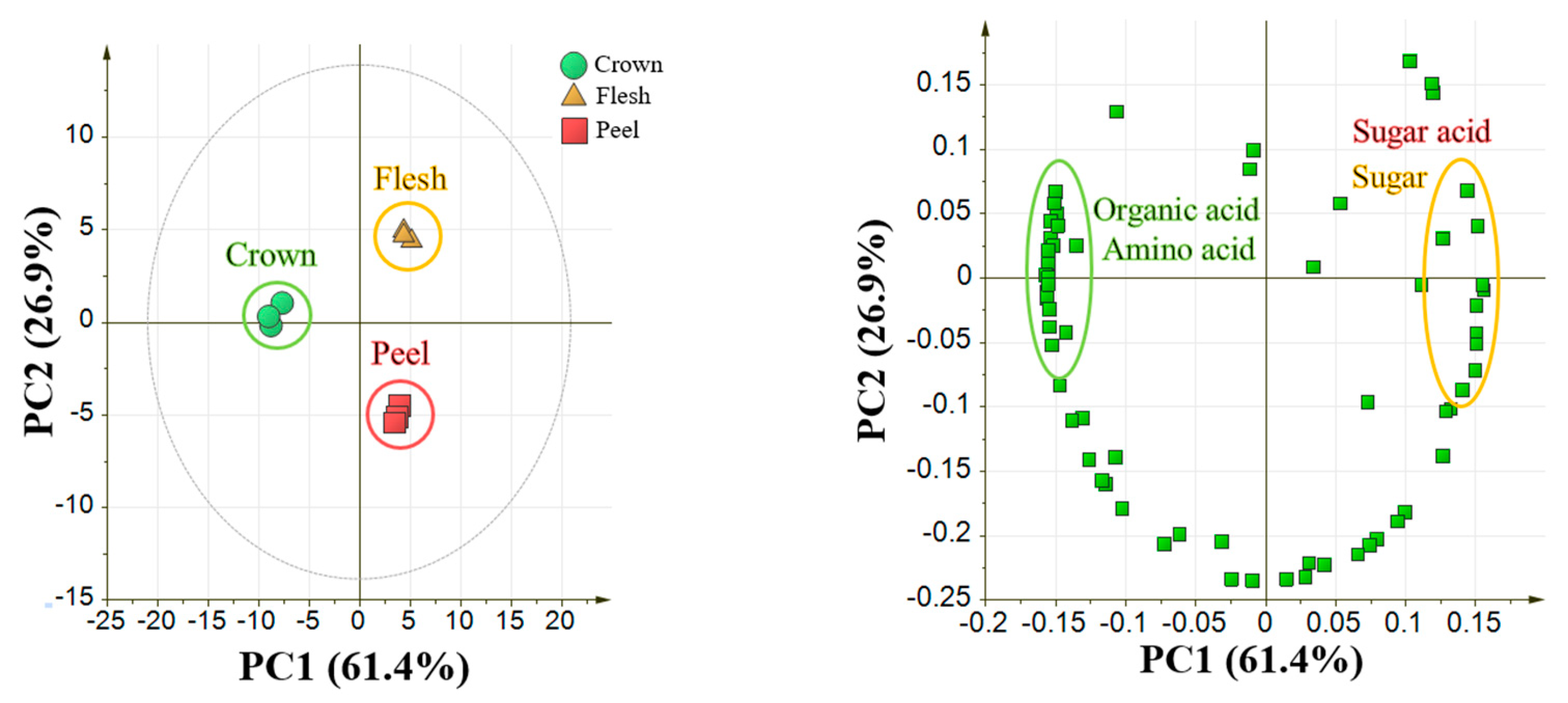
2.1. Optimization of Sample Preparation Methods in Pineapple Fruit
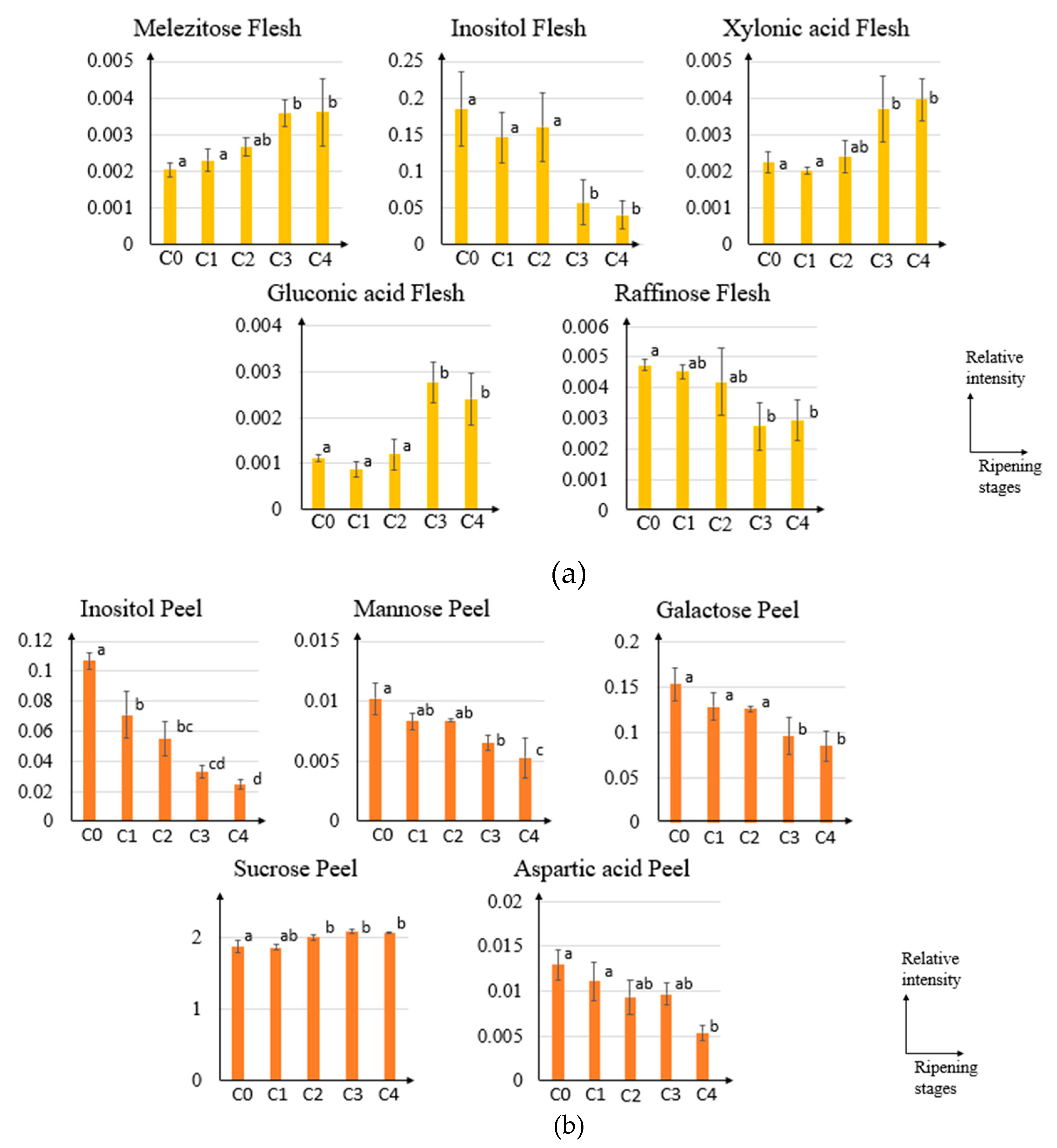
2.2. GC-MS and Principal Component Analysis of Pineapple from Different Ripening Stages
2.3. Orthogonal Projection to Latent Structures of Pineapple Ripening Process
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Optimization of Sample Preparation
4.2.1. Comparison between Food Processor and Freeze-Drying Methods
4.2.2. Comparison of Crown, Flesh, and Peel of Pineapple Fruit
4.3. Sample Preparation and Extraction of Pineapple from Different Ripening Stages
4.4. GC-MS Analysis
4.5. GC-MS Data Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mulderij, R. Overview Global Pineapple Market. Available online: https://www.freshplaza.com/article/2194086/overview-global-pineapple-market/ (accessed on 28 June 2019).
- Hui, Y.H.; Chen, F.; Nollet, L.M.L.; Guiné, R.P.F.; Martín-Belloso, O.; Mínguez-Mosquera, M.I.; Paliyath, G.; Pessoa, F.L.P.; Le Quéré, J.-L.; Sidhu, J.S. Handbook of Fruit and Vegetable Flavors; Wiley Online Library: Hoboken, NJ, USA, 2010; Volume 64. [Google Scholar]
- Kader, A.A. Fruit Maturity, Ripening, and Quality Relationship. In Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 1999; pp. 203–208. [Google Scholar]
- Symons, G.M.; Chua, Y.-J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal Changes during Non-Climacteric Ripening in Strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Agricultural Standards Unit United Nations Economic Commission for Europe. Standard on the Marketing and Commercial Quality Control of Pineapples—Explanatory Brochure; UNECE: Geneva, Switzerland, 2013. [Google Scholar]
- Ahmad, M.S.; Siddiqui, M.W. Postharvest Quality Assurance of Fruits; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Medina, J.D.L.C.; García, H.S. Post-Harvest Operations; Food and Agriculture Organization of the United Nations: Roma, Italy, 2005. [Google Scholar]
- Kamol, S.; Howlader, J.; Dhar, G.S.; Aklimuzzaman, M. Effect of Different Stages of Maturity and Postharvest Treatments on Quality and Storability of Pineapple. J. Bangladesh Agric. Univ. 2016, 12, 251–260. [Google Scholar] [CrossRef]
- Yun, Z.; Li, T.; Gao, H.; Zhu, H.; Gupta, V.K.; Jiang, Y.; Duan, X. Integrated Transcriptomic, Proteomic, and Metabolomics Analysis Reveals Peel Ripening of Harvested Banana under Natural Condition. Biomolecules 2019, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- White, I.R.; Blake, R.S.; Taylor, A.J.; Monks, P.S. Metabolite Profiling of the Ripening of Mangoes Mangifera Indica, L. Cv. ‘Tommy Atkins’ by Real-Time Measurement of Volatile Organic Compounds. Metabolomics 2016, 12, 1–11. [Google Scholar] [CrossRef]
- Aizat, W.M.; Dias, D.A.; Stangoulis, J.C.R.; Able, J.A.; Roessner, U.; Able, A.J. Metabolomics of Capsicum Ripening Reveals Modification of the Ethylene Related-Pathway and Carbon Metabolism. Postharvest Biol. Technol. 2014, 89, 19–31. [Google Scholar] [CrossRef]
- Diboun, I.; Mathew, S.; Al-Rayyashi, M.; Elrayess, M.; Torres, M.; Halama, A.; Méret, M.; Mohney, R.P.; Karoly, E.D.; Malek, J.; et al. Metabolomics of Dates (Phoenix Dactylifera) Reveals a Highly Dynamic Ripening Process Accounting for Major Variation in Fruit Composition. BMC Plant Biol. 2015, 15, 291. [Google Scholar] [CrossRef]
- Pedreschi, R.; Munoz, P.; Robledo, P.; Becerra, C.; Defilippi, B.G.; van Eekelen, H.D.L.M.; Mumm, R.; Westra, E.H.; de Vos, R.C.H. Metabolomics Analysis of Postharvest Ripening Heterogeneity of ‘Hass’ Avocadoes. Postharvest Biol. Technol. 2014, 92, 172–179. [Google Scholar] [CrossRef]
- Monti, L.L.; Bustamante, C.A.; Osorio, S.; Gabilondo, J.; Borsani, J.; Lauxmann, M.A.; Maulion, E.; Valentini, G.; Budde, C.O.; Fernie, A.R.; et al. Metabolic Profiling of a Range of Peach Fruit Varieties Reveals High Metabolic Diversity and Commonalities and Differences during Ripening. Food Chem. 2016, 190, 879–888. [Google Scholar] [CrossRef]
- Allwood, J.W.; Cheung, W.; Xu, Y.; Mumm, R.; De Vos, R.C.H.; Deborde, C.; Biais, B.; Maucourt, M.; Berger, Y.; Schaffer, A.A.; et al. Metabolomics in Melon: A New Opportunity for Aroma Analysis. Phytochemistry 2014, 99, 61–72. [Google Scholar] [CrossRef]
- Parijadi, A.A.R.; Putri, S.P.; Ridwani, S.; Dwivany, F.M.; Fukusaki, E. Metabolic Profiling of Garcinia Mangostana (Mangosteen) Based on Ripening Stages. J. Biosci. Bioeng. 2018, 125, 238–244. [Google Scholar] [CrossRef]
- Karagiannis, E.; Michailidis, M.; Karamanoli, K.; Lazaridou, A.; Minas, I.S.; Molassiotis, A. Postharvest Responses of Sweet Cherry Fruit and Stem Tissues Revealed by Metabolomic Profiling. Plant Physiol. Biochem. 2018, 127, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Jarret, D.A.; Morris, J.; Cullen, D.W.; Gordon, S.L.; Verrall, S.R.; Milne, L.; Hedley, P.E.; Allwood, J.W.; Brennan, R.M.; Hancock, R.D. A Transcript and Metabolite Atlas of Blackcurrant Fruit Development Highlights Hormonal Regulation and Reveals the Role of Key Transcription Factors. Front. Plant Sci. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Montecchiarini, M.L.; Margarit, E.; Morales, L.; Rivadeneira, M.F.; Bello, F.; Gollán, A.; Vázquez, D.; Podestá, F.E.; Tripodi, K.E.J. Proteomic and Metabolomic Approaches Unveil Relevant Biochemical Changes in Carbohydrate and Cell Wall Metabolisms of Two Blueberry (Vaccinium Corymbosum) Varieties with Different Quality Attributes. Plant Physiol. Biochem. 2019, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Dell, C.; Lieb, V.; Mayer-Ullmann, B.; Czerny, M.; Carle, R. Assignment of Distinctive Volatiles, Descriptive Sensory Analysis and Consumer Preference of Differently Ripened and Post-Harvest Handled Pineapple (Ananas Comosus [L.] Merr.) Fruits. Eur. Food Res. Technol. 2016, 242, 33–43. [Google Scholar] [CrossRef]
- Ogawa, E.M.; Costa, H.B.; Ventura, J.A.; Caetano, L.C.S.; Pinto, F.E.; Oliveira, B.G.; Barroso, M.E.S.; Scherer, R.; Endringer, D.C.; Romão, W. Chemical Profile of Pineapple Cv. Vitória in Different Maturation Stages Using Electrospray Ionization Mass Spectrometry. J. Sci. Food Agric. 2018, 98, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Steingass, C.B.; Grauwet, T.; Carle, R. Influence of Harvest Maturity and Fruit Logistics on Pineapple (Ananas Comosus [L.] Merr.) Volatiles Assessed by Headspace Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry (HS-SPME-GC/MS). Food Chem. 2014, 150, 382–391. [Google Scholar] [CrossRef]
- Steingass, C.B.; Jutzi, M.; Müller, J.; Carle, R.; Schmarr, H.G. Ripening-Dependent Metabolic Changes in the Volatiles of Pineapple (Ananas Comosus (L.) Merr.) Fruit: II. Multivariate Statistical Profiling of Pineapple Aroma Compounds Based on Comprehensive Two-Dimensional Gas Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 2609–2624. [Google Scholar] [CrossRef]
- Steingass, C.B.; Glock, M.P.; Schweiggert, R.M.; Carle, R. Studies into the Phenolic Patterns of Different Tissues of Pineapple (Ananas Comosus [L.] Merr.) Infructescence by HPLC-DAD-ESI-MS (n) and GC-MS Analysis. Anal. Bioanal. Chem. 2015, 407, 6463–6479. [Google Scholar] [CrossRef]
- Werth, M.T.; Halouska, S.; Shortridge, M.D.; Zhang, B.; Powers, R. Analysis of Metabolomic PCA Data Using Tree Diagrams. Anal. Biochem. 2010, 399, 58–63. [Google Scholar] [CrossRef]
- Teoh, S.T.; Putri, S.; Mukai, Y.; Bamba, T.; Fukusaki, E. A Metabolomics-Based Strategy for Identification of Gene Targets for Phenotype Improvement and Its Application to 1-Butanol Tolerance in Saccharomyces Cerevisiae. Biotechnol. Biofuels 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.; Zhao, Y.; Zha, L.; Yang, H.; Wu, Y. GC–MS-Based Nontargeted and Targeted Metabolic Profiling Identifies Changes in the Lentinula Edodes Mycelial Metabolome under High-Temperature Stress. Int. J. Mol. Sci. 2019, 20, 2330. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current Approaches and Challenges for the Metabolite Profiling of Complex Natural Extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.J.; Haselden, J.N. Metabolic Profiling as a Tool for Understanding Mechanisms of Toxicity. Toxicol. Pathol. 2008, 36, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hanifah, A.; Maharijaya, A.; Putri, S.P.; Laviña, W.A.; Sobir. Untargeted Metabolomics Analysis of Eggplant (Solanum Melongena, L.) Fruit and Its Correlation to Fruit Morphologies. Metabolites 2018, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Papadimitropoulos, M.-E.P.; Vasilopoulou, C.G.; Maga-Nteve, C.; Klapa, M.I. Untargeted GC-MS Metabolomics. Methods Mol. Biol. 2018, 1738, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, I.T.; Cadima, J.; Cadima, J. Principal Component Analysis: A Review and Recent Developments Subject Areas. Phil. Trans. R. Soc. A 2016, 374, 1–16. [Google Scholar] [CrossRef]
- Sanewski, G.M.; Bartholomew, D.P.; Paull, R.E. The Pineapple: Botany, Production and Uses, 2nd ed.; CABI: Wallingford, UK, 2018. [Google Scholar]
- Joy, P.P.; Rejuva TA, R. Harvesting and Post-Harvest Handling of Pineapple; Pineapple Research Station: Kerala, India, 2016. [Google Scholar]
- Alexander, D.L.J.; Tropsha, A.; Winkler, D.A. Beware of R(2): Simple, Unambiguous Assessment of the Prediction Accuracy of QSAR and QSPR Models. J. Chem. Inf. Model. 2015, 55, 1316–1322. [Google Scholar] [CrossRef]
- Fausto Rivero-Cruz, J.; Rodríguez de San Miguel, E.; Robles-Obregón, S.; Hernández-Espino, C.C.; Rivero-Cruz, B.E.; Pedraza-Chaverri, J.; Esturau-Escofet, N. Prediction of Antimicrobial and Antioxidant Activities of Mexican Propolis by 1H-NMR Spectroscopy and Chemometrics Data Analysis. Molecules 2017, 22, 1184. [Google Scholar] [CrossRef]
- Farcuh, M.; Li, B.; Rivero, R.M.; Shlizerman, L.; Sadka, A.; Blumwald, E. Sugar Metabolism Reprogramming in a Non-Climacteric Bud Mutant of a Climacteric Plum Fruit during Development on the Tree. J. Exp. Bot. 2017, 68, 5813–5828. [Google Scholar] [CrossRef]
- Siddique, S.; Endres, S.; Sobczak, M.; Radakovic, Z.S.; Fragner, L.; Grundler, F.M.W.; Weckwerth, W.; Tenhaken, R.; Bohlmann, H. Myo-Inositol Oxygenase Is Important for the Removal of Excess Myo-Inositol from Syncytia Induced by Heterodera Schachtii in Arabidopsis Roots. New Phytol. 2014, 201, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, A.R.; Parra, J.R.P. Insect Bioecology and Nutrition for Integrated Pest Management; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Lin, Y.-C.; Liu, Y.; Nakamura, Y. The Choline/Ethanolamine Kinase Family in Arabidopsis: Essential Role of CEK4 in Phospholipid Biosynthesis and Embryo Development. Plant Cell 2015, 27, 1497–1511. [Google Scholar] [CrossRef] [PubMed]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Musara, A. Determination of Ascrobic Acid in Some Citrus Fruits by Using Pencil Graphite Electrode. Ph.D. Thesis, Near East University, Nicosia, Turky, 2019. [Google Scholar]
- Paliyath, G.; Subramanian, J.; Lim, L.-T.; Subramanian, K.S.; Handa, A.K.; Matto, A.K. Postharvest Biology and Nanotechnology; New York Academy of Sciences: New York, NY, USA, 2019. [Google Scholar]
- Alkan, N.; Fortes, A.M. Insights into Molecular and Metabolic Events Associated with Fruit Response to Post-Harvest Fungal Pathogens. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Davidzon, M.; Alkan, N.; Kobiler, I.; Prusky, D. Acidification by Gluconic Acid of Mango Fruit Tissue during Colonization via Stem End Infection by Phomopsis Mangiferae. Postharvest Biol. Technol. 2010, 55, 71–77. [Google Scholar] [CrossRef]
- Moirangthem, K.; Tucker, G. How Do Fruits Ripen? Front. Young Minds 2018, 6, 1–7. [Google Scholar] [CrossRef]
- Dheilly, E.; Gall, S.L.; Guillou, M.-C.; Renou, J.-P.; Bonnin, E.; Orsel, M.; Lahaye, M. Cell Wall Dynamics during Apple Development and Storage Involves Hemicellulose Modifications and Related Expressed Genes. BMC Plant Biol. 2016, 16, 201. [Google Scholar] [CrossRef]
- Wu, Q.; Li, T.; Chen, X.; Wen, L.; Yun, Z.; Jiang, Y. Sodium Dichloroisocyanurate Delays Ripening and Senescence of Banana Fruit during Storage. Chem. Cent. J. 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Zgola-Grześkowiak, A.; Grześkowiak, T. Determination of Glutamic Acid and Aspartic Acid in Tomato Juice by Capillary Isotachophoresis. Int. J. Food Prop. 2012, 15, 628–637. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of Glutamate and Aspartate by Ultra-High Performance Liquid Chromatography. Molecules 2018, 23, 1389. [Google Scholar] [CrossRef]
- Folharini, Z.F.; Orlandi, C.R.; Martini, M.C.; Bruxel, F.; Altmayer, T.; Brietzke, D.T.; Hoehne, L. Nutritional Characterization of Vasconcellea Quercifolia, A.St-Hil.: Potential for the Development of Functional Food. Food Sci. Technol. 2019, 2061, 432–438. [Google Scholar] [CrossRef]
- Bernales, M.; Monsalve, L.; Ayala-Raso, A.; Valdenegro, M.; Martínez, J.P.; Travisany, D.; Defilippi, B.; González-Agüero, M.; Cherian, S.; Fuentes, L. Expression of Two Indole-3-Acetic Acid (IAA)-Amido Synthetase (GH3) Genes during Fruit Development of Raspberry (Rubus Idaeus Heritage). Sci. Hortic. 2019, 246, 168–175. [Google Scholar] [CrossRef]
- Iatridi, V.; Hayes, J.E.; Yeomans, M.R. Quantifying Sweet Taste Liker Phenotypes: Time for Some Consistency in the Classification Criteria. Nutrients 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Siti Roha, A.M.; Zainal, S.; Noriham, A.; Nadzirah, K.Z. Determination of Sugar Content in Pineapple Waste Variety N36. Int. Food Res. J. 2013, 20, 1941–1943. [Google Scholar]
- Jia, H.; Wang, Y.; Sun, M.; Li, B.; Han, Y.; Zhao, Y.; Li, X.; Ding, N.; Li, C.; Ji, W.; et al. Sucrose Functions as a Signal Involved in the Regulation of Strawberry Fruit Development and Ripening. New Phytol. 2013, 198, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Figueroa, C.R.; Valdenegro, M. Recent Advances in Hormonal Regulation and Cross-Talk during Non-Climacteric Fruit Development and Ripening. Horticulturae 2019, 5. [Google Scholar] [CrossRef]
- O’Donoghue, E.M.; Somerfield, S.D.; Watson, L.M.; Brummell, D.A.; Hunter, D.A. Galactose Metabolism in Cell Walls of Opening and Senescing Petunia Petals. Planta 2009, 229, 709–721. [Google Scholar] [CrossRef]
- Ogasawara, S.; Abe, K.; Nakajima, T. Pepper Beta-Galactosidase 1 (PBG1) Plays a Significant Role in Fruit Ripening in Bell Pepper (Capsicum Annuum). Biosci. Biotechnol. Biochem. 2007, 71, 309–322. [Google Scholar] [CrossRef]
- Osorio, S.; Scossa, F.; Fernie, A.R. Molecular Regulation of Fruit Ripening. Front. Plant Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Kumar, V.; Irfan, M.; Ghosh, S.; Chakraborty, N.; Chakraborty, S.; Datta, A. Fruit Ripening Mutants Reveal Cell Metabolism and Redox State during Ripening. Protoplasma 2016, 253, 581–594. [Google Scholar] [CrossRef]
- Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory Effects of Exogenously Applied Polyamines on Postharvest Physiology, Antioxidant System and Shelf Life of Fruits: A Review. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Golly, M.K.; Ma, H.; Sarpong, F.; Dotse, B.P.; Oteng-Darko, P.; Dong, Y. Shelf-Life Extension of Grape (Pinot Noir) by Xanthan Gum Enriched with Ascorbic and Citric Acid during Cold Temperature Storage. J. Food Sci. Technol. 2019, 56, 4867–4878. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Gao, H.; Liu, P.; Liu, S.; Luo, T.; Jin, S.; Xu, Q.; Xu, J.; Cheng, Y.; Deng, X. Comparative Proteomic and Metabolomic Profiling of Citrus Fruit with Enhancement of Disease Resistance by Postharvest Heat Treatment. BMC Plant Biol. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, C.A.; Monti, L.L.; Gabilondo, J.; Scossa, F.; Valentini, G.; Budde, C.O.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Differential Metabolic Rearrangements after Cold Storage Are Correlated with Chilling Injury Resistance of Peach Fruits. Front. Plant Sci. 2016, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Anjaritha, A.A.; Ridwani, S.; Dwivany, F.M.; Putri, S.P.; Fukusaki, E. A Metabolomics-Based Approach for the Evaluation of off-Tree Ripening Conditions and Different Postharvest Treatments in Mangosteen (Garcinia Mangostana). Metabolomics 2019, 15, 1–16. [Google Scholar] [CrossRef]
- Geladi, P.; Kowalski, B.R. Partial Least-Squares Regression: A Tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Nitta, K.; Laviña, W.A.; Pontrelli, S.; Liao, J.C.; Putri, S.P.; Fukusaki, E. Orthogonal Partial Least Squares / Projections to Latent Structures Regression-Based Metabolomics Approach for Identi Fi Cation of Gene Targets for Improvement of 1-Butanol Production in Escherichia Coli. J. Biosci. Bioeng. 2017, 124, 498–505. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikram, M.M.M.; Ridwani, S.; Putri, S.P.; Fukusaki, E. GC-MS Based Metabolite Profiling to Monitor Ripening-Specific Metabolites in Pineapple (Ananas comosus). Metabolites 2020, 10, 134. https://doi.org/10.3390/metabo10040134
Ikram MMM, Ridwani S, Putri SP, Fukusaki E. GC-MS Based Metabolite Profiling to Monitor Ripening-Specific Metabolites in Pineapple (Ananas comosus). Metabolites. 2020; 10(4):134. https://doi.org/10.3390/metabo10040134
Chicago/Turabian StyleIkram, Muhammad Maulana Malikul, Sobir Ridwani, Sastia Prama Putri, and Eiichiro Fukusaki. 2020. "GC-MS Based Metabolite Profiling to Monitor Ripening-Specific Metabolites in Pineapple (Ananas comosus)" Metabolites 10, no. 4: 134. https://doi.org/10.3390/metabo10040134
APA StyleIkram, M. M. M., Ridwani, S., Putri, S. P., & Fukusaki, E. (2020). GC-MS Based Metabolite Profiling to Monitor Ripening-Specific Metabolites in Pineapple (Ananas comosus). Metabolites, 10(4), 134. https://doi.org/10.3390/metabo10040134






