Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients—Comparison of Serum Metabolome Profiles
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Characteristics of the Patient Group
4.2. Material Collection
4.3. LC-MS Targeted Metabolomics
4.4. Analysis of Selected Amino Acids and Their Derivatives
4.5. Albumin and 3-Hydroxybutyrate Detection
4.6. Statistical and Bioinformatic Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Orth, M.; Lauber, K.; Niyazi, M.; Friedl, A.A.; Li, M.; Maihofer, C.; Schuttrumpf, L.; Ernst, A.; Niemoller, O.M.; Belka, C. Current concepts in clinical radiation oncology. Radiat. Environ. Biophys. 2014, 53, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Update on role of chemotherapy in head and neck squamous cell cancer. Indian J. Surg. Oncol. 2010, 1, 85–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yeh, S.-A. Radiotherapy for head and neck cancer. Semin. Plast. Surg. 2010, 24, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J.L.; Serkova, N.J.; Eckhardt, S.G. Clinical applications of metabolomics in oncology: A review. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 431–440. [Google Scholar] [CrossRef]
- Laiakis, E.C.; Mak, T.D.; Anizan, S.; Amundson, S.A.; Barker, C.A.; Wolden, S.L.; Brenner, D.J.; Fornace, A.J., Jr. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat. Res. 2014, 181, 350–361. [Google Scholar] [CrossRef]
- Patel, R.M.; Roback, J.D.; Uppal, K.; Yu, T.; Jones, D.P.; Josephson, C.D. Metabolomics profile comparisons of irradiated and nonirradiated stored donor red blood cells. Transfusion 2015, 55, 544–552. [Google Scholar] [CrossRef]
- Jelonek, K.; Pietrowska, M.; Ros, M.; Zagdanski, A.; Suchwalko, A.; Polanska, J.; Marczyk, M.; Rutkowski, T.; Skladowski, K.; Clench, M.R.; et al. Radiation-induced changes in serum lipidome of head and neck cancer patients. Int. J. Mol. Sci. 2014, 15, 6609–6624. [Google Scholar] [CrossRef]
- Ros-Mazurczyk, M.; Wojakowska, A.; Marczak, L.; Polanski, K.; Pietrowska, M.; Jelonek, K.; Dominczyk, I.; Hajduk, A.; Rutkowski, T.; Skladowski, K.; et al. Ionizing radiation affects profile of serum metabolites: Increased level of 3-hydroxybutyric acid in serum of cancer patients treated with radiotherapy. Acta Biochim. Pol. 2017, 64, 189–193. [Google Scholar]
- Jelonek, K.; Pietrowska, M.; Widlak, P. Systemic effects of ionizing radiation at the proteome and metabolome levels in the blood of cancer patients treated with radiotherapy: The influence of inflammation and radiation toxicity. Int. J. Radiat. Biol. 2017, 93, 683–696. [Google Scholar] [CrossRef]
- Jobard, E.; Blanc, E.; Negrier, S.; Escudier, B.; Gravis, G.; Chevreau, C.; Elena-Herrmann, B.; Tredan, O. A serum metabolomic fingerprint of bevacizumab and temsirolimus combination as first-line treatment of metastatic renal cell carcinoma. Br. J. Cancer 2015, 113, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. Mcr 2005, 3, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sathawane, R.; Mody, R. Correlation of radiotherapy with serum total and lipid-bound sialic acid in oscc patients. J. Indian Acad. Oral Med. Radiol. 2014, 26, 2–7. [Google Scholar] [CrossRef]
- Shaikh, S.; Channa, N.A.; Talpur, F.N.; Younis, M.; Tabassum, N. Radiotherapy improves serum fatty acids and lipid profile in breast cancer. Lipids Health Dis. 2017, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Widlak, P.; Jelonek, K.; Wojakowska, A.; Pietrowska, M.; Polanska, J.; Marczak, L.; Miszczyk, L.; Skladowski, K. Serum proteome signature of radiation response: Upregulation of inflammation-related factors and downregulation of apolipoproteins and coagulation factors in cancer patients treated with radiation therapy—A pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1108–1115. [Google Scholar] [CrossRef]
- Boguszewicz, L.; Bielen, A.; Mrochem-Kwarciak, J.; Skorupa, A.; Ciszek, M.; Heyda, A.; Wygoda, A.; Kotylak, A.; Skladowski, K.; Sokol, M. Nmr-based metabolomics in real-time monitoring of treatment induced toxicity and cachexia in head and neck cancer: A method for early detection of high risk patients. Metabolomics 2019, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Couch, M.E.; Dittus, K.; Toth, M.J.; Willis, M.S.; Guttridge, D.C.; George, J.R.; Barnes, C.A.; Gourin, C.G.; Der-Torossian, H. Cancer cachexia update in head and neck cancer: Definitions and diagnostic features. Head Neck 2015, 37, 594–604. [Google Scholar] [CrossRef]
- Ritchie, R.F.; Palomaki, G.E.; Neveux, L.M.; Navolotskaia, O.; Ledue, T.B.; Craig, W.Y. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: A practical, simple and clinically relevant approach in a large cohort. J. Clin. Lab. Anal. 1999, 13, 273–279. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, G.; Aitken, D.; Likhodii, S.; Liu, M.; Martin, G.; Furey, A.; Randell, E.; Rahman, P.; Jones, G.; et al. Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology (Oxf. Engl.) 2016, 55, 1566–1574. [Google Scholar] [CrossRef]
- Laiakis, E.C.; Canadell, M.P.; Grilj, V.; Harken, A.D.; Garty, G.Y.; Astarita, G.; Brenner, D.J.; Smilenov, L.; Fornace, A.J., Jr. Serum lipidomic analysis from mixed neutron/x-ray radiation fields reveals a hyperlipidemic and pro-inflammatory phenotype. Sci. Rep. 2019, 9, 4539. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The underconsumed and underappreciated essential nutrient. Nutr. Today 2018, 53, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Jackowski, S. Phosphatidylcholine and the cdp-choline cycle. Biochim. Biophys. Acta 2013, 1831, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Sonis, S.T.; Peterson, D.E. Management of oral mucositis in patients who have cancer. Dent. Clin. N. Am. 2008, 52, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.; Denaro, N.; Russi, E.G.; Pizzorni, N.; Bossi, P.; Merlotti, A.; Spadola Bissetti, M.; Numico, G.; Gava, A.; Orlandi, E.; et al. Dysphagia in head and neck cancer patients treated with radiotherapy and systemic therapies: Literature review and consensus. Crit. Rev. Oncol. Hematol. 2015, 96, 372–384. [Google Scholar] [CrossRef] [PubMed]
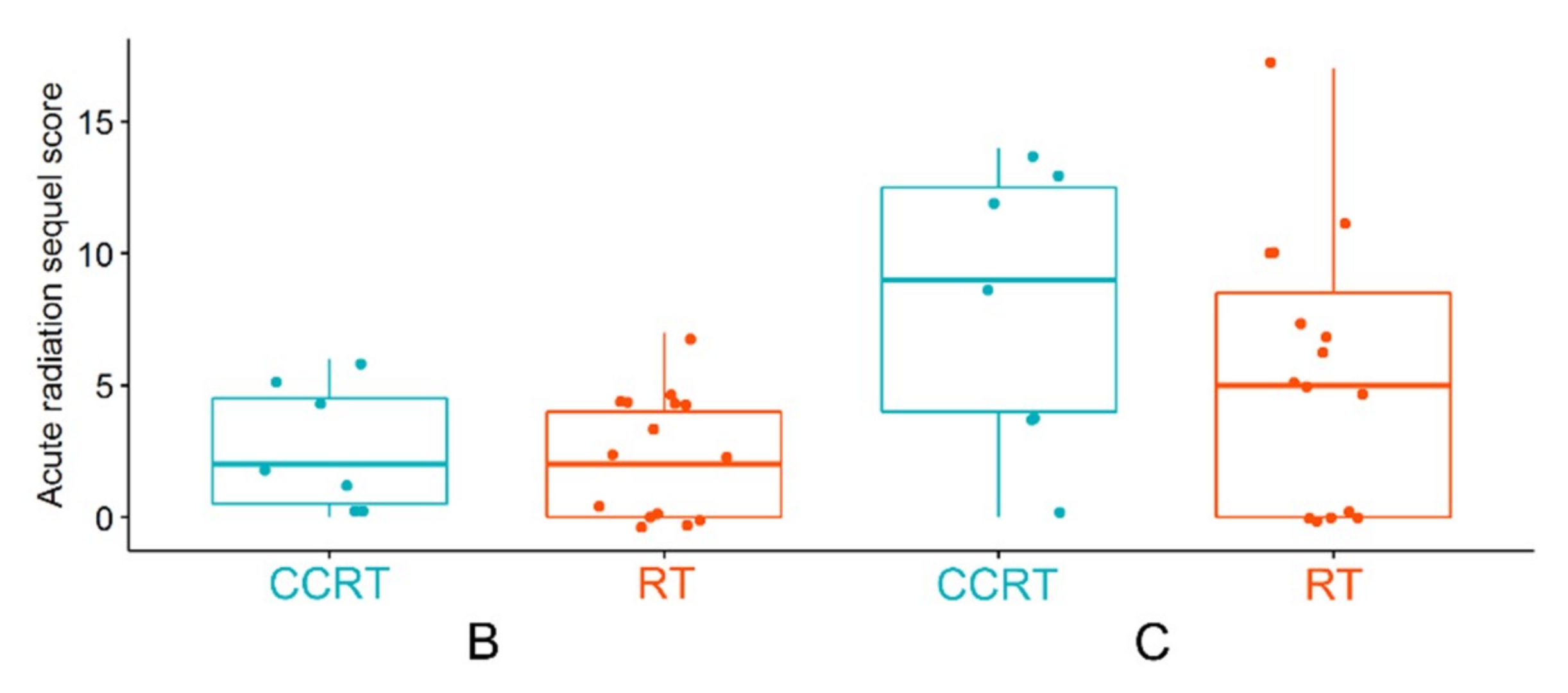
- Hajduk, A.; Składowski, K.; Boguszewicz, Ł.; Mrochem-Kwarciak, J.; Hutnik, M.; Lukaszczyk-Wideł, B.; Rutkowski, T.; Wygoda, A.; Przeorek, W.; Golen, M.; et al. Acute Radiaton Sequel evaluaton in head and neck cancer patients. A new concept of comprehensive scoring system—multiparametric monitoring. Eur. Arch. Otorhinolaryngol. 2012, 269, 1311. [Google Scholar]
- Olkowicz, M.; Debski, J.; Jablonska, P.; Dadlez, M.; Smolenski, R.T. Application of a new procedure for liquid chromatography/mass spectrometry profiling of plasma amino acid-related metabolites and untargeted shotgun proteomics to identify mechanisms and biomarkers of calcific aortic stenosis. J. Chromatogr. A 2017, 1517, 66–78. [Google Scholar] [CrossRef] [PubMed]
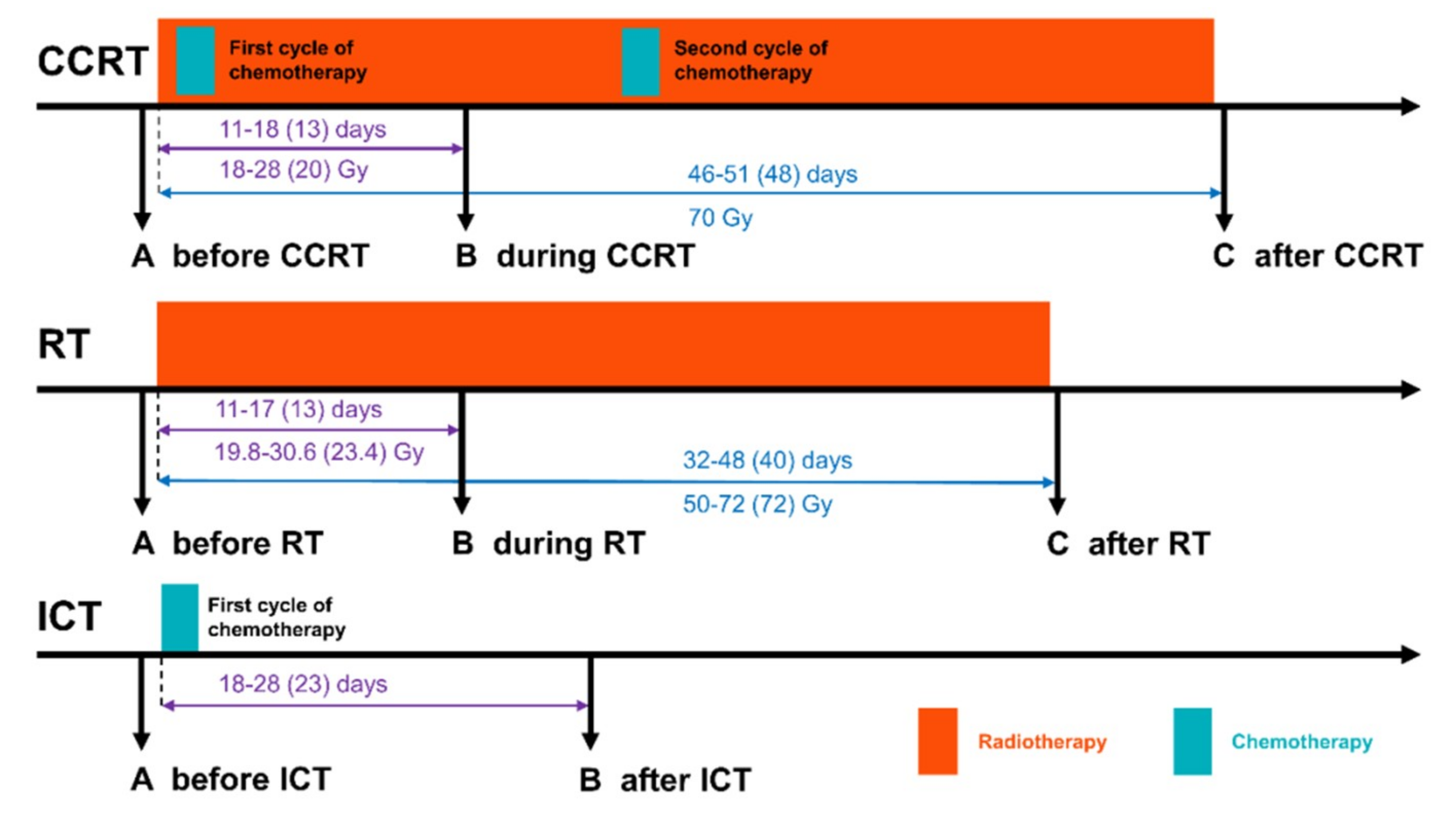
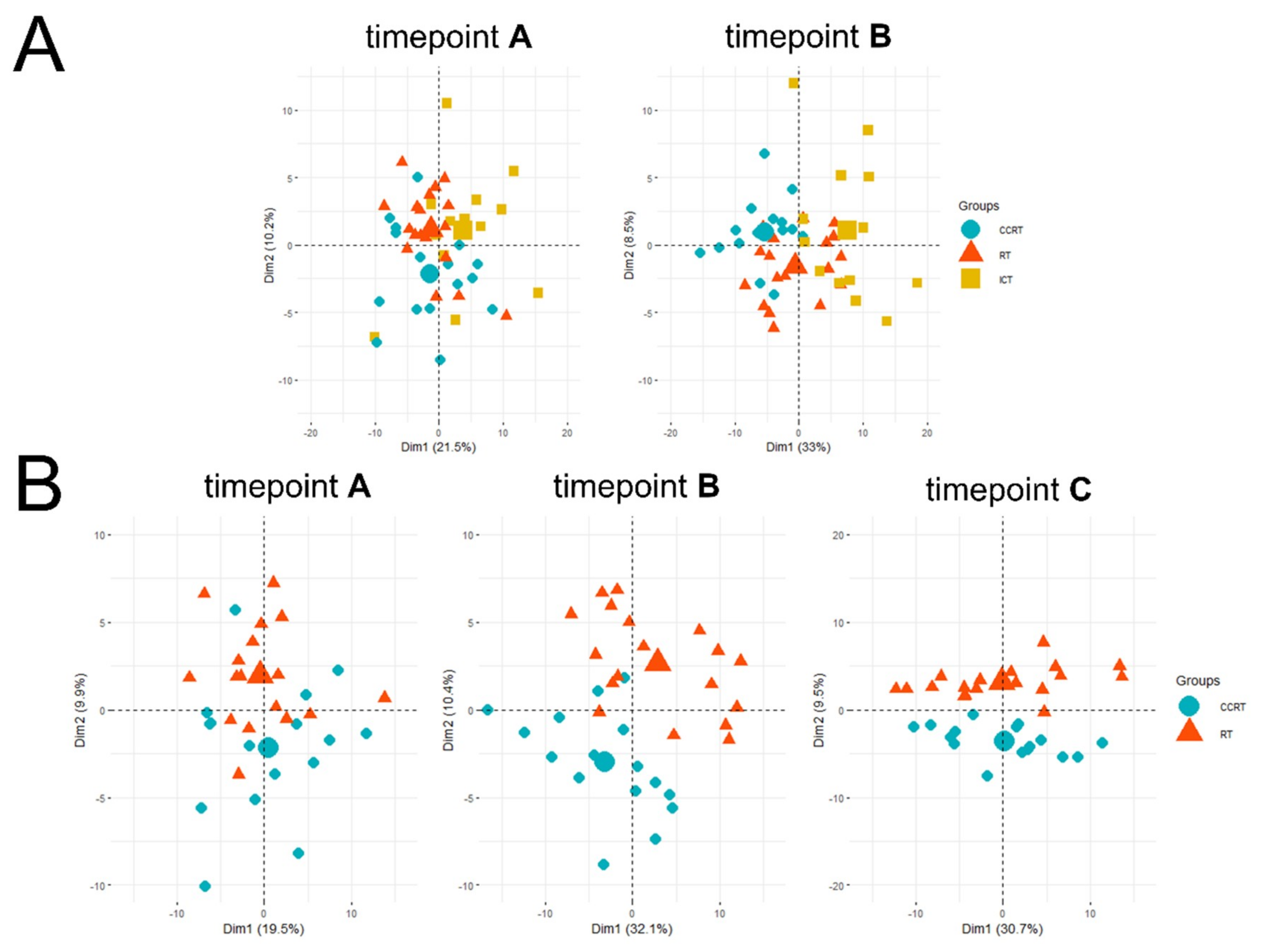
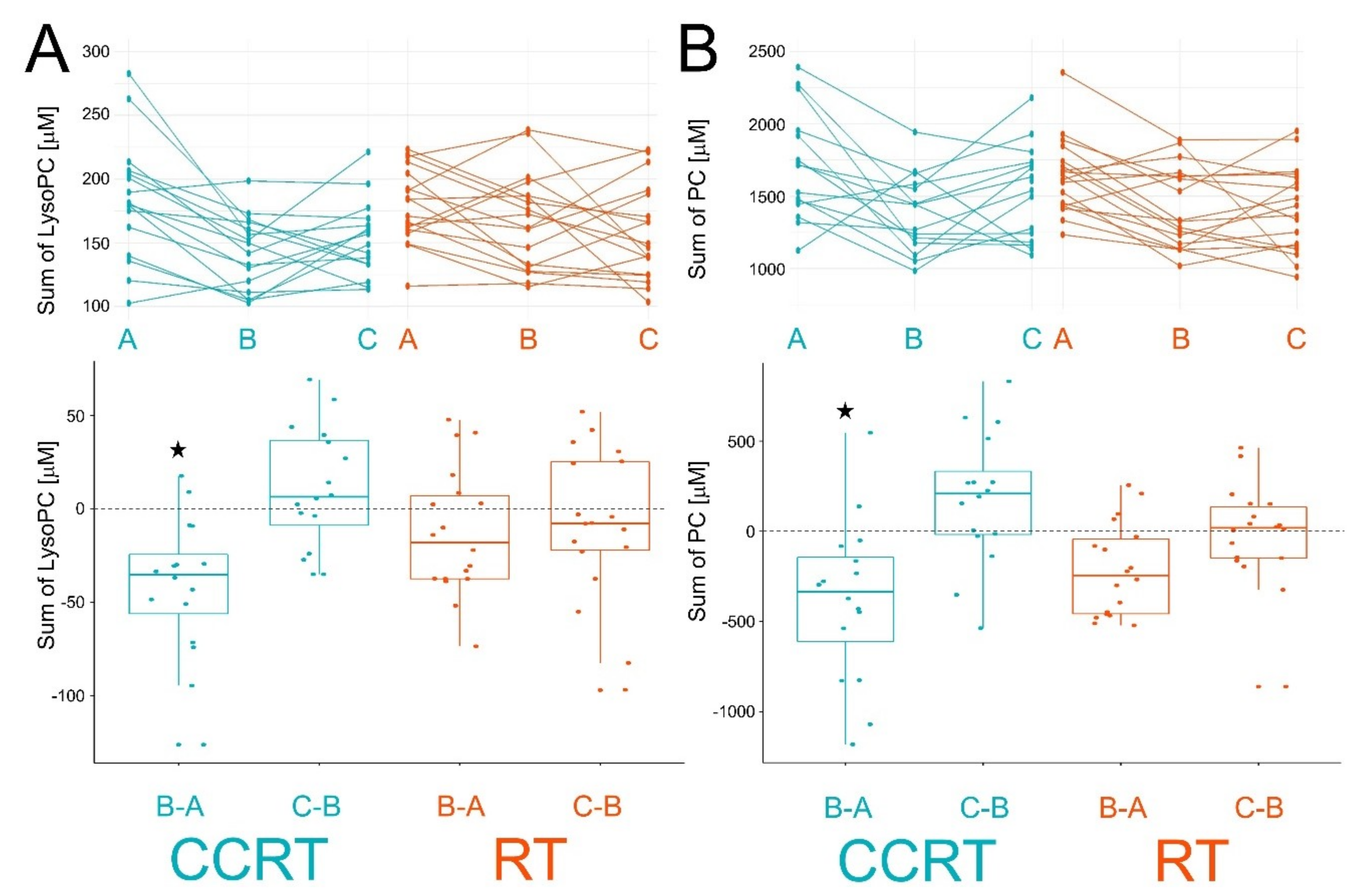
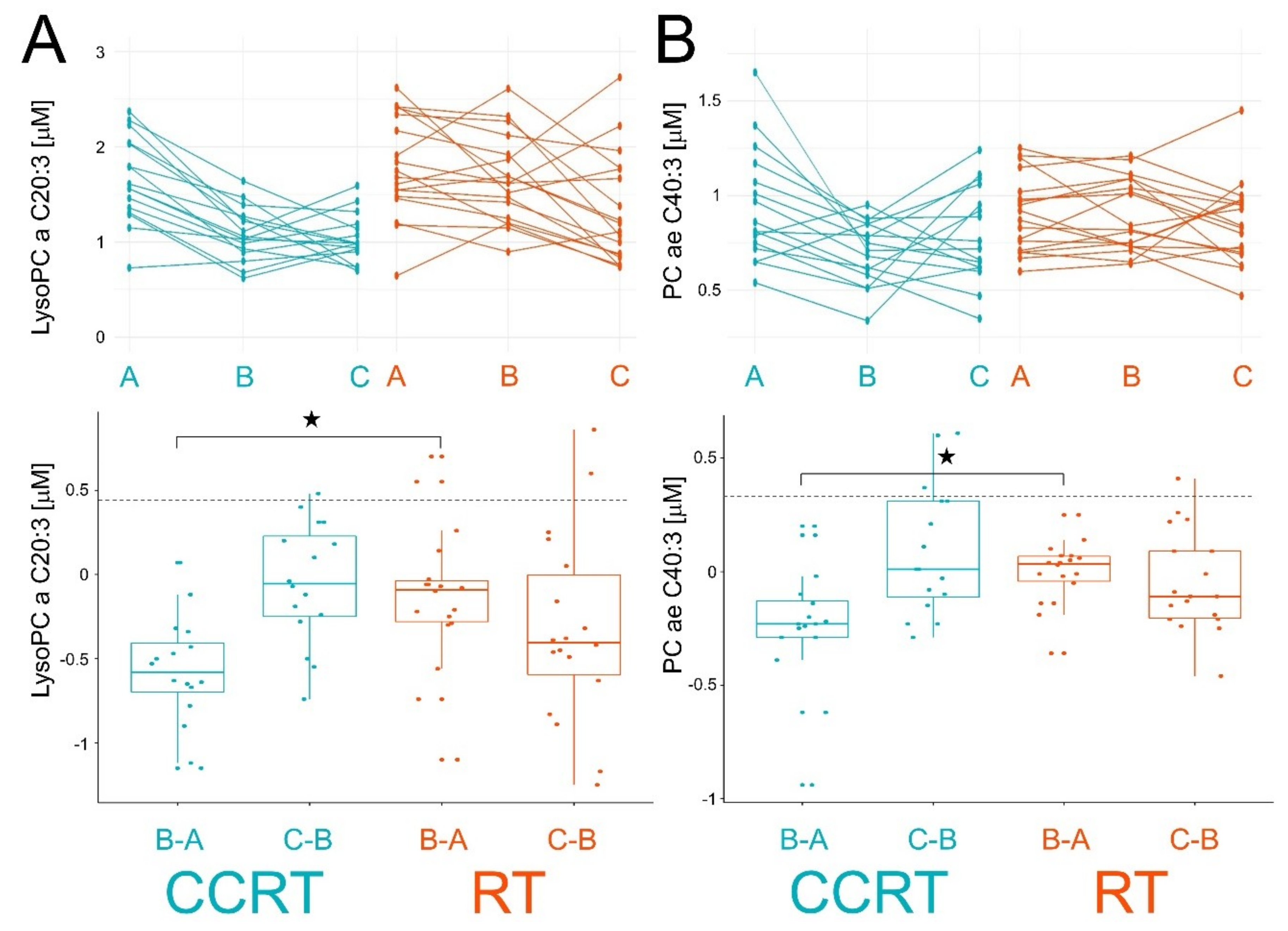
| Patient Groups | CCRT | RT | Pattern of Changes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level (Mean Value) | Change (Mean Value ± S.D.) | Level (Mean Value) | Change (Mean Value ± S.D.) | |||||||||
| Metabolites | A | B | C | B-A | C-B | A | B | C | B-A | C-B | CCRT | RT |
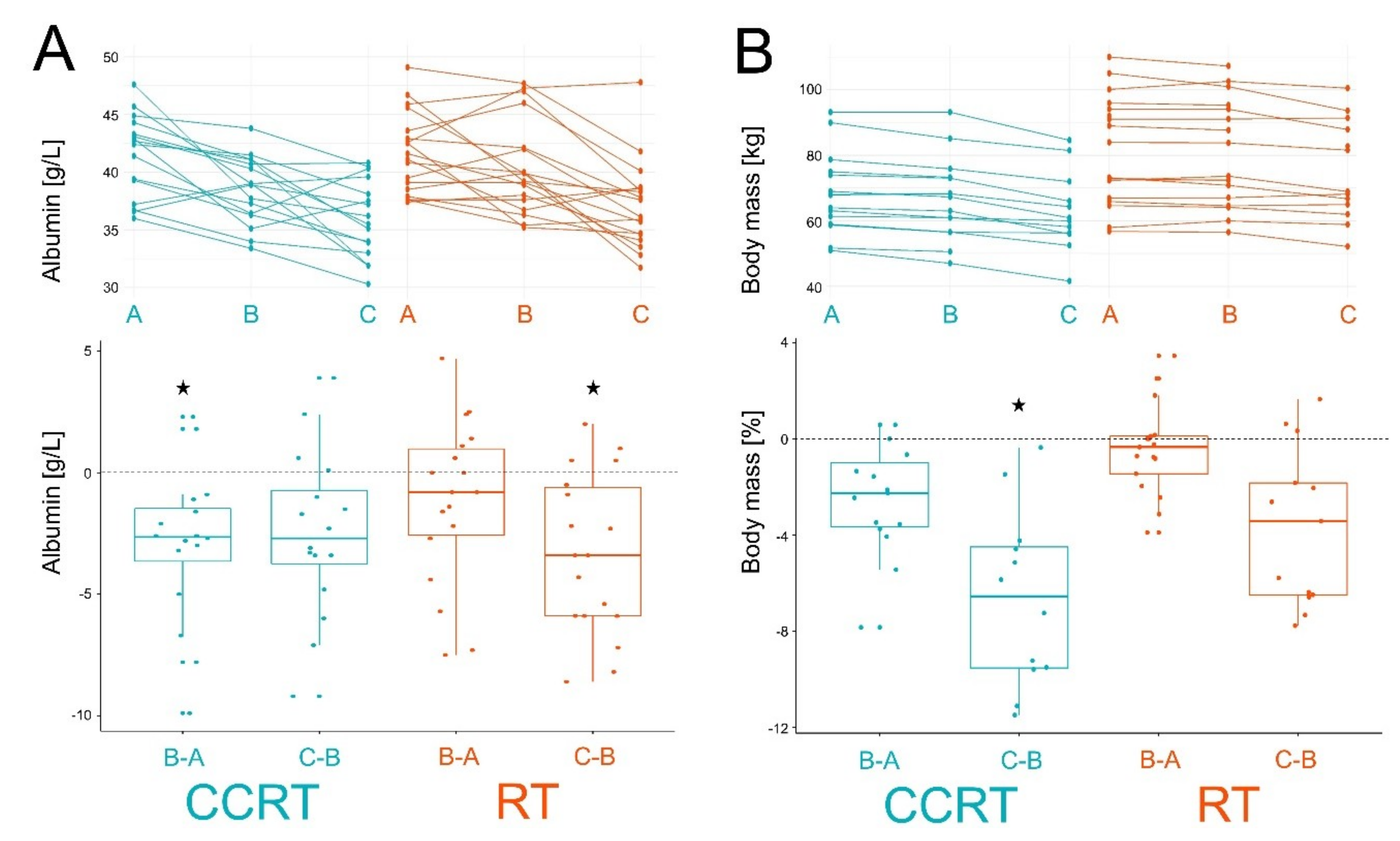
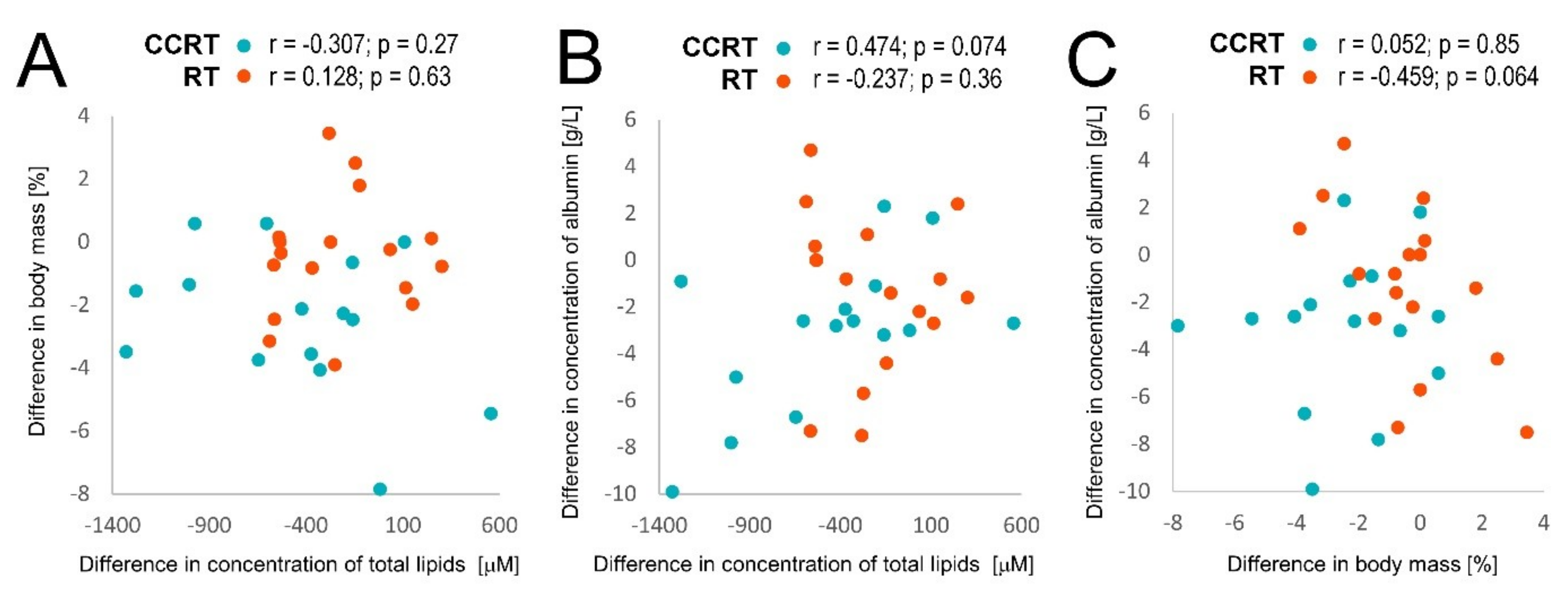
| ac C0 | 47.36 | 42.61 | 41.17 | −4.75 ± 11.42 | −1.44 ± 13.61 | 43.04 | 43.44 | 36.34 | 0.4 ± 9.37 | −7.1 * ± 9.95 | A = B > C | |
| ac C16 | 0.11 | 0.08 | 0.10 | −0.03 * ± 0.04 | 0.02 ± 0.04 | 0.11 | 0.09 | 0.10 | −0.01 ± 0.05 | 0.01 ± 0.06 | A > B = C | |
| ac C18:1 | 0.19 | 0.14 | 0.17 | −0.05 * ± 0.08 | 0.03 ± 0.1 | 0.15 | 0.12 | 0.14 | −0.03 ± 0.05 | 0.02 ± 0.04 | A > B = C | |
| Cit | 36.99 | 27.89 | 26.86 | −9.1 * ± 8.48 | −1.03 ± 9.09 | 36.12 | 33.49 | 27.69 | −2.63 ± 8.29 | −5.8 ± 6.36 | A > B = C | |
| His | 102.29 | 80.43 | 75.00 | −21.86 * ± 11.88 | −5.43 ± 16.09 | 104.68 | 95.38 | 83.02 | −9.29 ± 11.91 | −12.37 ± 18.83 | A > B = C | |
| Pro | 212.13 | 157.87 | 162.69 | −54.26 * ± 38.47 | 4.82 ± 47.2 | 221.00 | 211.28 | 189.72 | −9.72 ± 46.7 | −21.56 ± 49.33 | A > B = C | |
| kynurenine | 3.01 | 2.51 | 2.14 | −0.5 * ± 0.79 | −0.36 ± 0.75 | 3.20 | 3.13 | 2.38 | −0.07 ± 0.83 | −0.75 * ± 0.61 | A > B = C | A = B > C |
| lysoPC a C16:0 | 103.65 | 83.23 | 88.67 | −20.42 * ± 21.36 | 5.44 ± 16.54 | 99.78 | 92.29 | 89.77 | −7.49 ± 20.07 | −2.52 ± 21.64 | A > B = C | |
| lysoPC a C16:1 | 2.77 | 1.86 | 1.85 | −0.9 * ± 0.87 | −0.01 ± 0.42 | 2.75 | 2.43 | 2.15 | −0.32 ± 0.89 | −0.28 ± 0.82 | A > B = C | |
| lysoPC a C18:0 | 28.62 | 21.61 | 20.34 | −7.01 * ± 7.93 | −1.28 ± 7.32 | 28.64 | 26.73 | 22.12 | −1.92 ± 6.27 | −4.61 ± 7.18 | A > B = C | |
| lysoPC a C18:1 | 18.95 | 13.84 | 17.20 | −5.11 * ± 4.75 | 3.36 ± 5.9 | 17.77 | 16.30 | 16.22 | −1.47 ± 4.02 | −0.08 ± 5.29 | A > B = C | |
| lysoPC a C18:2 | 17.48 | 11.63 | 14.20 | −5.85 * ± 5.11 | 2.57 ± 4.63 | 16.22 | 15.22 | 14.44 | −1 ± 4.33 | −0.78 ± 6.49 | A > B = C | |
| lysoPC a C20:3 | 1.66 | 1.09 | 1.04 | −0.57 * ± 0.33 | −0.05 ± 0.36 | 1.79 | 1.66 | 1.33 | −0.13 ± 0.42 | −0.33 ± 0.56 | A > B = C | |
| lysoPC a C20:4 | 5.05 | 4.08 | 4.97 | −0.97 ± 1.33 | 0.89 * ± 1.37 | 5.90 | 5.58 | 5.86 | −0.32 ± 1.03 | 0.29 ± 2.01 | A = B<C | |
| PC aa C32:0 | 14.90 | 13.68 | 17.64 | −1.23 ± 4.54 | 3.97 * ± 4.35 | 12.96 | 12.11 | 13.89 | −0.85 ± 2.66 | 1.78 ± 2.85 | A = B<C | |
| PC aa C32:1 | 22.08 | 15.32 | 15.70 | −6.76 ± 10.39 | 0.38 ± 6.35 | 16.55 | 13.13 | 12.94 | −3.43 * ± 4.82 | −0.19 ± 7.21 | A > B = C | |
| PC aa C32:2 | 2.21 | 1.66 | 2.07 | −0.55 ± 0.64 | 0.42 ± 0.72 | 2.34 | 2.33 | 0.99 | −0.01 ± 1.55 | −1.34 * ± 1.71 | A = B > C | |
| PC aa C32:3 | 0.36 | 0.29 | 0.36 | −0.07 * ± 0.08 | 0.07 * ± 0.09 | 0.37 | 0.36 | 0.36 | −0.01 ± 0.08 | −0.01 ± 0.1 | A > B<C | |
| PC aa C34:2 | 332.25 | 247.81 | 308.13 | −66.75 * ± 100.8 | 46.19 * ± 73.68 | 295.44 | 245.61 | 265.72 | −41 * ± 49.04 | 28.33 ± 59.29 | A > B<C | A > B = C |
| PC aa C34:4 | 1.20 | 0.82 | 0.81 | −0.38 ± 0.49 | −0.01 ± 0.43 | 1.14 | 1.03 | 0.71 | −0.11 ± 0.25 | −0.32 * ± 0.45 | A = B > C | |
| PC aa C36:2 | 188.38 | 137.85 | 148.95 | −50.53 * ± 52.78 | 11.1 ± 49.89 | 181.61 | 155.31 | 136.73 | −26.31 ± 23.73 | −18.57 ± 36.15 | A > B = C | |
| PC aa C36:3 | 111.54 | 75.59 | 76.28 | −35.94 * ± 34.14 | 0.68 ± 29.13 | 109.86 | 93.58 | 78.48 | −16.28 * ± 19.03 | −15.1 ± 25.9 | A > B = C | A > B = C |
| PC aa C36:6 | 0.76 | 0.54 | 0.51 | −0.22 ± 0.3 | −0.03 ± 0.27 | 0.72 | 0.73 | 0.50 | 0.01 ± 0.29 | −0.22 * ± 0.28 | A = B > C | |
| PC aa C38:3 | 45.04 | 34.30 | 32.19 | −0.5 * ± 0.58 | 0.19 ± 0.83 | 47.89 | 41.80 | 33.03 | −0.23 ± 0.79 | −0.14 * ± 0.93 | A > B = C | A = B > C |
| PC aa C40:4 | 3.49 | 2.66 | 2.63 | −0.83 ± 1.06 | −0.03 ± 0.86 | 3.40 | 2.89 | 2.49 | −0.5 * ± 0.67 | −0.41 ± 0.79 | A > B = C | |
| PC aa C40:5 | 10.28 | 7.57 | 6.91 | −2.71 * ± 3.4 | −0.65 ± 2.77 | 10.26 | 9.26 | 7.74 | −1 ± 2.69 | −1.52 * ± 2.99 | A > B = C | A = B > C |
| PC aa C42:1 | 0.28 | 0.22 | 0.21 | −0.06 * ± 0.08 | −0.01 ± 0.09 | 0.27 | 0.23 | 0.23 | −0.04 ± 0.12 | 0 ± 0.11 | A > B = C | |
| PC aa C42:6 | 0.38 | 0.28 | 0.30 | −0.1 ± 0.12 | 0.02 ± 0.11 | 0.44 | 0.42 | 0.32 | −0.03 ± 0.15 | −0.1 * ± 0.13 | A = B > C | |
| PC ae C34:3 | 5.59 | 4.05 | 6.53 | −2.48 * ± 2.23 | 1.67 * ± 2.68 | 5.67 | 5.02 | 5.44 | −1.44 ± 2.08 | −0.29 ± 2.74 | A > B<C | |
| PC ae C36:2 | 9.24 | 7.08 | 8.77 | −2.15 * ± 1.71 | 1.69 ± 3.1 | 10.54 | 9.65 | 9.24 | −0.89 ± 2.23 | −0.41 ± 2.63 | A > B = C | |
| PC ae C36:3 | 5.62 | 3.54 | 4.15 | −2.08 * ± 1.36 | 0.61 ± 1.69 | 6.61 | 5.57 | 5.07 | −1.04 * ± 1.54 | −0.51 ± 1.77 | A > B = C | A > B = C |
| PC ae C36:4 | 15.63 | 11.42 | 12.08 | −4.21 * ± 3.5 | 0.66 ± 3.82 | 16.73 | 14.19 | 13.17 | −2.54 ± 4.14 | −1.02 ± 4 | A > B = C | |
| PC ae C38:2 | 1.57 | 1.13 | 1.10 | −0.44 * ± 0.45 | −0.02 ± 0.54 | 1.43 | 1.25 | 1.16 | −0.18 ± 0.26 | −0.08 ± 0.44 | A > B = C | |
| PC ae C38:3 | 3.33 | 2.39 | 2.70 | −0.94 * ± 0.8 | 0.31 ± 0.77 | 3.54 | 3.28 | 2.85 | −0.26 ± 0.69 | −0.44 ± 0.93 | A > B = C | |
| PC ae C38:4 | 11.26 | 8.77 | 9.64 | −2.5 * ± 2.28 | 0.87 ± 3.17 | 12.62 | 10.97 | 10.33 | −1.65 * ± 2.13 | −0.64 ± 2.06 | A > B = C | A > B = C |
| PC ae C38:5 | 16.56 | 12.74 | 14.99 | −3.81 * ± 3.23 | 2.25 ± 4.54 | 18.93 | 16.33 | 16.13 | −2.6 * ± 3.67 | −0.2 ± 3.84 | A > B = C | A > B = C |
| PC ae C40:3 | 0.94 | 0.71 | 0.79 | −0.24 * ± 0.27 | 0.09 ± 0.29 | 0.90 | 0.90 | 0.85 | 0 ± 0.14 | −0.05 ± 0.22 | A > B = C | |
| PC ae C42:2 | 0.57 | 0.43 | 0.40 | −0.14 * ± 0.15 | −0.03 ± 0.17 | 0.59 | 0.55 | 0.49 | −0.05 ± 0.15 | −0.06 ± 0.17 | A > B = C | |
| SM (OH) C14:1 | 6.23 | 5.64 | 7.14 | −0.59 ± 1.72 | 1.5 * ± 1.89 | 5.28 | 5.23 | 5.94 | −0.05 ± 1.01 | 0.72 ± 1 | A = B<C | |
| SM (OH) C16:1 | 3.20 | 3.17 | 4.15 | −0.03 ± 0.67 | 0.98 * ± 1.2 | 3.17 | 3.22 | 3.87 | 0.04 ± 0.89 | 0.66 * ± 0.8 | A = B<C | A = B<C |
| SM (OH) C22:1 | 10.50 | 8.13 | 8.73 | −2.38 * ± 2.18 | 0.6 ± 3.3 | 9.72 | 8.94 | 8.64 | −0.77 ± 2.3 | −0.31 ± 2.57 | A > B = C | |
| SM C16:1 | 16.58 | 14.85 | 19.13 | −1.73 ± 4.34 | 4.27 * ± 4.83 | 15.71 | 14.42 | 16.54 | −1.28 ± 3.06 | 2.12 ± 2.83 | A = B<C | |
| SM C18:0 | 29.49 | 29.20 | 39.38 | −0.29 ± 9.17 | 10.18 * ± 11.71 | 26.62 | 25.94 | 32.77 | −0.68 ± 7.49 | 6.82 * ± 5.69 | A = B<C | A = B<C |
| SM C24:0 | 21.65 | 15.79 | 16.06 | −5.86 * ± 4.96 | 0.27 ± 5.79 | 18.77 | 16.57 | 15.49 | −2.21 ± 3.86 | −1.08 ± 3.59 | A > B = C | |
| SM C24:1 | 63.66 | 56.36 | 67.73 | −7.31 ± 14.77 | 11.37 ± 16.82 | 58.63 | 55.34 | 65.98 | −3.29 ± 14.52 | 10.64 * ± 9.45 | A = B<C | |
| Metabolites | RT | CCRT |
| Ratio B/A | ||
| PC aa C34:2 | 0.847 | 0.809 |
| PC aa C36:3 | 0.854 | 0.713 |
| PC ae C36:3 | 0.854 | 0.652 |
| PC ae C38:4 | 0.876 | 0.779 |
| PC ae C38:5 | 0.873 | 0.776 |
| Ratio C/B | ||
| SM (OH) C16:1 | 1.245 | 1.387 |
| SM C18:0 | 1.301 | 1.421 |
| Ratio C/A | ||
| C0 | 0.869 | 0.895 |
| His | 0.797 | 0.742 |
| Pro | 0.868 | 0.792 |
| Kynurenine | 0.775 | 0.756 |
| lysoPC a C20:3 | 0.787 | 0.668 |
| PC aa C36:3 | 0.714 | 0.719 |
| PC aa C38:3 | 0.687 | 0.733 |
| PC aa C40:5 | 0.774 | 0.720 |
| PC ae C36:3 | 0.783 | 0.780 |
| PC ae C38:3 | 0.815 | 0.843 |
| PC ae C38:4 | 0.835 | 0.872 |
| SM (OH) C16:1 | 1.270 | 1.322 |
| SM C18:0 | 1.289 | 1.347 |
| SM C24:0 | 0.825 | 0.756 |
| Characteristics | CCRT | RT | ICT |
|---|---|---|---|
| Number of patients | 16 | 18 | 13 |
| Age [years] (median) | 49–75 (58) | 55–77 (63) | 46–80 (63) |
| Gender: male/female | 13/3 | 12/6 | 11/2 |
| Tumor localization | |||
| Oral cavity | 0 | 2 | 3 |
| Tonsil | 2 | 3 | 3 |
| Pharynx | 7 | 1 | 7 |
| Larynx | 7 | 12 | 0 |
| TNM staging | |||
| T1 | 0 | 1 | 0 |
| T2 | 4 | 11 | 1 |
| T3 | 7 | 6 | 6 |
| T4 | 5 | 0 | 6 |
| N0 | 6 | 16 | 1 |
| N1 | 2 | 1 | 1 |
| N2 | 8 | 1 | 7 |
| N3 | 0 | 0 | 4 |
| Radiotherapy | |||
| Total dose [Gy] (median) | 70 | 50-72 (72) | - |
| 7x1,8Gy/week | 0 | 10 | - |
| 5x2Gy/week | 16 | 2 | - |
| 5x2,2Gy/week | 0 | 4 | - |
| 5x2,5Gy/week | 0 | 2 | - |
| Chemotherapy | |||
| Cisplatin (100 mg/m2) | 16 | - | 5 |
| Cisplatin (100 mg/m2) + 5-fluorouracil (800 mg/m2) | 0 | - | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelonek, K.; Krzywon, A.; Jablonska, P.; Slominska, E.M.; Smolenski, R.T.; Polanska, J.; Rutkowski, T.; Mrochem-Kwarciak, J.; Skladowski, K.; Widlak, P. Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients—Comparison of Serum Metabolome Profiles. Metabolites 2020, 10, 60. https://doi.org/10.3390/metabo10020060
Jelonek K, Krzywon A, Jablonska P, Slominska EM, Smolenski RT, Polanska J, Rutkowski T, Mrochem-Kwarciak J, Skladowski K, Widlak P. Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients—Comparison of Serum Metabolome Profiles. Metabolites. 2020; 10(2):60. https://doi.org/10.3390/metabo10020060
Chicago/Turabian StyleJelonek, Karol, Aleksandra Krzywon, Patrycja Jablonska, Ewa M. Slominska, Ryszard T. Smolenski, Joanna Polanska, Tomasz Rutkowski, Jolanta Mrochem-Kwarciak, Krzysztof Skladowski, and Piotr Widlak. 2020. "Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients—Comparison of Serum Metabolome Profiles" Metabolites 10, no. 2: 60. https://doi.org/10.3390/metabo10020060
APA StyleJelonek, K., Krzywon, A., Jablonska, P., Slominska, E. M., Smolenski, R. T., Polanska, J., Rutkowski, T., Mrochem-Kwarciak, J., Skladowski, K., & Widlak, P. (2020). Systemic Effects of Radiotherapy and Concurrent Chemo-Radiotherapy in Head and Neck Cancer Patients—Comparison of Serum Metabolome Profiles. Metabolites, 10(2), 60. https://doi.org/10.3390/metabo10020060







