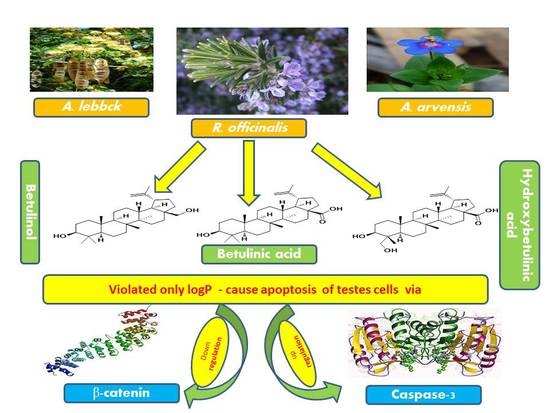
Testicular Caspase-3 and β-Catenin Regulators Predicted via Comparative Metabolomics and Docking Studies
Abstract
:1. Introduction
2. Results
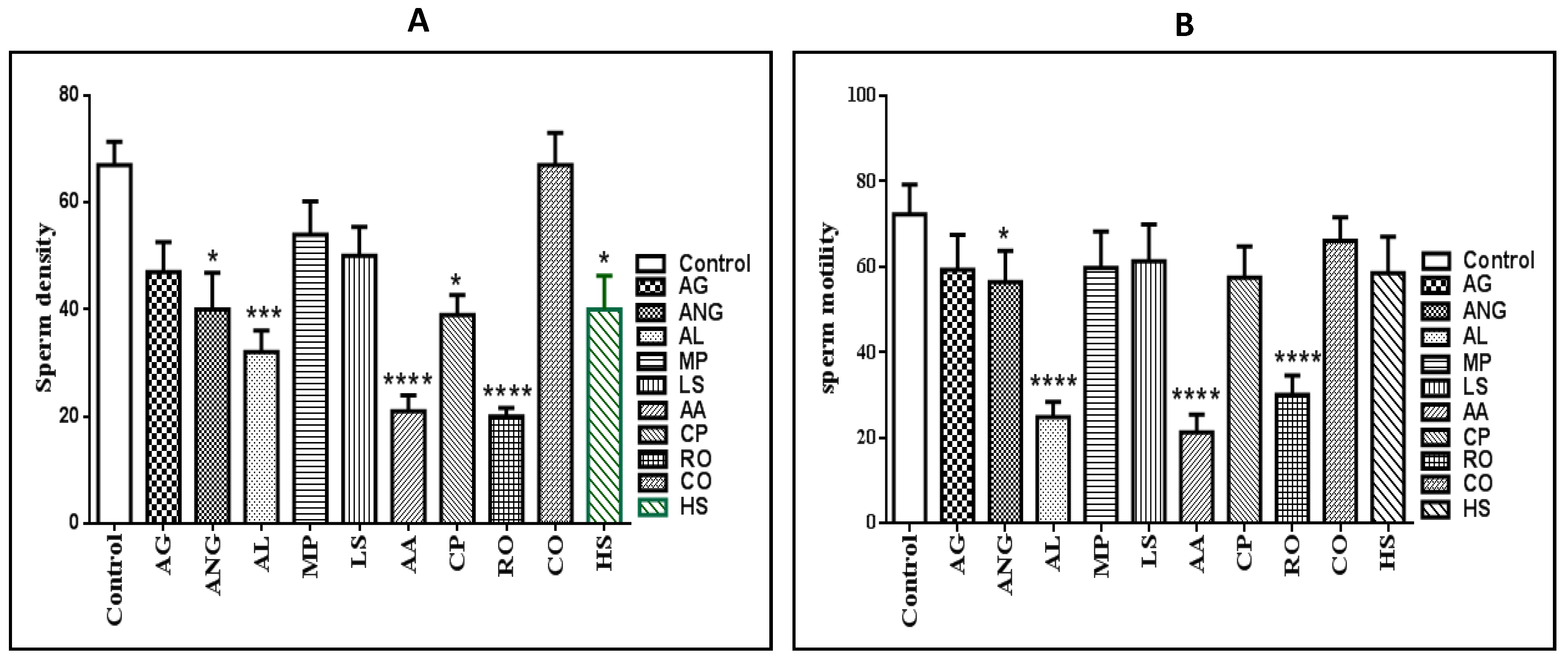
2.1. Sperm Density and Motility
2.2. Hormonal Assay and Effect on Organ Weights
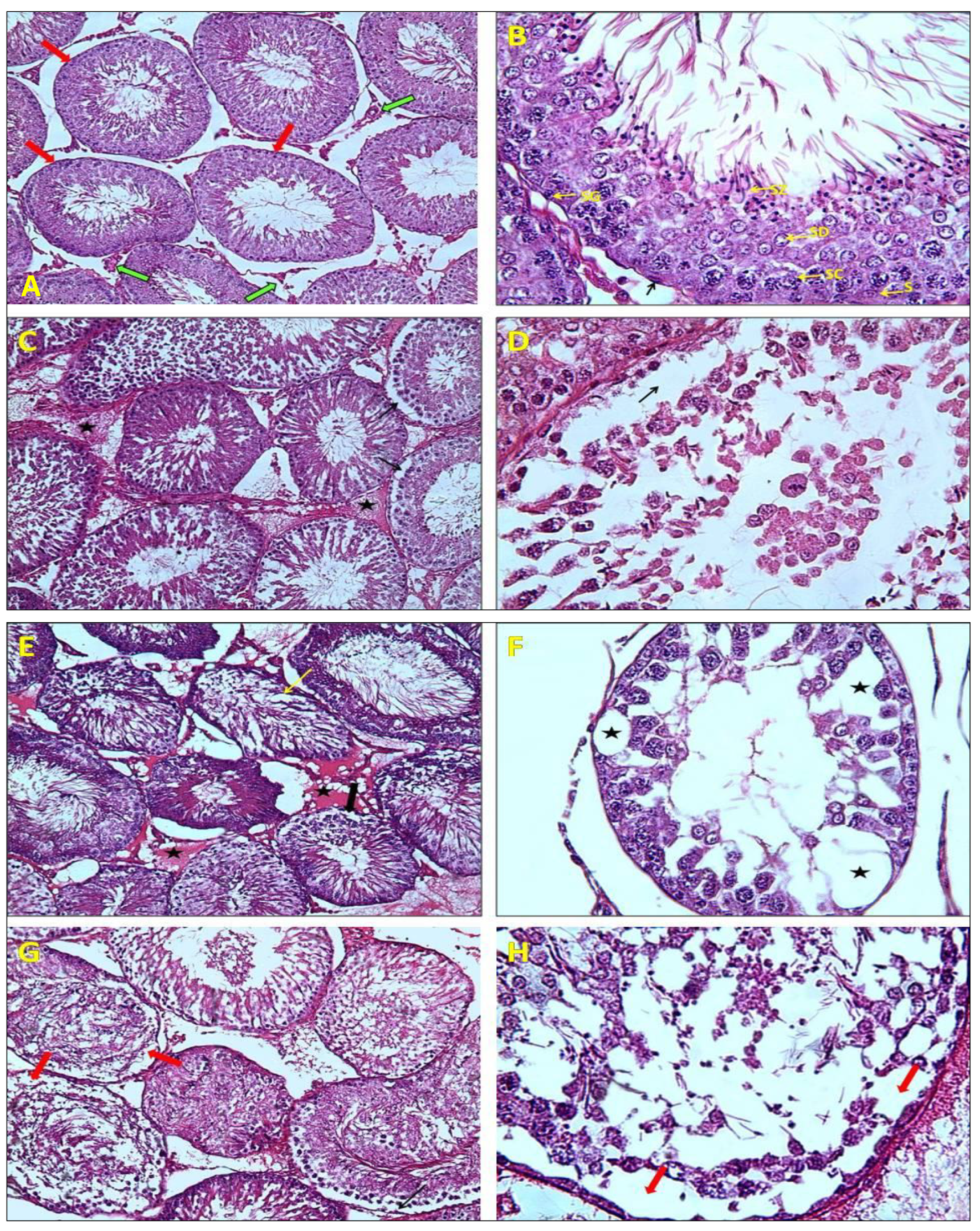
2.3. Histological Analysis
2.4. Multivariate Analysis
2.5. Chemical Diversity of Natural Products in Plant Extracts
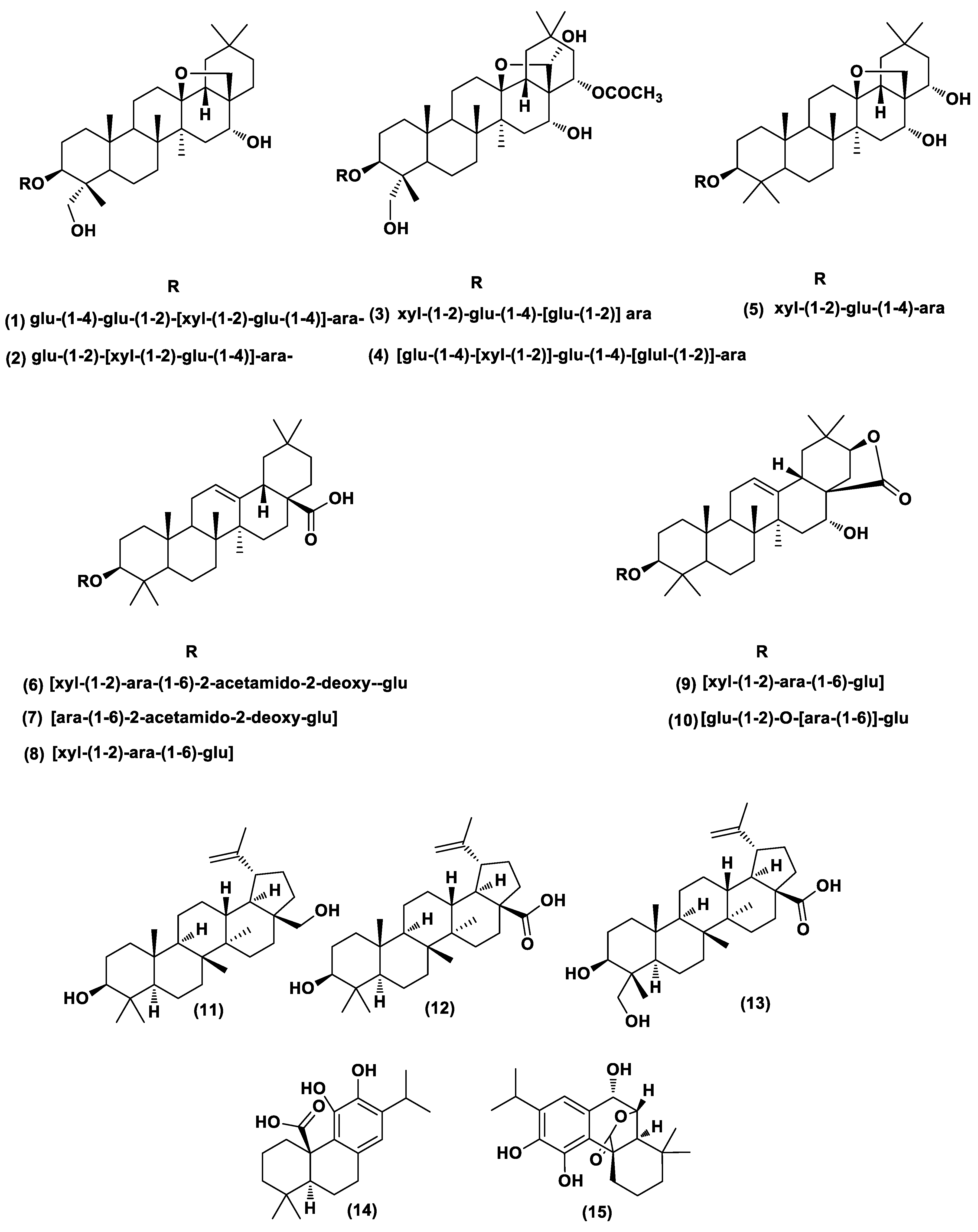
2.6. Dereplication of A. arvensis, A. lebbeck, and R. officinalis Extracts
2.7. Modelling Study
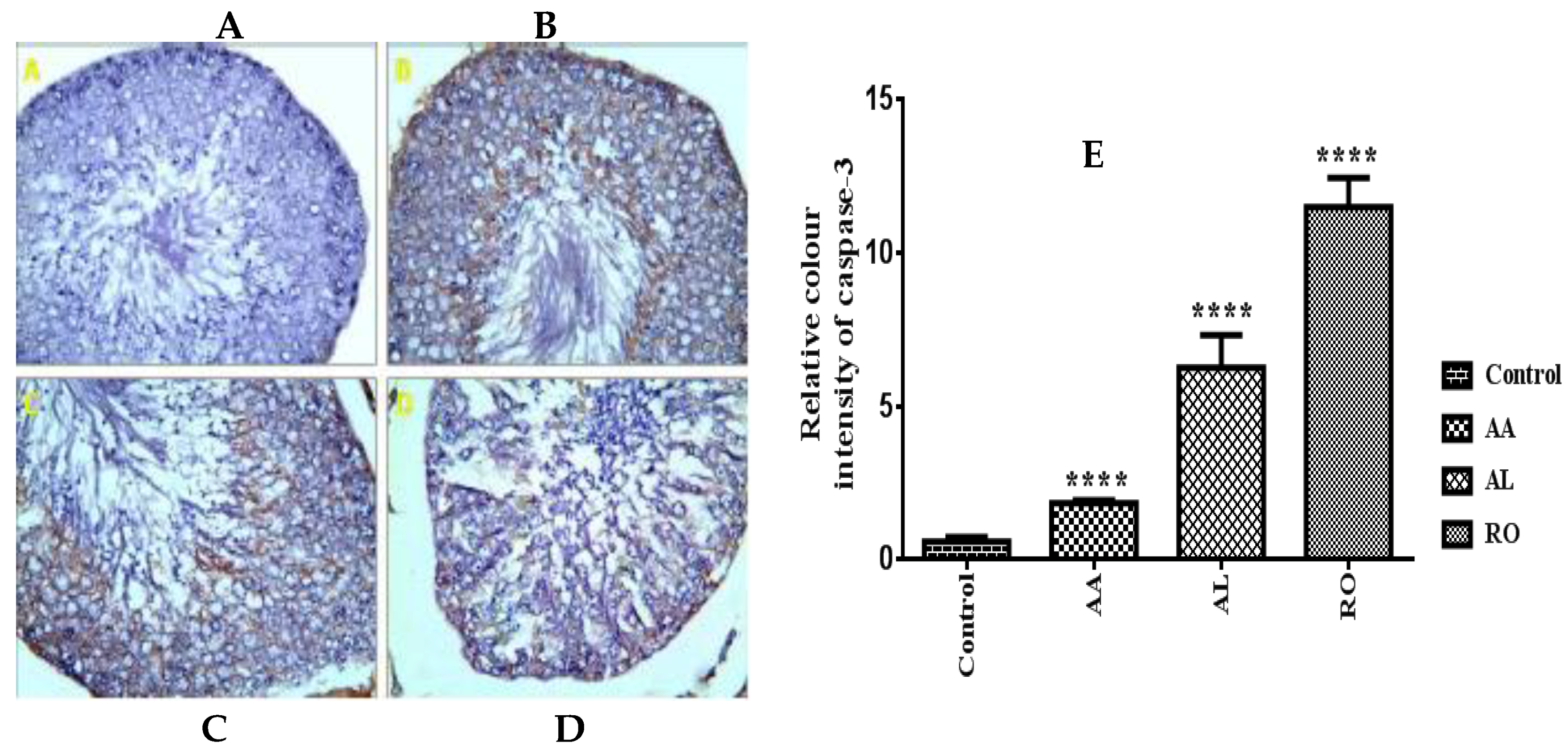
2.8. Immunohistochemical Assay
2.8.1. β-Catenin
2.8.2. Caspase-3
2.9. Rule of Five and Veber’s Oral Bioavailability Rule of High Features Compounds
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Plant Extraction
4.4. Oral Acute Toxicity Study
4.5. Animals Grouping, Modelling and Drugs Administration
4.6. Hormonal Assay
4.7. Semen Analysis
4.8. Histological Analysis
4.9. Metabolomics and PCA Analyses
4.10. Modelling Study
4.11. Immunohistochemical Assay
4.11.1. Immunohistochemical Procedure
4.11.2. Morphometric Study
4.12. Rule of Five and Veber’s Oral Bioavailability Rule of High Feature Compounds
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LD50 | Lethal dose 50 |
| FSH | Follicular stimulating hormone |
| PBS | Phosphate buffer saline |
| HBA | Hydrogen bond acceptor |
| tPSA | Two additional descriptors topological polar surface area |
| LH | Luteinizing hormone |
| PCA | Principle component analysis |
| DAB | 3,3′Diaminobenzidine |
| HBD | Hydrogen bond donor |
| ANOVA | One way analysis of variance |
References
- Ramakrishnan, P.S. Increasing population and declining biological resources in the context of global change and globalization. J. Biosci. 2001, 26, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Srivastava, S.; Kulshreshta, D.K.; Gupta, C.M. Traditional Remedies for Fertility Regulation. Curr. Med. Chem. 2004, 11, 1431–1450. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.C.D.; Vaithinathan, S.; Jubendradass, R.; Mathur, P.P. Effects of plants and plant products on the testis. Asian J. Androl. 2010, 12, 468–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, B.C.; Achari, B.; Yoshikawa, K.; Arihar, S. Saponins from Albizia lebbeck. Phytochemistry 1995, 38, 1287–1291. [Google Scholar] [CrossRef]
- Shoji, N.; Umeyama, A.; Yoshikawa, K.; Arihara, S. Structures of anagallosaponins I-V and their companion substances from Anagallis arvensis L. Chem. Pharm. Bull. 1994, 42, 1750–1755. [Google Scholar] [CrossRef] [Green Version]
- D’Ambrosio, M.; Ciocarlan, A.; Colombo, E.; Guerriero, A.; Pizza, C.; Sangiovanni, E.; Dell’Agli, M. Structure and cytotoxic activity of sesquiterpene glycoside esters from Calendula officinalis L.: Studies on the conformation of viridiflorol. Phytochemistry 2015, 117, 1–9. [Google Scholar] [CrossRef]
- Babu, N.P.; Pandikumar, P.; Ignacimuthu, S. Anti-inflammatory activity of Albizia lebbeck Benth., an ethnomedicinal plant, in acute and chronic animal models of inflammation. J. Ethnopharmacol. 2009, 125, 356–360. [Google Scholar] [CrossRef]
- Venkatesh, P.; Mukherjee, P.K.; Kumar, N.S.; Bandyopad, A.; Fukui, H.; Mizuguchi, H.; Islam, N. Anti-allergic activity of standardized extract of Albizia lebbeck with reference to catechin as a phytomarker. Immunopharmacol. Immunotoxicol. 2010, 32, 272–276. [Google Scholar] [CrossRef]
- Ahmed, D.; Kumar, V.; Verma, A.; Gupta, P.S.; Kumar, H.; Dhingra, V. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. Complement. Altern. Med. 2014, 14, 243. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Chaudhary, R.; Yadav, R.K.; Verma, S.K.; Dobhal, M.P. Effect of saponins of Albizia lebbeck (L.) Benth bark on the reproductive system of male albino rats. J. Ethnopharmacol. 2005, 96, 31–36. [Google Scholar] [CrossRef]
- Setty, B.S.; Kamboj, V.P.; Garg, H.S.; Khanna, N.M. Spermicidal potential of saponins isolated from indian medicinal plants. Contraception 1976, 14, 571–578. [Google Scholar] [CrossRef]
- Tiwari, S. Plants: A rich source of herbal medicine. J. Nat. Prod. 2008, 1, 27–35. [Google Scholar]
- Banerji, R.; Srivastava, A.K.; Misra, G.; Nigam, S.K.; Singh, S.; Nigam, S.C.; Saxena, R.C. Steroid and triterpenoid saponins as spermicidal agents. Indian Drugs 1979, 17, 6–8. [Google Scholar]
- García, D.; Sánchez, E.; Crespo, M.; Carballo, C. Estudio farmacognóstico de caléndula (Calendula officinalis L.). Rev. Cuba. Plantas Med. 1996, 1, 21–25. [Google Scholar]
- Maria, Y.; Dias, C.; Vicentini, F.T.M.C.; Nomizo, A.; Fernanda, R.; José, M.; Fonseca, V. Protective effect of Calendula officinalis extract against UVB-induced oxidative stress in skin: Evaluation of reduced glutathione levels and matrix metalloproteinase secretion. J. Ethnopharmacol. 2010, 127, 596–601. [Google Scholar]
- Kalvatchev, Z.; Walder, R.; Garzaro, D. Anti-HIV activity of extracts from Calendula officinalis flowers. Biomed. Pharmacother. 1997, 51, 176–180. [Google Scholar] [CrossRef]
- Parkhurst, R.M.; Stolzenberg, S.J. Saponin-Containing Spermatocidal Compositions. U.S. Patent 3,886,272, 27 May 1975. [Google Scholar]
- Yıldız, L.; Bas, K.S.; Tütem, E. Combined HPLC-CUPRAC (cupric ion reducing antioxidant capacity) assay of parsley, celery leaves, and nettle. Talanta 2008, 77, 304–313. [Google Scholar] [CrossRef]
- Abdulmanea, K.; Prokudina, E.A.; Lanková, P.; Vaní, L. Immunochemical and HPLC identification of isoflavonoids in the Apiaceae family. Biochem. Syst. Ecol. 2012, 45, 237–243. [Google Scholar] [CrossRef]
- Figueroa, M.G.; Rocha-guzmán, N.E.; Mercado-silva, E.; Loarca-piña, G.; Reynoso-camacho, R. Effect of chemical elicitors on peppermint (Mentha piperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem. 2014, 156, 273–278. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-Inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef] [Green Version]
- Baananou, S.; Bouftira, I.; Mahmoud, A. Antiulcerogenic and antibacterial activities of Apium graveolens essential oil and extract. Nat. Prod. Res. 2013, 27, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.; Kang, J.S.; Kim, G.; Park, I. Insecticidal and acetylcholine esterase inhibition activity of Apiaceae plant essential oils and their constituents against adults of German cockroach (Blattella germanica). Agric. Food Chem. 2012, 60, 7194–7203. [Google Scholar] [CrossRef] [PubMed]
- Asadi-samani, M.; Kafash-farkhad, N.; Azimi, N.; Fasihi, A.; Alinia-ahandani, E.; Rafieian-kopaei, M. Medicinal plants with hepatoprotective activity in Iranian folk medicine. Asian Pac. J. Trop. Biomed. 2015, 5, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Jorge, V.-G.; Ángel, J.-R.L.; Adrián, T.-S.; Francisco, A.-C.; Anuar, S.-G.; Samuel, E.-S.; Ángel, S.-O.; Emmanuel, H.-N. Vasorelaxant activity of extracts obtained from Apium graveolens: Possible source for vasorelaxant molecules isolation with potential antihypertensive effect. Asian Pac. J. Trop. Biomed. 2013, 3, 776–779. [Google Scholar] [CrossRef] [Green Version]
- Modaresi, M.; Ghalamkari, G.; Jalalizand, A. The effect of celery (Apium graveolens) extract on the reproductive hormones in male mice. APCBEE Procedia 2012, 4, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Shekhawat, G.S. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn. Rev. 2010, 4, 179–184. [Google Scholar]
- Orhan, I.E.; Senol, F.S. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. dill) samples cultivated under organic and conventional agricultural conditions. Food Chem. Toxicol. 2013, 59, 96–103. [Google Scholar] [CrossRef]
- Monsefi, M.; Zahmati, M.; Masoudi, M.; Javidnia, K. Effects of Anethum graveolens L. on fertility in male rats. Eur. J. Contracept. Reprod. Health Care 2011, 16, 488–497. [Google Scholar] [CrossRef]
- Kizil, S.; Hasimi, N.; Tolan, V.; Kilinc, E.; Yuksel, U. Mineral content, essential oil components and biological activity of two Mentha species (M. piperita L., M. spicata L.). Turk. J. Field Crops 2010, 15, 148–153. [Google Scholar]
- Sharma, A.; Sharma, M.K.; Kumar, M. Protective effect of Mentha piperita against arsenic-induced toxicity in liver of swiss albino mice. Basic Clin. Pharmacol. Toxicol. 2007, 100, 249–257. [Google Scholar] [CrossRef]
- Heshmati, A.; Dolatian, M.; Mojab, F. The effect of peppermint (Mentha piperita) capsules on the severity of primary dysmenorrhea. J. Herb. Med. 2016, 6, 137–141. [Google Scholar] [CrossRef]
- Akdogan, M.; Ozguner, M.; Kocak, A.; Oncu, M.; Cicek, E. Effects of peppermint teas on plasma luteinizing hormone levels and testicular tissue in rats. Urology 2004, 64, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Buch, J.G.; Dikshit, R.K.; Mansuri, S.M. Effect of certain volatile oils on ejaculated human spermatozoa. Indian J. Med. Res. 1988, 87, 361–363. [Google Scholar] [PubMed]
- Chopa, C.S.; Descamps, L.R. Composition and biological activity of essential oils against Metopolophium dirhodum (Hemiptera: Aphididae) cereal crop pest. Pest Manag. Sci. 2012, 68, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Linares, I.B.; Arráez-Román, D.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time. J. Chromatogr. A 2011, 1218, 7682–7690. [Google Scholar] [CrossRef] [Green Version]
- González-Trujano, M.E.; Peña, E.I.; Martínez, A.L.; Moreno, J.; Guevara-Fefer, P.; Deciga-Campos, M.; López-Muñoz, F.J. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Aqel, M.B. Relaxant effect of the volatile oil of Romarinus officinalis on tracheal smooth muscle. J. Ethmopharmacol. 1991, 33, 57–62. [Google Scholar] [CrossRef]
- Nusier, M.K.; Bataineh, H.N.; Daradkah, H.M. Adverse effects of rosemary (Rosmarinus officinalis L.) on reproductive function in adult male rats. Exp. Biol. Med. 2007, 232, 809–813. [Google Scholar]
- Alonso-villaverde, C.; Joven, J.; Menøndez, J.A.; Segura-carretero, A.; Fernµndez-gutiørrez, A. Short communication direct characterization of aqueous extract of Hibiscus sabdariffa using HPLC with diode array detection coupled to ESI and ion trap MS. J. Sep. Sci. 2009, 32, 3441–3448. [Google Scholar]
- Ali, B.H.; Al Wabel, N.; Blunden, G. Phytochemical, Pharmacological and toxicological aspects of Hibiscus sabdariffa L.: A Review. Phyther. Res. 2005, 375, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.I. Effect of extract of Hibiscus on the ultrastructure of the testis in adult mice. Acta Histochem. 2012, 114, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Orisakwe, O.E.; Husaini, D.C.; Afonne, O.J. Testicular effects of sub-chronic administration of Hibiscus sabdariffa calyx aqueous extract in rats. Reprod. Toxicol. 2004, 18, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; Nelson, C.J.; Lee, S.M. Cardenolides in the latex and leaves of seven Asclepias species and Calotropis procera. Phytochemistry 1982, 21, 2343–2348. [Google Scholar] [CrossRef]
- Nenaah, G.E. Potential of using flavonoids, latex and extracts from Calotropis procera (Ait.) as grain protectants against two coleopteran pests of stored rice. Ind. Crop. Prod. 2013, 45, 327–334. [Google Scholar] [CrossRef]
- Sharma, N.; Jacob, D. Inhibition of fertility and functional alteration in the genital organs of male swiss albino mouse after administration of Calotropis procera flower extract. Pharm. Biol. 2001, 39, 403–407. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Qureshi, N.M.; Arshad, R.; Begum, R. A study on the antisperm activity in extracts from different parts of Calotropis procera. Pak. J. Zool. 1991, 23, 161–166. [Google Scholar]
- Sarma, K.; Roychoudhury, S.; Sankar Bora, S.; Dehury, B.; Parida, P.; Das, S.; Das, R.; Dohutia, C.; Nath, S.; Deb, B. Molecular modeling and dynamics simulation analysis of KATNAL1 for identification of novel inhibitor of sperm maturation. Comb. Chem. High Throughput Screen. 2017, 20, 82–92. [Google Scholar] [CrossRef]
- Michalska, K.; Stojakowska, A.; Malarz, J.; Doležalová, I.; Lebeda, A.; Kisiel, W. Systematic implications of sesquiterpene lactones in Lactuca species. Biochem. Syst. Ecol. 2009, 37, 174–179. [Google Scholar] [CrossRef]
- Giordani, R.; Siepaio, M.; Moulin-Traffort, J.; Regli, P. Antifungal action of latex saps from Lactuca sativa L. and Asclepias curassavica L. Mycosis 1990, 33, 383–392. [Google Scholar]
- Harsha, S.N.; Anilakumar, K.R. Anxiolytic property of hydro-alcohol extract of Lactuca sativa and its effect on behavioral activities of mice. J. Biomed. Res. 2013, 27, 37. [Google Scholar] [PubMed]
- Ghorbani, A.; Rakhshandeh, H.; Reza, H. Potentiating Effects of Lactuca sativa on Pentobarbital-Induced Sleep. Iran. J. Pharm. Res. 2013, 12, 401–406. [Google Scholar] [PubMed]
- Ahangarpour, A.; Oroojan, A.A.; Radan, M. Effect of aqueous and hydro-alcoholic extracts of lettuce (Lactuca sativa) seed on testosterone level and spermatogenesis in NMRI mice. Iran. J. Reprod. Med. 2014, 12, 65–72. [Google Scholar] [PubMed]
- Bobzin, S.C.; Yang, S.; Kasten, T.P. LC-NMR: A new tool to expedite the dereplication and identification of natural products. J. Ind. Microbiol. Biotechnol. 2000, 25, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Tawfike, A.F.; Viegelmann, C.; Edrada-Ebel, R. Metabolomics and dereplication strategies in natural products. In Metabolomics Tools for Natural Product Discovery; Humana Press: Totowa, NJ, USA, 2013; pp. 227–244. [Google Scholar]
- Blunt, J. MarinLit; University of Canterbury: Christchurch, New Zealand, 2012. [Google Scholar]
- Laatsch, H. Antibase Version 4.0—The Natural Compound Identifier; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Int. J. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Werf, M.J.; Jellema, R.H.; Hankemeier, T. Microbial metabolomics: Replacing trial-and-error by the unbiased selection and ranking of targets. J. Ind. Microbiol. Biotechnol. 2005, 32, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, S.K.; Goel, N.; Singh, N. Influence of Albizia lebbeck Saponin and Its Fractions on In vitro gas production kinetics, rumen methanogenesis, and rumen fermentation characteristics. Vet. Sci. 2014, 2014, e498218. [Google Scholar]
- Soberón, J.R.; Sgariglia, M.A.; Pastoriza, A.C.; Soruco, E.M.; Jäger, N.; Labadie, G.R.; Sampietro, D.A.; Vattuone, M.A. Antifungal activity and cytotoxicity of extracts and triterpenoid saponins obtained from the aerial parts of Anagallis arvensis L. J. Ethnopharmacol. 2017, 203, 233–240. [Google Scholar] [CrossRef]
- Cleland, J.; Bernstein, S.; Ezeh, A.; Faundes, A.; Glasier, A.; Innis, J. Family planning: The unfinished agenda. Lancet 2006, 368, 1810–1827. [Google Scholar] [CrossRef]
- Bleakley, H.; Canning, D.; Field, E.; Finkelstein, A.; Mullainathan, S.; Newhouse, J.; Olken, B.; Pop-eleches, C.; Urdinola, P.; Zaslavsky, A. Contraception as development? New evidence from family planning in Colombia. Econ. J. 2005, 120, 709–736. [Google Scholar]
- Ueda, M.; Tokunaga, T.; Okazaki, M.; Noriko, U. A potential source of bioactive saponin from the leaves of Albizzia lebbeck. Nat. Prod. Res. 2003, 17, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Shoji, N.; Umeyama, A.; Yoshikawa, K.; Arihara, S. Triterpenoid glycosides from Anagallis arvensis. Phytochemistry 1994, 37, 1397–1402. [Google Scholar] [CrossRef]
- Machado, D.G.; Cunha, M.P.; Neis, V.B.; Balen, G.O.; Colla, A.; Bettio, L.E.B.; Oliveira, Á.; Pazini, F.L.; Dalmarco, J.B.; Simionatto, E.L. Antidepressant-like effects of fractions, essential oil, carnosol and betulinic acid isolated from Rosmarinus officinalis L. Food Chem. 2013, 136, 999–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razboršek, M.I.; Vončina, D.B.; Doleček, V.; Vončina, E. Determination of major phenolic acids, phenolic diterpenes and triterpenes in Rosemary (Rosmarinus officinalis L.) by gas chromatography and mass spectrometry. Acta Chim. Slov. 2007, 54, 60–67. [Google Scholar]
- Mahmoud, A.A.; Al-Shihry, S.S.; Son, B.W. Diterpenoid quinones from Rosemary (Rosmarinus officinalis L.). Phytochemistry 2005, 66, 1685–1690. [Google Scholar] [CrossRef]
- Wellwood, C.R.L.; Cole, R.A. Relevance of carnosic acid concentrations to the selection of rosemary, Rosmarinus officinalis (L.), accessions for optimization of antioxidant yield. J. Agric. Food Chem. 2004, 52, 6101–6107. [Google Scholar] [CrossRef]
- Petiwala, S.M.; Johnson, J.J. Diterpenes from rosemary (Rosmarinus officinalis): Defining their potential for anti-cancer activity. Cancer Lett. 2015, 367, 93–102. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Shi, D.; Chen, Y. Adult exposure to sasanguasaponin induces spermatogenic cell apoptosis in vivo through increased oxidative stress in male mice. Toxicol. Ind. Health 2010, 26, 691–700. [Google Scholar]
- Gupta, R.S.; Bhatnager, A.K.; Joshi, Y.C.; Sharma, R.; Sharma, A. Suppression of fertility in male albino rats following α-amyrin acetate administration. Pharm. Biol. 2004, 42, 98–104. [Google Scholar] [CrossRef]
- Gupta, R.S.; Bhatnager, A.K.; Joshi, Y.C.; Sharma, M.C.; Khushalani, V.; Kachhawa, J.B.S. Induction of antifertility with lupeol acetate in male albino rats. Pharmacology 2005, 75, 57–62. [Google Scholar] [CrossRef]
- Padashetty, S.A.; Mishra, S.H. Effect of terpenoidal fraction of Echinops echinatus roots on reproductive parameters of male rats. J. Nat. Med. 2007, 61, 452–457. [Google Scholar] [CrossRef]
- Gupta, R.; Kachhawa, J.B.S.; Gupta, R.S.; Sharma, A.K.; Sharma, M.C.; Dobhal, M.P. Phytochemical evaluation and antispermatogenic activity of Thevetia peruviana methanol extract in male albino rats. Hum. Fertil. 2011, 14, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-P.; GU, Z.-P.; You, Y.X.G.-D.; Cao, L.; Mao, B.-Y.; Qian, S.-Z. Effects of tripchlorolide on the epididymides and testes of rats. Pituitary 1999, 28, 25–79. [Google Scholar]
- Ni, B.; Zhu, T.; Jiang, Z.; Zhang, R.; Zhang, T.; Zhang, L. In vitro and in silico approaches for analyzing the toxicological effect of triptolide on Cx43 in Sertoli cells. Toxicol. Mech. Methods 2008, 18, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chan, H.C. Gossypol as a male antifertility agent-why studies should have been continued. Int. J. Androl. 1998, 21, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, E.M. Gossypol: A contraceptive for men. Contraception 2002, 65, 259–263. [Google Scholar] [CrossRef]
- Wang, X.; Howell, C.P.; Chen, F. Gossypol—A polyphenolic compound from cotton plant. Adv. Food Nutr. Res. 2009, 58, 215–263. [Google Scholar]
- Türk, G.; Ateşşahin, A.; Sönmez, M.; Çeribaşi, A.O.; Yüce, A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil. Steril. 2008, 89, 1474–1481. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.C.B.; Cajueiro, J.F.P.; Silva, S.V.; Soares, P.C.; Guerra, M.M.P. Effect of antioxidants resveratrol and quercetin on in vitro evaluation of frozen ram sperm. Theriogenology 2012, 77, 1722–1726. [Google Scholar] [CrossRef]
- Öǧretmen, F.; Inanan, B.E.; Öztürk, M. Protective effects of propolis on cryopreservation of common carp (Cyprinus carpio) sperm. Cryobiology 2014, 68, 107–112. [Google Scholar] [CrossRef]
- Yousef, M.I.; Salama, A.F. Propolis protection from reproductive toxicity caused by aluminium chloride in male rats. Food Chem. Toxicol. 2009, 47, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, A.; García-Caballero, M.; Medina, M.Á.; Quesada, A.R. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur. J. Nutr. 2013, 52, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-C.; Lee, M.-F.; Tsai, M.-L.; Lai, C.-S.; Lee, J.H.; Ho, C.-T.; Pan, M.-H. Rosmanol potently induces apoptosis through both the mitochondrial apoptotic pathway and death receptor pathway in human colon adenocarcinoma COLO 205 cells. Food Chem. Toxicol. 2011, 49, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.E.; Carothers, A.M.; Weyant, M.J.; Redston, M.; Bertagnolli, M.M. Carnosol inhibits β-catenin tyrosine phosphorylation and prevents adenoma formation in the C57bl/6j/Min/+ (Min/+) mouse. Cancer Res. 2005, 1097–1105. [Google Scholar]
- De Roche, M.; Rutherford, T.J.; Gupta, D.; Veprintsev, D.B.; Saxty, B.; Freund, S.M.; Bienz, M. An intrinsically labile α-helix abutting the BCL9-binding site of β-catenin is required for its inhibition by carnosic acid. Nat. Commun. 2012, 3, 610–680. [Google Scholar]
- Fulda, S.; Debatin, K. Betulinic acid induces apoptosis through a direct effect on mitochondria in neuroectodermal tumors. Med. Pediatr. Oncol. 2000, 35, 616–618. [Google Scholar] [CrossRef]
- Wang, D.; Liu, J.; Yin, M.; Li, X.; Lou, G.; Liu, Y.; Chen, X. Betulin induces apoptosis of HeLa cell lines in vitro and its possible mechanism. Tumor 2012, 32, 234–238. [Google Scholar]
- Haridas, V.; Higuchi, M.; Jayatilake, G.S.; Bailey, D.; Mujoo, K.; Blake, M.E.; Arntzen, C.J.; Gutterman, J.U. Avicins: Triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc. Natl. Acad. Sci. USA 2001, 98, 5821–5826. [Google Scholar] [CrossRef] [Green Version]
- Han, L.-T.; Li, J.; Huang, F.; Yu, S.-G.; Fang, N.-B. Triterpenoid saponins from Anemone flaccida induce apoptosis activity in HeLa cells. J. Asian Nat. Prod. Res. 2009, 11, 122–127. [Google Scholar] [CrossRef]
- Bian, Q.; Liu, P.; Gu, J.; Song, B. Tubeimoside-1 inhibits the growth and invasion of colorectal cancer cells through the Wnt/β-catenin signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 12517–12524. [Google Scholar]
- Lee, K.M.; Yun, J.H.; Lee, D.H.; Park, Y.G.; Son, K.H.; Nho, C.W.; Kim, Y.S. Chikusetsusaponin IVa methyl ester induces cell cycle arrest by the inhibition of nuclear translocation of β-catenin in HCT116 cells. Biochem. Biophys. Res. Commun. 2015, 459, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wang, H.; Guo, G.; Wang, S.; He, L.; Chen, H.; Wu, J. Male germ cell apoptosis and epigenetic histone modification induced by Tripterygium wilfordii Hook F. PLoS ONE 2011, 6, e0020751. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-F.; Lee-Chang, J.S.; Harris, K.Y.; Sinha-Hikim, A.P.; Rao, M.K. Role of β-catenin in post-meiotic male germ cell differentiation. PLoS ONE 2011, 6, e28039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurshid, A.; Vishal, M.B.; Mohammad, H.B.; Ashwini, K.S.; Saif, K.; Mohammad, A.K. Identification of potent caspase-3 inhibitors for treatment of multi-neurodegenerative diseases using pharmacophore modeling and docking approaches. CNS Neurol. Disord. Targets 2014, 13, 1346–1353. [Google Scholar]
- OECD. Guidance document on acute oral toxicity testing. In Environmental Health and Safety Monograph Series on Testing and Assessment; No. 23; OECD: Paris, France, 2000. [Google Scholar]
- Garber, J.; Barbee, R.; Bielitzki, J.; Clayton, L.; Donovan, J.; Hendriksen, C.F. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academic Press: Washington, DC, USA, 2010; ISBN 0309186633. [Google Scholar]
- Bearden, H.J.; Fuquay, J.W. Applied Animal Reproduction; Reston Publishing Company, Inc.: Reston, VA, USA, 1980; ISBN 0835902498. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Elsevier Health Sciences: London, UK, 2008; ISBN 0443102791. [Google Scholar]
- Abdelhafez, O.H.; Fawzy, M.A.; Fahim, J.R.; Desoukey, S.Y.; Krischke, M.; Mueller, M.J.; Abdelmohsen, U.R. Hepatoprotective potential of Malvaviscus arboreus against carbon tetrachloride-induced liver injury in rats. PLoS ONE 2018, 13, e020236. [Google Scholar] [CrossRef]
- Suvarna, K.; Layton, C.; Bancroft, J. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone of Elsevier: Philadelphia, PA, USA, 2013. [Google Scholar]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Ebejer, J.-P.; Charlton, M.H.; Finn, P.W. Are the physicochemical properties of antibacterial compounds really different from other drugs? J. Cheminform. 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]




| No | Accurate m/z | Suggested Formula | Ion Adduct | Tentative Detection b | Intensity | Plant Extract |
|---|---|---|---|---|---|---|
| 1 | 1225.6210 | C58H96O27 | [M + H]+ | Anagallisin A | 8.8 × 105 | AA |
| 2 | 1062.5593 | C52H86O22 | [M + H]+ | Anagallisin C | 4.4 × 107 | AA |
| 3 | 1136.5636 | C54H88O25 | [M + H]+ | Anagallosaponin II | 1.2 × 107 | AA |
| 4 | 1282.6160 | C60H98O29 | [M + H]+ | Anagallosaponin IX | 2.3 × 107 | AA |
| 5 | 900.5069 | C46H76O17 | [M + H]+ | Anagallosaponin VI | 3.6 × 106 | AA |
| 6 | 923.5234 | C48H77NO16 | [M + H]+ | Albiziatrioside A | 6.8 × 106 | AL |
| 7 | 792.4895 | C43H69NO12 | [M + H]+ | Albiziabioside A | 1.1 × 104 | AL |
| 8 | 883.5055 | C46H74O16 | [M + H]+ | Pitheduloside C | 2.2 × 104 | AL |
| 9 | 897.4844 | C46H72O17 | [M + H]+ | Albiziasaponin A | 3 × 104 | AL |
| 10 | 927.4945 | C47H74O18 | [M + H]+ | Albiziasaponin B | 1.9 × 104 | AL |
| 11 | 443.3881 | C30H50O2 | [M + H]+ | Betulinol | 5.6 × 104 | RO |
| 12 | 457.3677 | C30H48O3 | [M + H]+ | Betulinic acid | 5.6 × 104 | RO |
| 13 | 473.3622 | C30H48O4 | [M + H]+ | Hydroxybetulinic acid | 3.4 × 104 | RO |
| 14 | 333.2062 | C20H28O4 | [M + H]+ | Carnosic acid | 9.6 × 106 | RO |
| 15 | 346.1781 | C20H26O5 | [M + H]+ | Rosmanol | 1.4 × 107 | RO |
| Compound Name | Binding Energy Score * | Average Number of Poses per Run |
|---|---|---|
| 23-OH-betulinic acid | −7.474 | 10 |
| Albizeasaponin A | −15.227 | 10 |
| Albiziabioside A | −10.731 | 10 |
| Albiziasaponin B | −14.591 | 10 |
| Albiziatrioside A | −8.712 | 10 |
| Anagallicin A | −11.562 | 10 |
| Anagallicin C | −12.587 | 10 |
| Anagallosaponin II | −9.921 | 10 |
| Anagallosaponin IX | −13.087 | 10 |
| Anagallosaponin VI | −10.545 | 10 |
| Betulinic acid | −7.628 | 10 |
| Betulinol | −9.518 | 9 |
| Carnosic acid | −9.362 | 8 |
| Pitheduloside C | −10.143 | 10 |
| Rosmanol | −9.902 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hifnawy, M.S.; Aboseada, M.A.; Hassan, H.M.; AboulMagd, A.M.; Tohamy, A.F.; Abdel-Kawi, S.H.; Rateb, M.E.; El Naggar, E.M.B.; Liu, M.; Quinn, R.J.; et al. Testicular Caspase-3 and β-Catenin Regulators Predicted via Comparative Metabolomics and Docking Studies. Metabolites 2020, 10, 31. https://doi.org/10.3390/metabo10010031
Hifnawy MS, Aboseada MA, Hassan HM, AboulMagd AM, Tohamy AF, Abdel-Kawi SH, Rateb ME, El Naggar EMB, Liu M, Quinn RJ, et al. Testicular Caspase-3 and β-Catenin Regulators Predicted via Comparative Metabolomics and Docking Studies. Metabolites. 2020; 10(1):31. https://doi.org/10.3390/metabo10010031
Chicago/Turabian StyleHifnawy, Mohammed S., Mahmoud A. Aboseada, Hossam M. Hassan, Asmaa M. AboulMagd, Adel F. Tohamy, Samraa H. Abdel-Kawi, Mostafa E. Rateb, El Moataz Bellah El Naggar, Miaomiao Liu, Ronald J. Quinn, and et al. 2020. "Testicular Caspase-3 and β-Catenin Regulators Predicted via Comparative Metabolomics and Docking Studies" Metabolites 10, no. 1: 31. https://doi.org/10.3390/metabo10010031
APA StyleHifnawy, M. S., Aboseada, M. A., Hassan, H. M., AboulMagd, A. M., Tohamy, A. F., Abdel-Kawi, S. H., Rateb, M. E., El Naggar, E. M. B., Liu, M., Quinn, R. J., Alhadrami, H. A., & Abdelmohsen, U. R. (2020). Testicular Caspase-3 and β-Catenin Regulators Predicted via Comparative Metabolomics and Docking Studies. Metabolites, 10(1), 31. https://doi.org/10.3390/metabo10010031