Assessment of Metabolic Profiles in Florets of Carthamus Species Using Ultra-Performance Liquid Chromatography-Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. Floret Colors and Leaf Shapes of Safflower Species
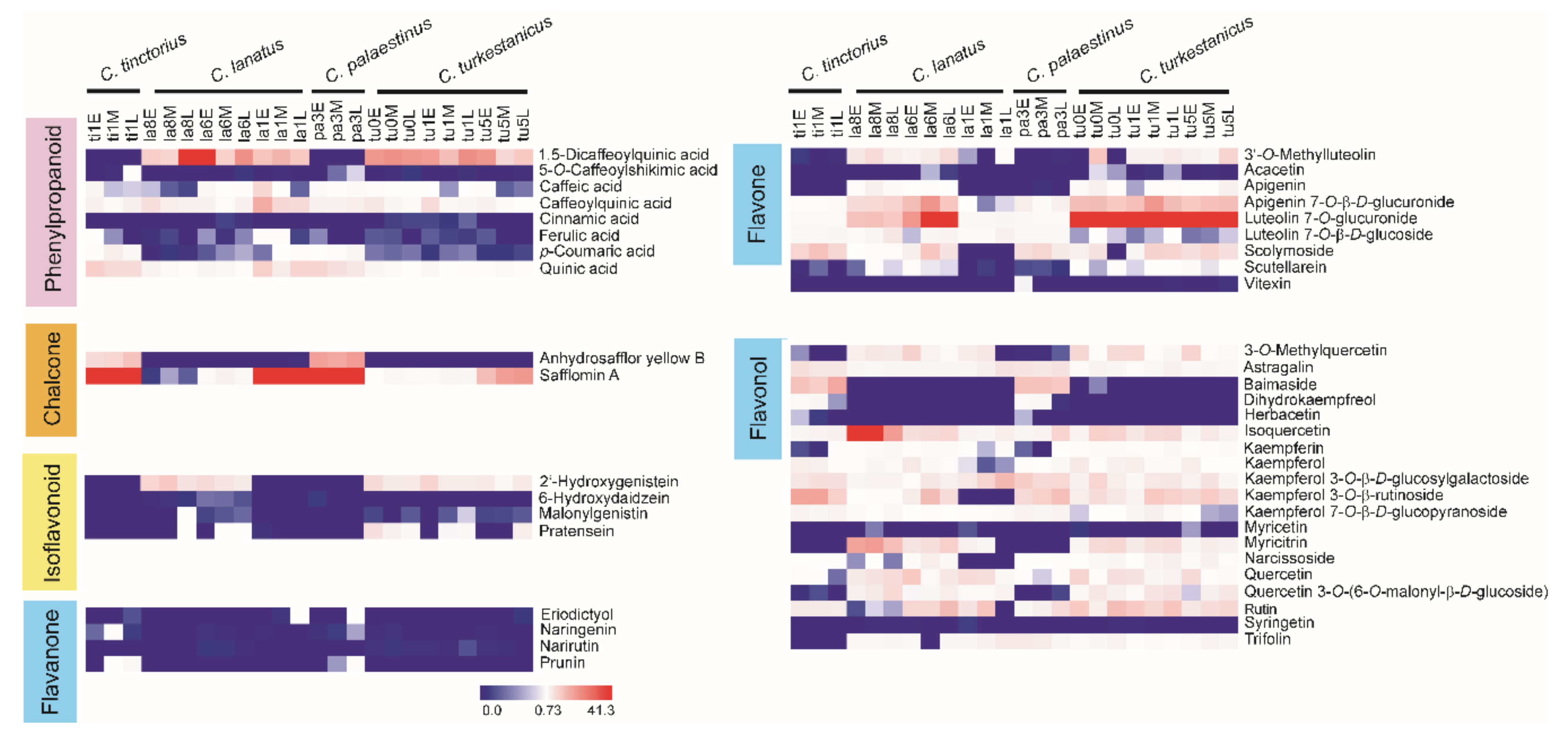
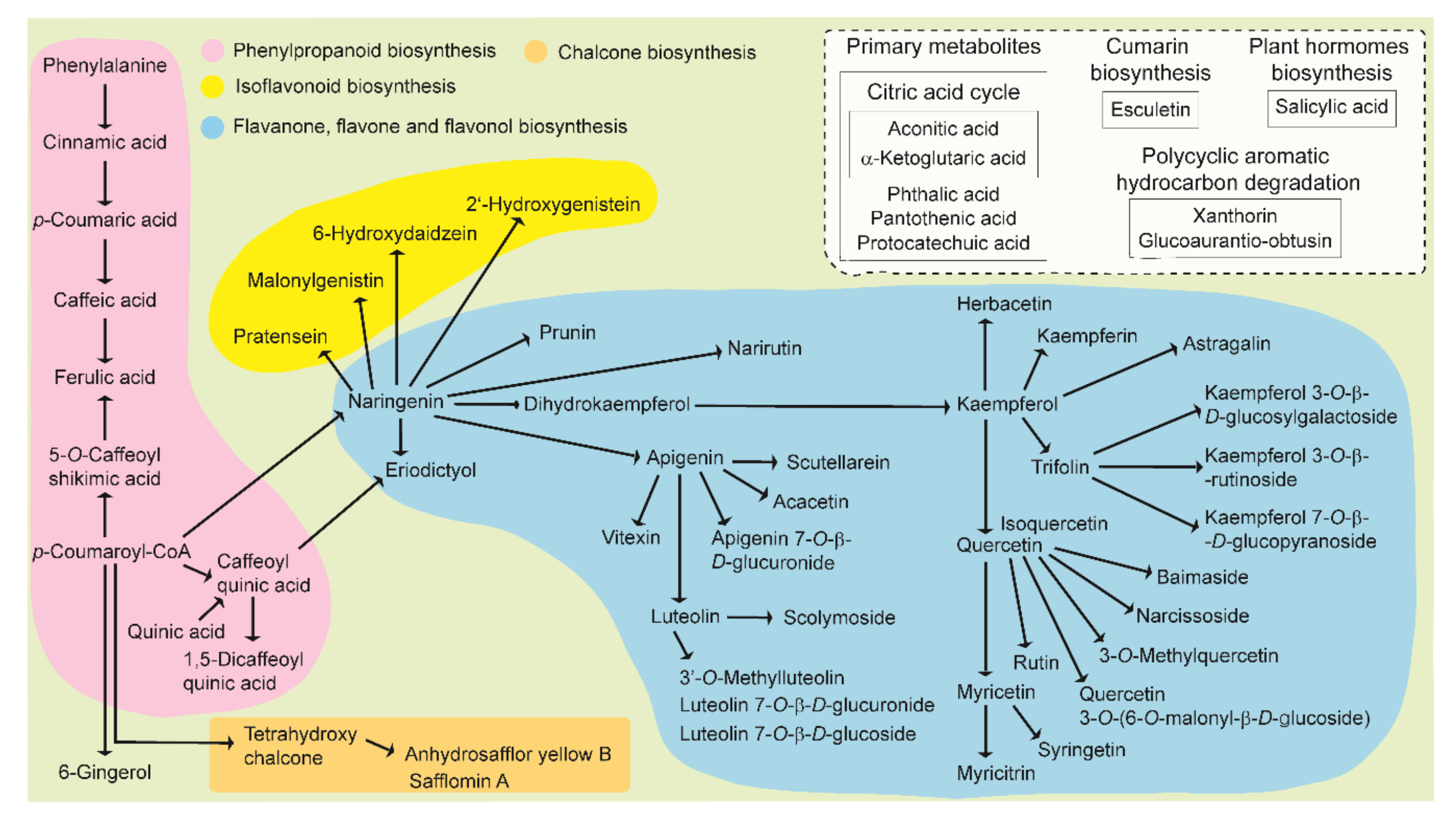
2.2. Metabolites of Safflower Species
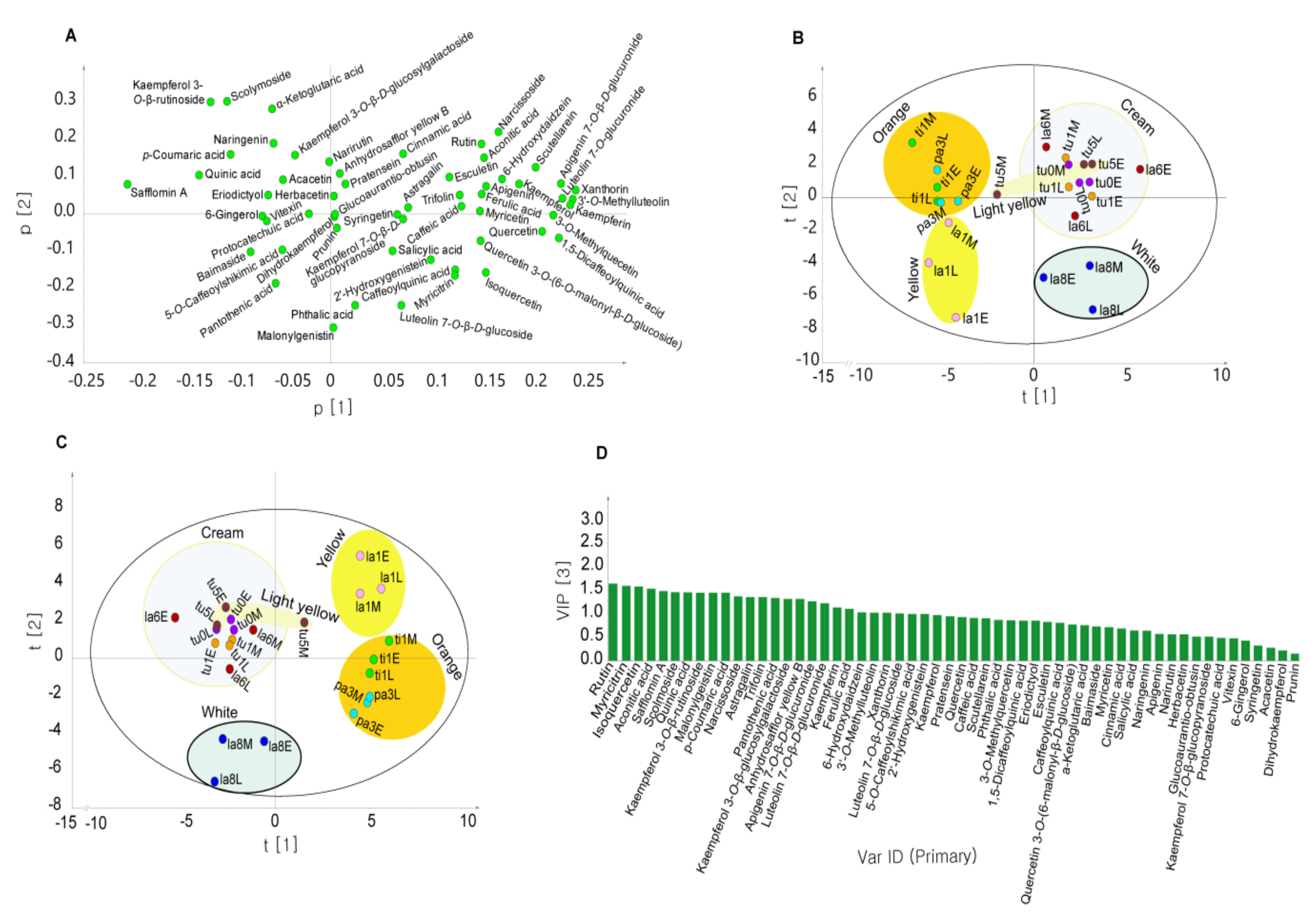
2.3. Classification of Safflower Species Based on Their Characteristic Chemical Components Using Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA)
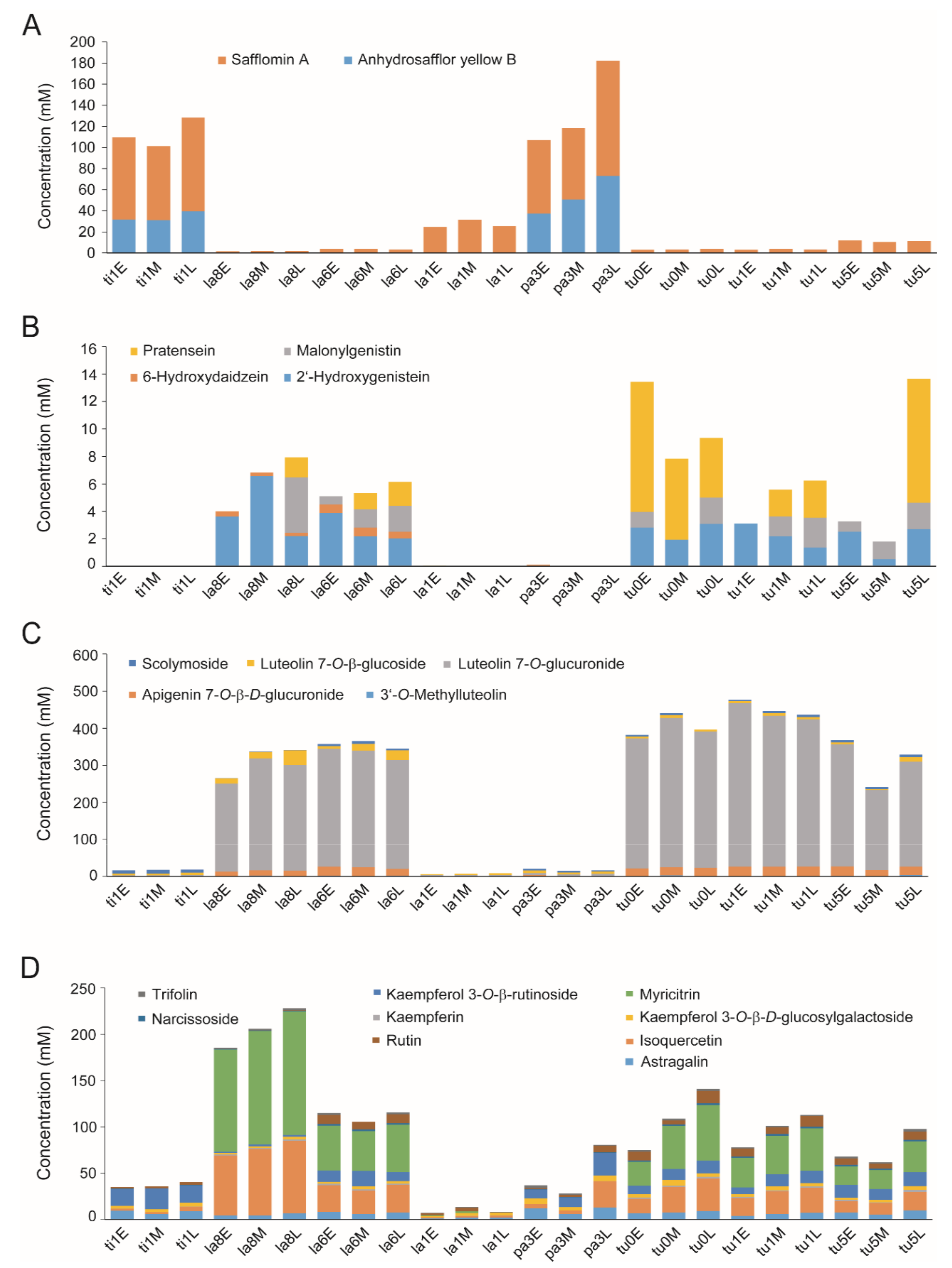
2.4. Quantification of Metabolites with High VIP Scores
3. Discussion
4. Materials and Methods
4.1. Plant Samples and Chemicals
4.2. Sample Preparation and Extraction
4.3. LC-ESI-QTOF-MS Analysis of Metabolites
4.4. Qualitative Analysis and Data Processing
4.5. Quantification of Metabolites and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turgumbayeva, A.A.; Ustenova, G.O.; Yeskalieva, B.K.; Ramazanova, B.A.; Rahimov, K.D.; Aisa, H.; Juszkiewicz, K.T. Volatile oil composition of Carthamus tinctorius L. flowers grown in Kazakhstan. Ann. Agric. Environ. Med. 2018, 25, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Lee, S.; Yeo, Y.; Hahn, B. A metabolic perspective and opportunities in pharmacologically important safflower. Metabolites 2020, 10, 253. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Kazemivash, N. Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin. J. Integr. Med. 2013, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Dajue, L.; Mündel, H.-H. Safflower (Carthamus tinctorius L.). Promoting the Conservation and Use of under-Utilized and Neglected Crops. 7; International Plant Genetic Resources Institute: Rome, Italy, 1996; ISBN 9290432977. [Google Scholar]
- Park, S.D.; Park, K.S.; Kim, K.J.; Kim, J.C.; Yoon, J.T.; Khan, Z. Effect of sowing time on development of safflower anthracnose disease and degree of resistance in various cultivars. J. Phytopathol. 2005, 153, 48–51. [Google Scholar] [CrossRef]
- Knowles, P.F. Safflower. Adv. Agron. 1959, 10, 289–323. [Google Scholar]
- Ashri, A.; Knowles, P.F. Cytogenetics of safflower (Carthamus L.) species and their hybrids. Agron. J. 1960, 52, 11–17. [Google Scholar] [CrossRef]
- Khidir, M.O. Evolution of the genetic system of safflower (Carthamus L.). Genetica 1969, 40, 84–88. [Google Scholar] [CrossRef]
- Khidir, O.; Knowles, P.F. Cytogenetic studies of Carthamus species (Compositae) with 32 pairs of chromosomes. I. Intrasectional hybridization. Am. J. Bot. 1970, 57, 123–129. [Google Scholar] [CrossRef]
- Khidir, M.O.; Knowles, P.F. Cytogenetic studies of Carthamus species (Compositae) with 32 pairs of chromosomes. II. Intersectional hybridization. Can. J. Genet. Cytol. 1970, 12, 90–99. [Google Scholar] [CrossRef]
- Vilatersana, R.; Brysting, A.K.; Brochmann, C. Molecular evidence for hybrid origins of the invasive polyploids Carthamus creticus and C. turkestanicus (Cardueae, Asteraceae). Mol. Phylogenet. Evol. 2007, 44, 610–621. [Google Scholar] [CrossRef]
- Estilai, A.; Knowles, P.F. Relationship of Carthamus leucocaulos to other Carthamus species (Compositae). Can. J. Genet. Cytol. 1978, 20, 221–233. [Google Scholar] [CrossRef]
- Sehgal, D.; Raina, S.N. Carthamus. In Wild Crop Relatives: Genomic and Breeding Resources. Oilseeds; Kole, C., Ed.; Springer-Verlag: Berlin, Germany, 2011; pp. 63–95. ISBN 9783642148712. [Google Scholar]
- Baker, H.G. Taxonomy and the biological species concepts in cultivated plants. In Genetic Resources in Plants—Their Exploration and Conservation; Frankel, O.H., Bennett, E., Eds.; Blackwell Scientific Publications: Oxford, UK, 1970; pp. 49–65. [Google Scholar]
- Harvey, B.L.; Knowles, P.F. Natural and artificial alloploids with 22 pairs of chromosoms in the genus Carthamus (Compositae). Can. J. Genet. Cytol. 1965, 7, 126–139. [Google Scholar] [CrossRef]
- Efron, Y.; Peleg, M.; Ashri, A. Alcohol dehydrogenase allozymes in the safflower Carthamus L. Biochem. Genet. 1973, 9, 299–308. [Google Scholar] [CrossRef]
- Sasanuma, T.; Sehgal, D.; Sasakuma, T.; Raina, S.N. Phylogenetic analysis of Carthamus species based on the nucleotide sequence of the nuclear SACPD gene and chloroplast trnL–trnF IGS region. Genome 2008, 51, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Ambreen, H.; Kumar, S.; Variath, M.T.; Joshi, G.; Bali, S.; Agarwal, M.; Kumar, A.; Jagannath, A.; Goel, S. Development of genomic microsatellite markers in Carthamus tinctorius L. (safflower) using next generation sequencing and assessment of their cross-species transferability and utility for diversity analysis. PLoS ONE 2015, 10, e0135443. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Burke, J.M. DNA sequence diversity and the origin of cultivated safflower (Carthamus tinctorius L.; Asteraceae). BMC Plant Biol. 2007, 7, 60. [Google Scholar] [CrossRef]
- Agrawal, R.; Tsujimoto, H.; Tandon, R.; Rao, S.R.; Raina, S.N. Species-genomic relationships among the tribasic diploid and polyploid Carthamus taxa based on physical mapping of active and inactive 18S–5.8S–26S and 5S ribosomal RNA gene families, and the two tandemly repeated DNA sequences. Gene 2013, 521, 136–144. [Google Scholar] [CrossRef]
- Sehgal, D.; Rajpal, V.R.; Raina, S.N. Chloroplast DNA diversity reveals the contribution of two wild species to the origin and evolution of diploid safflower (Carthamus tinctorius L.). Genome 2008, 51, 638–643. [Google Scholar] [CrossRef]
- Sehgal, D.; Raina, S.N.; Devarumath, R.M.; Sasanuma, T.; Sasakuma, T. Nuclear DNA assay in solving issues related to ancestry of the domesticated diploid safflower (Carthamus tinctorius L.) and the polyploid (Carthamus) taxa, and phylogenetic and genomic relationships in the genus Carthamus L. (Asteraceae). Mol. Phylogenet. Evol. 2009, 53, 631–644. [Google Scholar] [CrossRef]
- Kazuma, K.; Takahashi, T.; Sato, K.; Takeuchi, H.; Matsumoto, T.; Okuno, T. Quinochalcones and flavonoids from fresh florets in different cultivars of Carthamus tinctorius L. Biosci. Biotechnol. Biochem. 2000, 64, 1588–1599. [Google Scholar] [CrossRef]
- Golkar, P.; Arzani, A.; Rezaei, A.M. Inheritance of flower colour and spinelessness in safflower (Carthamus tinctorius L.). J. Genet. 2010, 89, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Flemmer, A.C.; Franchini, M.C.; Lindström, L.I. Description of safflower (Carthamus tinctorius) phenological growth stages according to the extended BBCH scale. Ann. Appl. Biol. 2015, 166, 331–339. [Google Scholar] [CrossRef]
- Kumazawa, T.; Sato, S.; Kanenari, D.; Kunimatsu, A.; Hirose, R.; Matsuba, S.; Obara, H.; Suzuki, M.; Sato, M.; Onodera, J. Precursor of carthamin, a constituent of safflower. Chem. Lett. 1994, 23, 2343–2344. [Google Scholar] [CrossRef]
- Mohammadi, M.; Tavakoli, A. Effect of harvest time of spring safflower (Carthamus tinctorius L.) florets on the production of red and yellow pigments. Qual. Assur. Saf. Crop. Foods 2015, 7, 581–588. [Google Scholar] [CrossRef]
- Pu, Z.-J.; Yue, S.-J.; Zhou, G.-S.; Yan, H.; Shi, X.-Q.; Zhu, Z.-H.; Huang, S.-L.; Peng, G.-P.; Chen, Y.-Y.; Bai, J.-Q.; et al. The comprehensive evaluation of safflowers in different producing areas by combined analysis of color, chemical compounds, and biological activity. Molecules 2019, 24, 3381. [Google Scholar] [CrossRef]
- Xu, M.; Du, C.; Zhang, N.; Shi, X.; Wu, Z.; Qiao, Y. Color spaces of safflower (Carthamus tinctorius L.) for quality assessment. J. Tradit. Chinese Med. Sci. 2016, 3, 168–175. [Google Scholar] [CrossRef]
- Tu, Y.; Li, D.; Fan, L.; Jia, X.; Guo, D.; Xin, H.; Guo, M. DOXC-class 2-oxoglutarate-dependent dioxygenase in safflower: Gene characterization, transcript abundance, and correlation with flavonoids. Biochem. Syst. Ecol. 2018, 80, 14–20. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene alcohols from the flowers of Compositae and their anti-inflammatory effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Zhang, H.L.; Nagatsu, A.; Watanabe, T.; Sakakibara, J.; Okuyama, H. Antioxidative compounds isolated from safflower (Carthamus tinctorius L.) oil cake. Chem. Pharm. Bull. (Tokyo) 1997, 45, 1910–1914. [Google Scholar] [CrossRef]
- Takii, T.; Hayashi, M.; Hiroma, H.; Chiba, T.; Kawashima, S.; Zhang, H.L.; Nagatsu, A.; Sakakibara, J.; Onozaki, K. Serotonin derivative, N-(p-coumaroyl)serotonin, isolated from safflower (Carthamus tinctorius L.) oil cake augments the proliferation of normal human and mouse fibroblasts in synergy with basic fibroblast growth factor (bFGF) or epidermal growth factor (EGF). J. Biochem. 1999, 125, 910–915. [Google Scholar] [PubMed]
- Hotta, Y.; Nagatsu, A.; Liu, W.; Muto, T.; Narumiya, C.; Lu, X.; Yajima, M.; Ishikawa, N.; Miyazeki, K.; Kawai, N.; et al. Protective effects of antioxidative serotonin derivatives isolated from safflower against postischemic myocardial dysfunction. Mol. Cell. Biochem. 2002, 238, 151–162. [Google Scholar] [CrossRef]
- Mitova, M.; Taskova, R.; Popov, S.; Berger, R.G.; Krings, U.; Handjieva, N. GC/MS analysis of some bioactive constituents from Carthamus lanatus L. Z. Nat. C 2003, 58, 697–703. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taskova, R.; Mitovab, M.; Mikhova, B.; Duddeck, H. Bioactive phenolics from Carthamus lanatus L. Z. Nat. C 2003, 58, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Mikhova, B.; Duddeck, H.; Taskova, R.; Mitova, M.; Alipieva, K. Oxygenated bisabolane fucosides from Carthamus lanatus L. Z. Nat. C 2004, 59, 244–248. [Google Scholar] [CrossRef]
- Koyama, N.; Kuribayashi, K.; Seki, T.; Kobayashi, K.; Furuhata, Y.; Suzuki, K.; Arisaka, H.; Nakano, T.; Amino, Y.; Ishii, K. Serotonin derivatives, major safflower (Carthamus tinctorius L.) seed antioxidants, inhibit low-density lipoprotein (LDL) oxidation and atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2006, 54, 4970–4976. [Google Scholar] [CrossRef]
- Hao, G.Z.; In, G.Q.; Ai, Y.G.; Uo, L.G. Structural identification of a new tri-p-coumaroylspermidine with serotonin transporter inhibition from safflower. Chem. Pharm. Bull. (Tokyo) 2010, 58, 950–952. [Google Scholar]
- Zhou, X.; Tang, L.; Xu, Y.; Zhou, G.; Wang, Z. Towards a better understanding of medicinal uses of Carthamus tinctorius L. in traditional Chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2014, 151, 27–43. [Google Scholar] [CrossRef]
- Obara, H.; Onodera, Ju. Structure of carthamin. Chem. Lett. 1979, 8, 201–204. [Google Scholar] [CrossRef]
- Kazuma, K.; Shirai, E.; Wada, M.; Umeo, K.; Sato, A.; Matsumoto, T.; Okuno, T. Structure of precarthamin, a biosynthetic precursor of carthamin. Biosci. Biotechnol. Biochem. 1995, 59, 1588–1590. [Google Scholar] [CrossRef]
- Takahashi, Y.; Saito, K.; Yanagiya, M.; Ikura, M.; Hikichi, K.; Matsumoto, T.; Wada, M. Chemical constitution of safflower yellow B, a quinochalcone C-glycoside from the flower petals of Carthamus tinctorius L. Tetrahedron Lett. 1984, 25, 2471–2474. [Google Scholar] [CrossRef]
- Ondera, J.; Obara, H.; Osone, M.; Maruyama, Y.; Sato, S. The structure of safflomin-A, a component of safflower yellow. Chem. Lett. 1981, 10, 433–436. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miyasaka, N.; Tasaka, S.; Miura, I.; Urano, S.; Ikura, M.; Hikichi, K.; Matsumoto, T.; Wada, M. Constitution of two coloring matters in the flower petals of Carthamus tinctorius L. Tetrahedron Lett. 1982, 23, 5163–5166. [Google Scholar] [CrossRef]
- Goda, Y.; Suzuki, J.; Maitani, T. Structure of safflomin A and content of safflomin (safflor yellow) in commercial safflower yellow product. Jpn. J. Food Chem. 1997, 4, 54–58. [Google Scholar]
- Onodera, J.; Obara, H.; Hirose, R.; Matsuba, S.; Sato, N.; Sato, S.; Suzuki, M. The structure of safflomin C, a constituent of safflower. Chem. Lett. 1989, 18, 1571–1574. [Google Scholar] [CrossRef]
- Meselhy, M.R.; Kadota, S.; Momose, Y.; Hattori, M.; Namba, T. Tinctormine, a novel Ca2+ antagonist N-containing quinochalcone C-glycoside from Carthamus tinctorius L. Chem. Pharm. Bull. (Tokyo) 1992, 40, 3355–3357. [Google Scholar] [CrossRef]
- Yin, H.-B.; He, Z.-S. A novel semi-quinone chalcone sharing a pyrrole ring C-glycoside from Carthamus tinctorius. Tetrahedron Lett. 2000, 41, 1955–1958. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, X.-L.; Shi, H.; Xiao, Y.-S.; Ke, Y.-X.; Xue, X.-Y.; Zhang, F.-F.; Liang, X.-M. Characterization of C-glycosyl quinochalcones in Carthamus tinctorius L. by ultraperformance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 1275–1287. [Google Scholar] [CrossRef]
- Yue, S.-J.; Qu, C.; Zhang, P.-X.; Tang, Y.-P.; Jin, Y.; Jiang, J.-S.; Yang, Y.-N.; Zhang, P.-C.; Duan, J.-A. Carthorquinosides A and B, quinochalcone C-glycosides with diverse dimeric skeletons from Carthamus tinctorius. J. Nat. Prod. 2016, 79, 2644–2651. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.-N.; Jiang, J.; Feng, Z.-M.; Zhang, P.-C. Saffloflavonesides A and B, two rearranged derivatives of flavonoid C-glycosides with a furan − tetrahydrofuran ring from Carthamus tinctorius. Org. Lett. 2014, 16, 5714–5717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Z.; Chen, H.; Qiao, L.; Xu, N.; Cao, J.-Q.; Pei, Y.-H. Two new compounds from Carthamus tinctorius. J. Asian Nat. Prod. Res. 2008, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chang, E.J.; Kim, H.J.; Park, J.H.; Choi, S.W. Antioxidative flavonoids from leaves of Carthamus tinctorius. Arch. Pharm. Res. 2002, 25, 313–319. [Google Scholar] [CrossRef]
- Shin-jun, Y.; Yu-ping, T.; Lin-yan, W.; Hao, T.; Shu-Jiao, L.; Pei, L.; Shu-lan, S.; Jin-ao, D. Separation and evaluation of antioxidant constituents from Carthamus tinctorius. China J. Chin. Mater. Med. 2014, 39, 3295–3300. [Google Scholar] [CrossRef]
- Rahamatalla, A.B.; Babiker, E.E.; Krishna, A.G.; ELTinay, A.H. Changes in fatty acids composition during seed growth and physicochemical characteristics of oil extracted from four safflower cultivars. Plant Foods Hum. Nutr. 2001, 56, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Gullich, L.M.D.; Bilibio, D.; Zanella, O.; Bender, J.P.; Carniel, N.; Priamo, W.L. Pressurized liquid extraction and chemical characterization of safflower oil: A comparison between methods. Food Chem. 2016, 213, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, H.; Lan, T.; Wu, D.; Pan, L.; Chen, Z. Preparation of high-purity trilinolein and triolein by enzymatic esterification reaction combined with column chromatography. J. Oleo Sci. 2019, ess18142. [Google Scholar] [CrossRef]
- Zhou, Y.-Z.; Qiao, L.; Chen, H.; Li, R.-F.; Hua, H.-M.; Pei, Y.-H. New aromatic glucosides from Carthamus tinctorius. J. Asian Nat. Prod. Res. 2008, 10, 817–821. [Google Scholar] [CrossRef]
- Huang, X.-X.; Yan, Z.-Y.; Liu, S.; Wang, X.-B.; Song, S.-J. Investigation of chemical constituents of safflower and their tyrosinase inhibitory activity. J. Asian Nat. Prod. Res. 2019, 31, 248–256. [Google Scholar] [CrossRef]
- Li, W.-C.; Wang, X.-Y.; Lin, P.-C.; Hu, N.; Zhang, Q.-L.; Suo, Y.-R.; Ding, C.-X. Preparative separation and purification of four cis–trans isomers of coumaroylspermidine analogs from safflower by high-speed counter-current chromatography. J. Chromatogr. B 2013, 938, 75–79. [Google Scholar] [CrossRef]
- Kim, H.J.; Bae, Y.C.; Park, R.W.; Choi, S.W.; Cho, S.H.; Choi, Y.S.; Lee, W.J. Bone-protecting effect of safflower seeds in ovariectomized rats. Calcif. Tissue Int. 2002, 71, 88–94. [Google Scholar] [CrossRef]
- Radhika, K.; Sujatha, M.; Rao, T.N. Thidiazuron stimulates adventitious shoot regeneration in different safflower explants. Biol. Plant. 2006, 50, 174–179. [Google Scholar] [CrossRef]
- Schweiggert, R.M. Perspective on the ongoing replacement of artificial and animal-based dyes with alternative natural pigments in foods and beverages. J. Agric. Food Chem. 2018, 66, 3074–3081. [Google Scholar] [CrossRef]
- Nie, P.; Zhang, L.; Zhang, W.; Rong, W.; Zhi, J. The effects of hydroxysafflor yellow A on blood pressure and cardiac function. J. Ethnopharmacol. 2012, 139, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Tang, Y.; Li, S.; Duan, J. Chemical and biological properties of quinochalcone C-glycosides from the florets of Carthamus tinctorius. Molecules 2013, 18, 15220–15254. [Google Scholar] [CrossRef]
- Delshad, E.; Yousefi, M.; Sasannezhad, P.; Rakhshandeh, H.; Ayati, Z. Medical uses of Carthamus tinctorius L. (safflower): A comprehensive review from traditional medicine to modern medicine. Electron. Physician 2018, 10, 6672–6681. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, Z.; Miao, L.; Wang, L. Effects of extracts and isolated compounds from safflower on some index of promoting blood circulation and regulating menstruation. J. Ethnopharmacol. 2016, 191, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Balabanos, D.; Savva, S.; Skaperda, Z.; Priftis, A.; Kerasioti, E.; Mikropoulou, E.V.; Vougogiannopoulou, K.; Mitakou, S.; Halabalaki, M.; et al. Extracts from the Mediterranean food plants Carthamus lanatus, Cichorium intybus, and Cichorium spinosum enhanced GSH levels and increased Nrf2 expression in human endothelial cells. Oxid. Med. Cell. Longev. 2018, 2018, 6594101. [Google Scholar] [CrossRef] [PubMed]
- Topashka-ancheva, M.N.; Taskova, R.M.; Handjieva, N. V Mitogenic effect of Carthamus lanatus extracts, fractions and constituents. Fitoterapia 2006, 77, 608–610. [Google Scholar] [CrossRef]
- Bocheva, A.; Mikhova, B.; Taskova, R.; Mitova, M.; Duddeck, H. Antiinflammatory and analgesic effects of Carthamus lanatus aerial parts. Fitoterapia 2003, 74, 559–563. [Google Scholar] [CrossRef]
- Jalil, S.; Mikhova, B.; Taskova, R.; Mitova, M.; Duddeck, H.; Choudhary, M.I.; Atta-ur, R. In vitro anti-inflammatory effect of Carthamus lanatus L. Z. Nat. C 2003, 58, 830–832. [Google Scholar]
- Taskova, R.; Mitova, M.; Najdenski, H.; Tzvetkova, I.; Duddeck, H. Antimicrobial activity and cytotoxicity of Carthamus lanatus. Fitoterapia 2002, 73, 540–543. [Google Scholar] [CrossRef]
- Ashri, A. Evaluation of the germplasm collection of safflower, Carthamus tinctirius L. V. distribution and regional divergence for morphological characters. Euphytica 1975, 24, 651–659. [Google Scholar] [CrossRef]
- Shinwari, Z.K.; Rehman, H.; Rabbani, M.A. Morphological traits based genetic diversity in safflower (Carthamus tinctorius L.). Pakistan J. Bot. 2014, 46, 1389–1395. [Google Scholar]
- Mukta, N.; Reddy, A.P.; Lavanya, C.; Dudhe, M.Y. Variability for DUS characteristics in released varieties of safflower (Carthamus tinctorius L.) in India. J. Oilseeds Res. 2012, 29, 133–135. [Google Scholar]
- Dwivedi, S.L.; Upadhyaya, H.D.; Hegde, D.M. Development of core collection using geographic information and morphological descriptors in safflower (Carthamus tinctorius L.) germplasm. Genet. Resour. Crop Evol. 2005, 52, 821–830. [Google Scholar] [CrossRef]
- Khan, M.A.; von Witzke-Ehbrecht, S.; Maass, B.L.; Becker, H.C. Relationships among different geographical groups, agro-morphology, fatty acid composition and RAPD marker diversity in Safflower (Carthamus tinctorius). Genet. Resour. Crop Evol. 2009, 56, 19–30. [Google Scholar] [CrossRef]
- Yang, W.; Qiao, X.; Bo, T.; Wang, Q.; Guo, D.; Ye, M. Low energy induced homolytic fragmentation of flavonol 3-O-glycosides by negative electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 28, 385–395. [Google Scholar] [CrossRef]
- Yao, C.; Yang, W.; Si, W.; Shen, Y.; Zhang, N.; Chen, H.; Pan, H.; Yang, M.; Wu, W.; Guo, D. An enhanced targeted identification strategy for the selective identification of flavonoid O-glycosides from Carthamus tinctorius by integrating offline two-dimensional liquid chromatography / linear ion-trap-orbitrap mass spectrometry, high-resolution. J. Chromatogr. A 2017, 1491, 87–97. [Google Scholar] [CrossRef]
- De Villiers, A.; Venter, P.; Pasch, H. Recent advances and trends in the liquid-chromatography–mass spectrometry analysis of flavonoids. J. Chromatogr. A 2016, 1430, 16–78. [Google Scholar] [CrossRef]
- Mirzajani, F.; Zeinali, S.M.; Goodarzi, R. Identification of hydroxy-safflor yellow A, safflor yellow B, and precarthaminin safflower using LC/ESI—MSMS. J. Food Meas. Charact. 2015, 9, 332–336. [Google Scholar] [CrossRef]
- Salem, N.; Msaada, K.; Hamdaoui, G.; Limam, F.; Marzouk, B. Variation in phenolic composition and antioxidant activity during flower development of safflower (Carthamus tinctorius L.). J. Agric. Food Chem. 2011, 59, 4455–4463. [Google Scholar] [CrossRef]
- Steberl, K.; Hartung, J.; Graeff-Hönninger, S. Impact of cultivar, harvest date and threshing parameter settings on floret and carthamidin yield of safflower. Agronomy 2020, 10, 1272. [Google Scholar] [CrossRef]
- Chakradhari, S.; Perkons, I.; Mišina, I.; Sipeniece, E.; Radziejewska, E. Profiling of the bioactive components of safflower seeds and seed oil: Cultivated (Carthamus tinctorius L.) vs. wild (Carthamus oxyacantha M. Bieb.). Eur. Food Res. Technol. 2020, 246, 449–459. [Google Scholar] [CrossRef]
- Yeloojeh, K.A.; Saeidi, G.; Sabzalian, M.R. Drought stress improves the composition of secondary metabolites in safflower flower at the expense of reduction in seed yield and oil content. Ind. Crop. Prod. 2020, 154, 112496. [Google Scholar] [CrossRef]
- Anna, V.S.; Gurak, P.D.; Marczak, L.D.F.; Tessaro, I.C. Dyes and pigments tracking bioactive compounds with colour changes in foods—A review. Dye. Pigment. 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrncic, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and analysis of polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Wang, R.; Xian, B.; Ren, C.; Liu, Q.; Wu, Q. Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment. BMC Plant Biol. 2020, 20, 353. [Google Scholar] [CrossRef]
- Bolstad, B.M.; Irizarry, R.A.; Astrand, M.; Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–377. [Google Scholar] [CrossRef]

| Species | C. tinctorius PI 592391 | C. lanatus PI 202728 | C. lanatus PI 235666 | C. lanatus W6 16791 | C. palaestinus PI 235663 | C. turkestanicus PI 426180 | C. turkestanicus PI 426181 | C. turkestanicus PI 426425 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | L | E | M | |||
| C. tinctorius PI 592391 | M | 0.998** | |||||||||||||||||||||||
| L | 0.998 ** | 0.996 ** | |||||||||||||||||||||||
| C. lanatus PI 202728 | E | −0.077 | −0.083 | −0.076 | Key | ||||||||||||||||||||
| M | −0.088 | −0.092 | −0.086 | 0.976 ** | −1 | −0.75 | −0.5 | −0.25 | 0 | 0.25 | 0.5 | 0.75 | 1 | ||||||||||||
| L | −0.081 | −0.087 | −0.079 | 0.940 ** | 0.914 ** | ||||||||||||||||||||
| C. lanatus PI 235666 | E | 0.008 | 0.015 | 0.002 | 0.710 ** | 0.742 ** | 0.747 ** | ||||||||||||||||||
| M | 0.092 | 0.116 | 0.086 | 0.665 ** | 0.678 ** | 0.647 ** | 0.827 ** | ||||||||||||||||||
| L | 0.007 | 0.016 | 0.007 | 0.773 ** | 0.789 ** | 0.806 ** | 0.913 ** | 0.908 ** | |||||||||||||||||
| C. lanatus W6 16791 | E | 0.891 ** | 0.878 ** | 0.891 ** | 0.051 | 0.022 | 0.088 | 0.187 | 0.099 | 0.138 | |||||||||||||||
| M | 0.949 ** | 0.939 ** | 0.954 ** | 0.007 | −0.001 | 0.042 | 0.119 | 0.108 | 0.110 | 0.959 ** | |||||||||||||||
| L | 0.963 ** | 0.952 ** | 0.966 ** | −0.018 | −0.028 | 0.002 | 0.045 | 0.067 | 0.045 | 0.938 ** | 0.989 ** | ||||||||||||||
| C. palaestinus PI 235663 | E | 0.993 ** | 0.988 ** | 0.995 ** | −0.063 | −0.077 | −0.067 | −0.001 | 0.068 | 0.005 | 0.895 ** | 0.957 ** | 0.973 ** | ||||||||||||
| M | 0.992 ** | 0.988 ** | 0.996 ** | −0.081 | −0.090 | −0.083 | −0.014 | 0.058 | −0.009 | 0.881 ** | 0.946 ** | 0.958 ** | 0.996 ** | ||||||||||||
| L | 0.992 ** | 0.990 ** | 0.996 ** | −0.060 | −0.071 | −0.063 | 0.008 | 0.087 | 0.017 | 0.888 ** | 0.954 ** | 0.963 ** | 0.995 ** | 0.997 ** | |||||||||||
| C. turkestanicus PI 426180 | E | −0.018 | −0.014 | −0.023 | 0.620 ** | 0.647 ** | 0.683 ** | 0.917 ** | 0.815 ** | 0.893 ** | 0.153 | 0.093 | 0.029 | −0.022 | −0.037 | −0.021 | |||||||||
| M | 0.004 | 0.014 | 0.004 | 0.683 ** | 0.706 ** | 0.699 ** | 0.796 ** | 0.895 ** | 0.933 ** | 0.076 | 0.072 | 0.038 | 0.006 | −0.011 | 0.008 | 0.879 ** | |||||||||
| L | −0.007 | −0.004 | −0.005 | 0.735 ** | 0.753 ** | 0.749 ** | 0.894 ** | 0.840 ** | 0.905 ** | 0.117 | 0.109 | 0.041 | 0.000 | −0.015 | 0.013 | 0.917 ** | 0.848 ** | ||||||||
| C. turkestanicus PI 426181 | E | −0.013 | −0.011 | −0.016 | 0.689 ** | 0.709 ** | 0.708 ** | 0.920 ** | 0.843 ** | 0.914 ** | 0.149 | 0.093 | 0.033 | −0.012 | −0.028 | −0.011 | 0.935 ** | 0.882 ** | 0.907 ** | ||||||
| M | 0.054 | 0.068 | 0.051 | 0.695 ** | 0.709 ** | 0.678 ** | 0.825 ** | 0.954 ** | 0.922 ** | 0.100 | 0.098 | 0.063 | 0.048 | 0.032 | 0.055 | 0.862 ** | 0.949 ** | 0.877 ** | 0.922 ** | ||||||
| L | 0.025 | 0.040 | 0.025 | 0.682 ** | 0.687 ** | 0.714 ** | 0.854 ** | 0.945 ** | 0.955 ** | 0.107 | 0.100 | 0.043 | 0.021 | 0.008 | 0.033 | 0.889 ** | 0.946 ** | 0.904 ** | 0.915 ** | 0.972 ** | |||||
| C. turkestanicus PI 426425 | E | 0.355 ** | 0.363 ** | 0.349 ** | 0.550 ** | 0.585 ** | 0.574 ** | 0.900 ** | 0.830 ** | 0.832 ** | 0.444 ** | 0.424 ** | 0.374 ** | 0.346 ** | 0.328 * | 0.348 * | 0.865 ** | 0.786 ** | 0.829 ** | 0.874 ** | 0.839 ** | 0.832 ** | |||
| M | 0.522 ** | 0.538 ** | 0.517 ** | 0.493 ** | 0.490 ** | 0.498 ** | 0.702 ** | 0.866 ** | 0.775 ** | 0.509 ** | 0.538 ** | 0.511 ** | 0.511 ** | 0.492 ** | 0.517 * | 0.712 ** | 0.782 ** | 0.720 ** | 0.740 ** | 0.852 ** | 0.840 ** | 0.891 ** | |||
| L | 0.346 ** | 0.357 ** | 0.343 ** | 0.565 ** | 0.607 ** | 0.564 ** | 0.766 ** | 0.860 ** | 0.865 ** | 0.362 ** | 0.389 ** | 0.356 ** | 0.340 * | 0.321 * | 0.345 ** | 0.790 ** | 0.894 ** | 0.780 ** | 0.789 ** | 0.873 ** | 0.865 ** | 0.875 ** | 0.898 ** | ||
| Accession Number | Species | Flower Color at Middle Stage of Development | Spine Length | Type | Leaf Shape | Leaf Apices |
|---|---|---|---|---|---|---|
| PI 202728 | C. lanatus | White | Long | Wild | Lanceolate | Attenuate |
| PI 235666 | C. lanatus | Cream | Long | Wild | Lanceolate | Attenuate |
| W6 16791 | C. lanatus | Yellow | Long | Wild | Lanceolate | Attenuate |
| PI 235663 | C. palaestinus | Orange | Very short | Wild | Lanceolate | Acuminate |
| PI 426180 | C. turkestanicus | Cream | Long | Wild | Lanceolate | Attenuate |
| PI 426181 | C. turkestanicus | Cream | Long | Wild | Lanceolate | Attenuate |
| PI 426425 | C. turkestanicus | Light-yellow | Long | Wild | Lanceolate | Attenuate |
| PI 592391 | C. tinctorius | Orange | Very short | Cultivar | Lanceolate | Acuminate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Assefa, A.D.; Song, J.; Mani, V.; Park, S.; Lee, S.-K.; Lee, K.; Kim, D.-G.; Hahn, B.-S. Assessment of Metabolic Profiles in Florets of Carthamus Species Using Ultra-Performance Liquid Chromatography-Mass Spectrometry. Metabolites 2020, 10, 440. https://doi.org/10.3390/metabo10110440
Kim J, Assefa AD, Song J, Mani V, Park S, Lee S-K, Lee K, Kim D-G, Hahn B-S. Assessment of Metabolic Profiles in Florets of Carthamus Species Using Ultra-Performance Liquid Chromatography-Mass Spectrometry. Metabolites. 2020; 10(11):440. https://doi.org/10.3390/metabo10110440
Chicago/Turabian StyleKim, Jiseon, Awraris Derbie Assefa, Jaeeun Song, Vimalaj Mani, Soyoung Park, Seon-Kyeong Lee, Kijong Lee, Dong-Gwan Kim, and Bum-Soo Hahn. 2020. "Assessment of Metabolic Profiles in Florets of Carthamus Species Using Ultra-Performance Liquid Chromatography-Mass Spectrometry" Metabolites 10, no. 11: 440. https://doi.org/10.3390/metabo10110440
APA StyleKim, J., Assefa, A. D., Song, J., Mani, V., Park, S., Lee, S.-K., Lee, K., Kim, D.-G., & Hahn, B.-S. (2020). Assessment of Metabolic Profiles in Florets of Carthamus Species Using Ultra-Performance Liquid Chromatography-Mass Spectrometry. Metabolites, 10(11), 440. https://doi.org/10.3390/metabo10110440







