Natural Antioxidants from Acmella oleracea Extract as Dermatocosmetic Actives
Abstract
1. Introduction
2. Materials and Methods
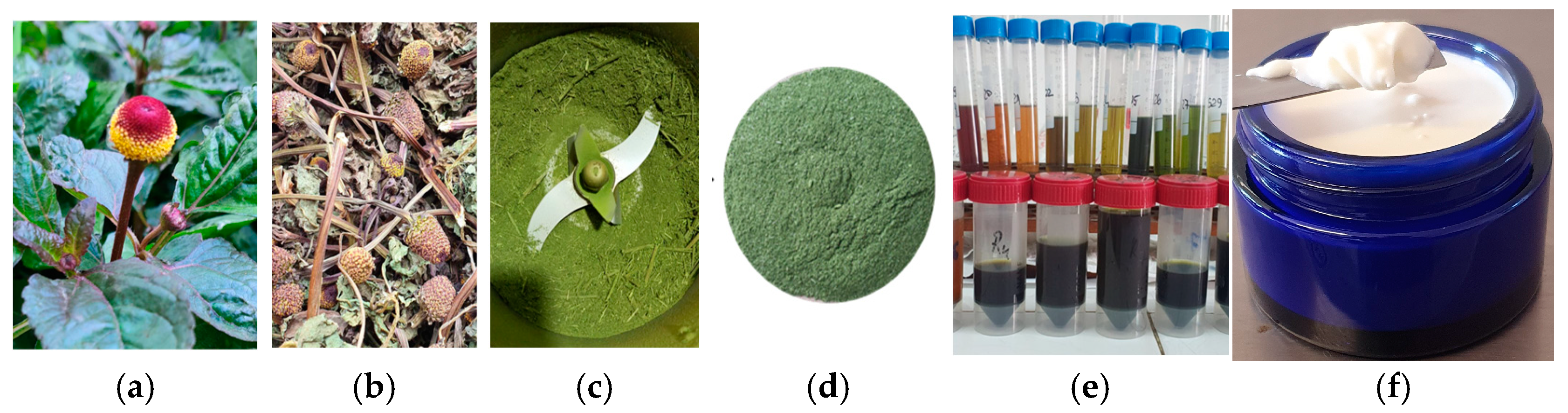
2.1. Plant Material
2.2. A. oleracea Extract
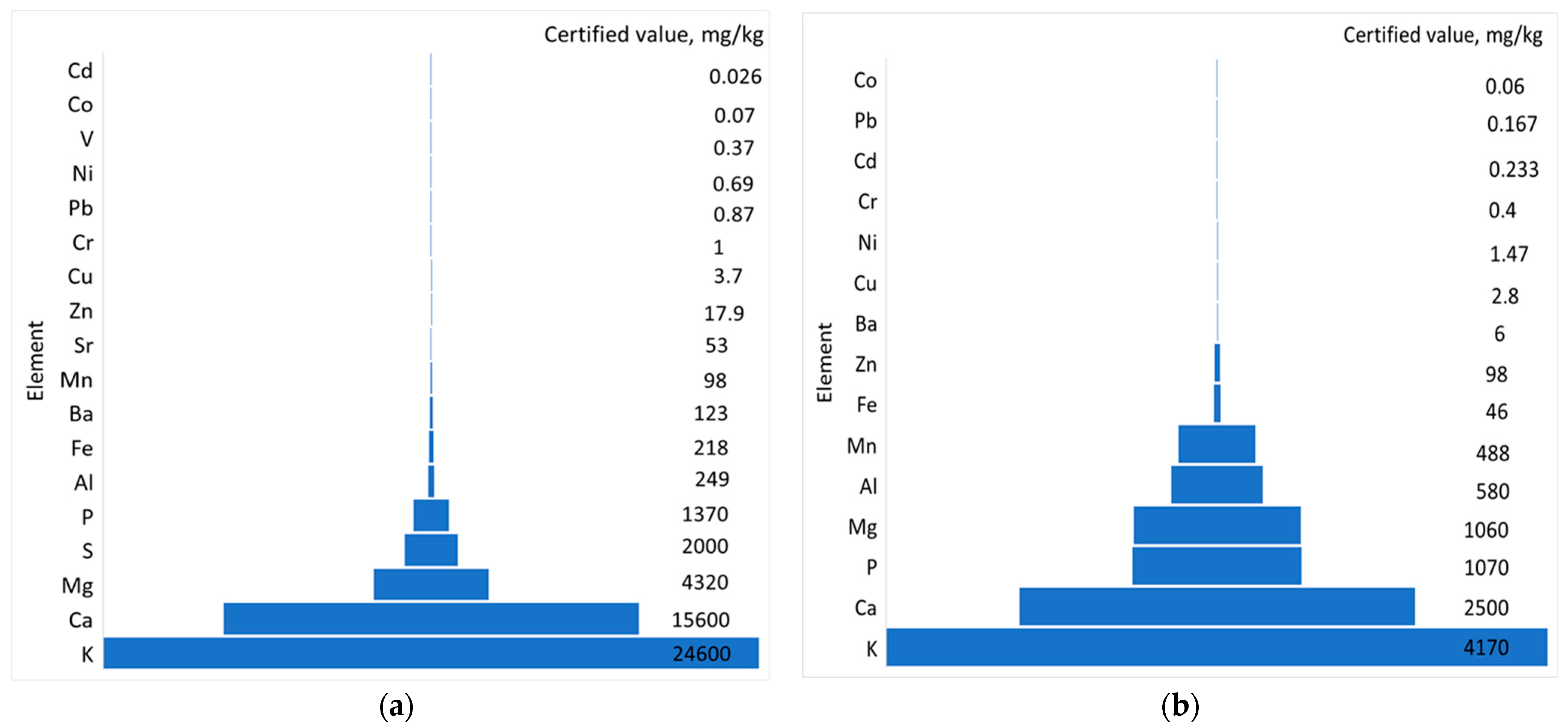
2.3. Evaluation of the Mineral Content of the Dried Plant
2.4. Evaluation of the Main Phytoconstituents of the Extract
2.5. Evaluation of the Antioxidant Activity of the Extract
2.6. The Methodology of Obtaining the Emulsion Based on the Extract of A. oleraceea
2.7. Characterization of Emulsion
2.7.1. Microbiological Control
2.7.2. Rheological Measurements
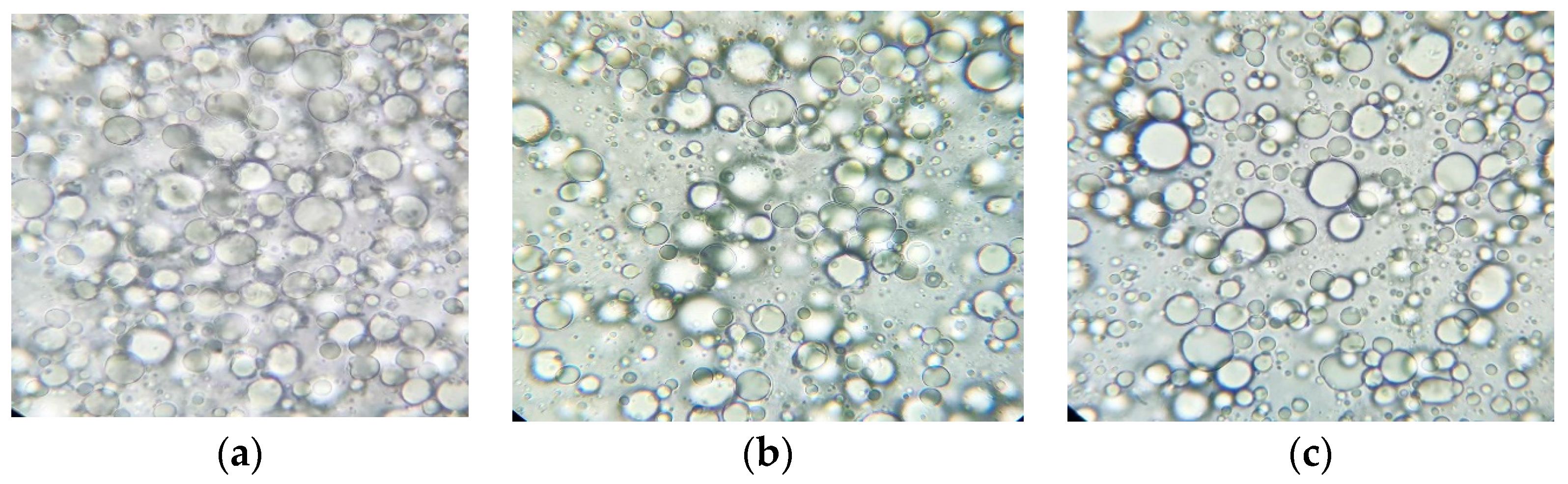
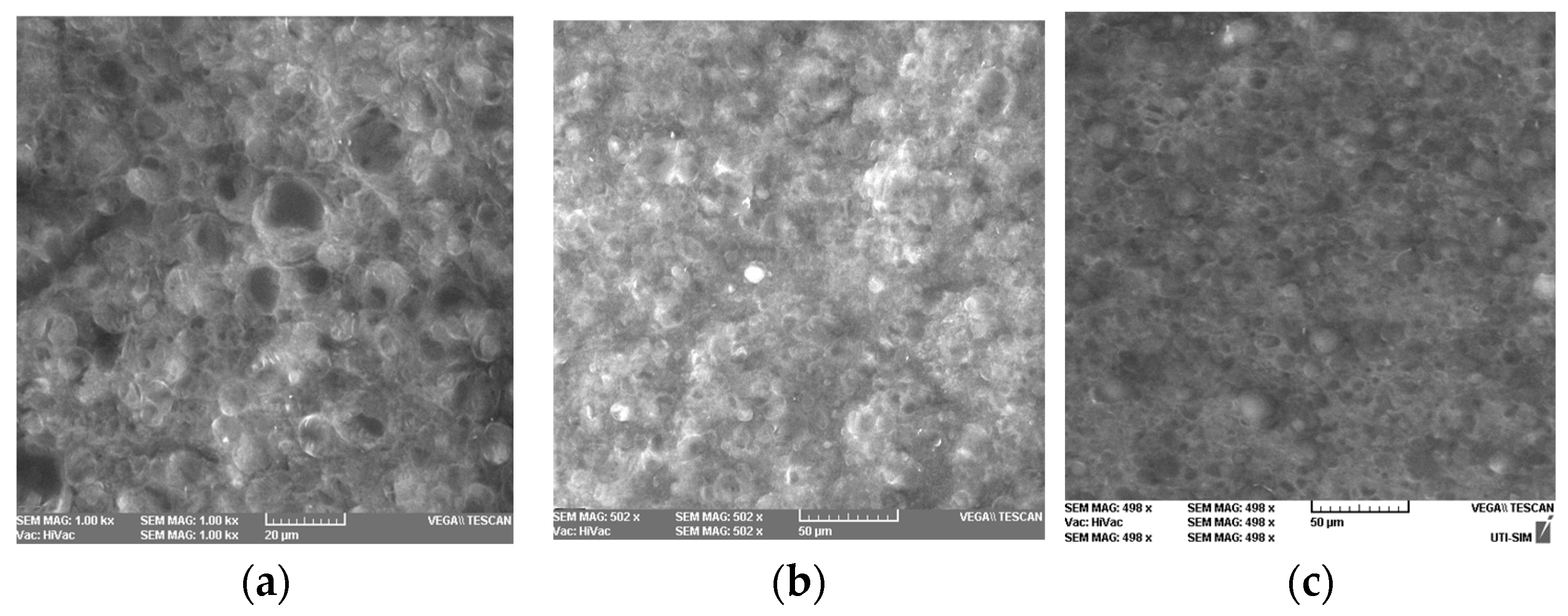
2.7.3. Analysis of Homogeneity of the Emulsion
2.7.4. In Vitro Evaluation of the Emulsion
3. Results
3.1. Determination of Minerals from the Initial Plant Sample
3.2. Vegetal Extract Characterized
3.3. Characterization the Obtained Emulsion
3.3.1. Microbiological Control
3.3.2. Analysis of Homogeneity of the Emulsion
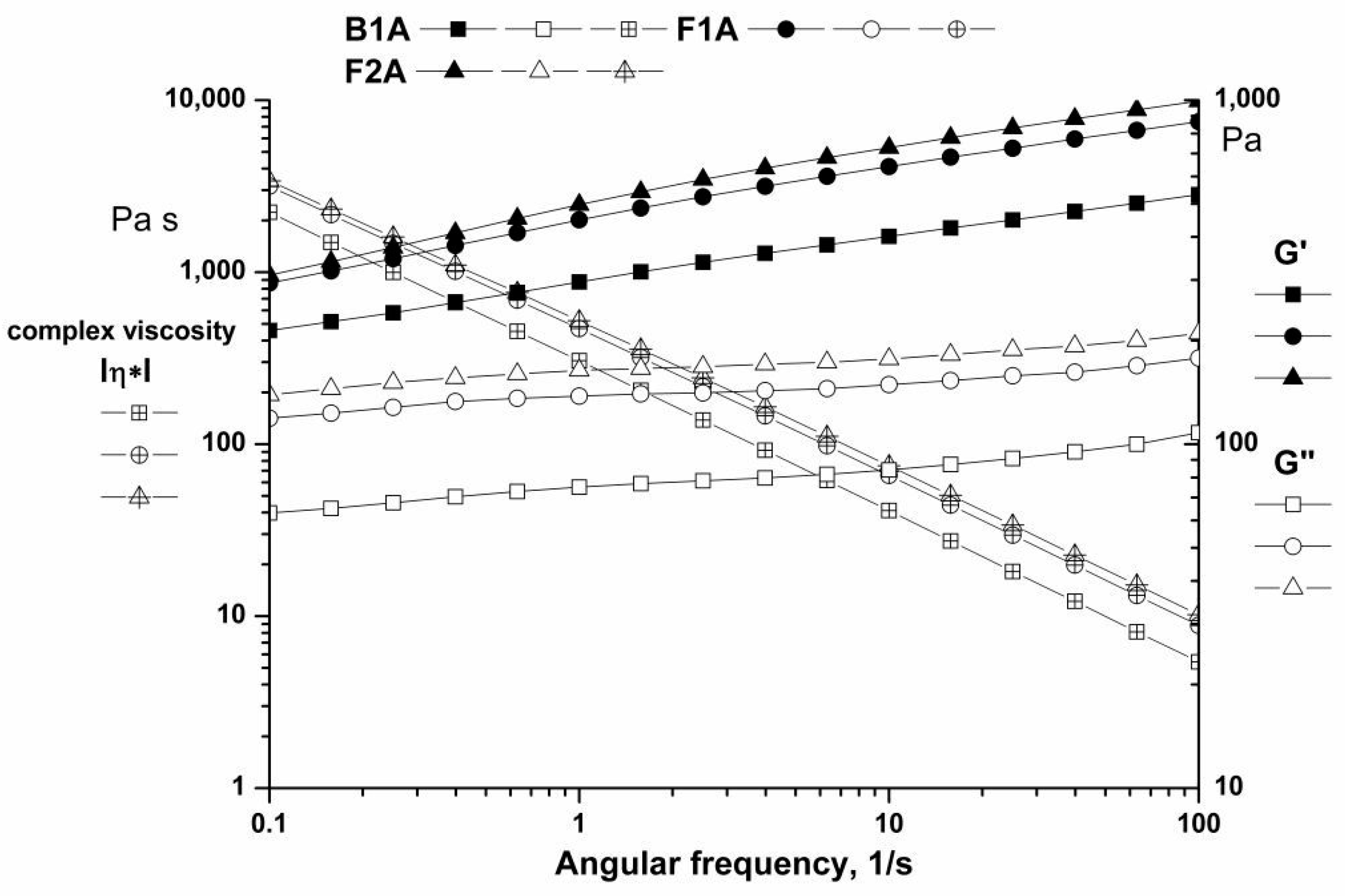
3.3.3. Rheological Tests
The Amplitude Sweep
The Frequency Sweep
Dynamic Temperature Sweep Tests
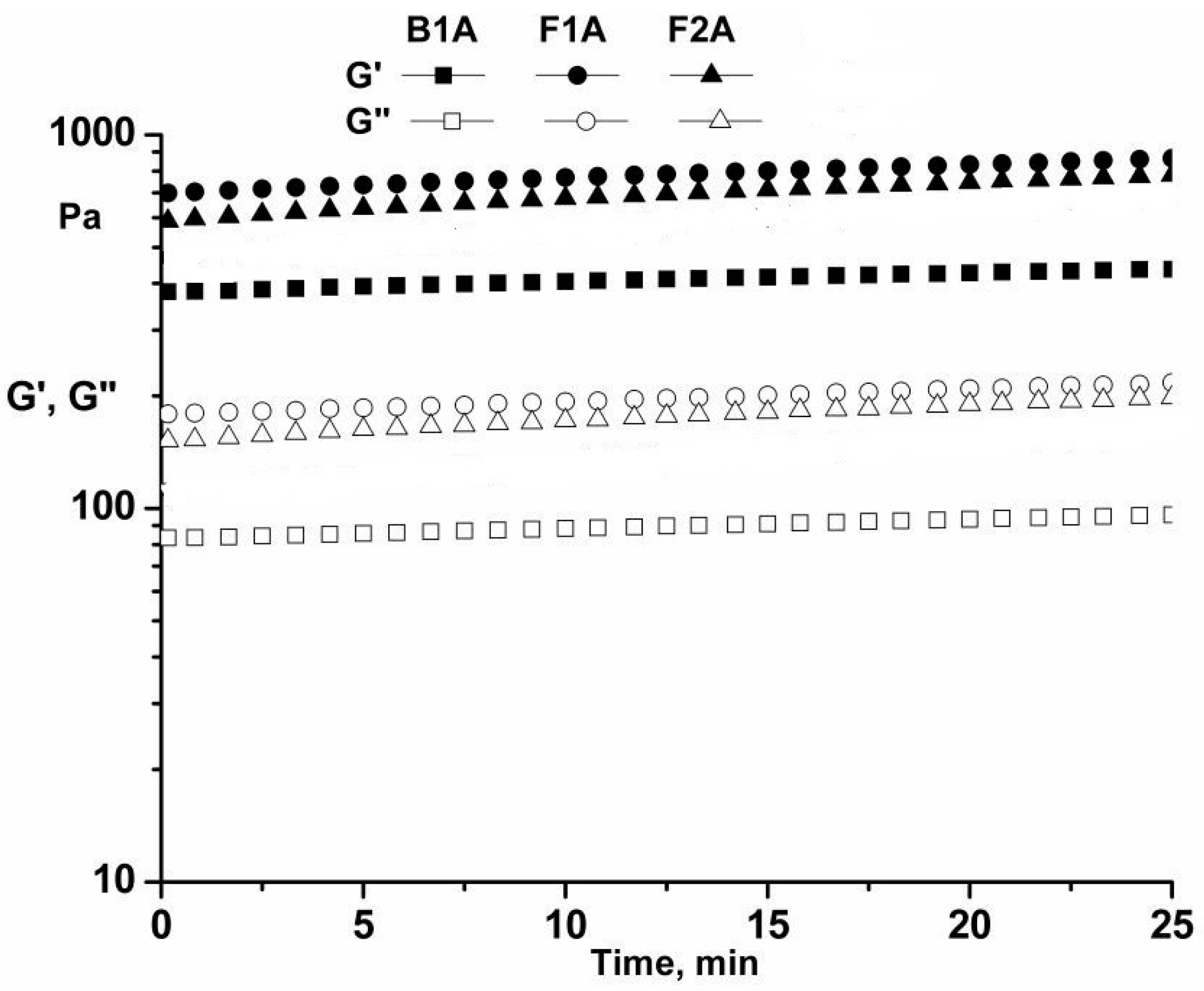
Time Sweep Tests
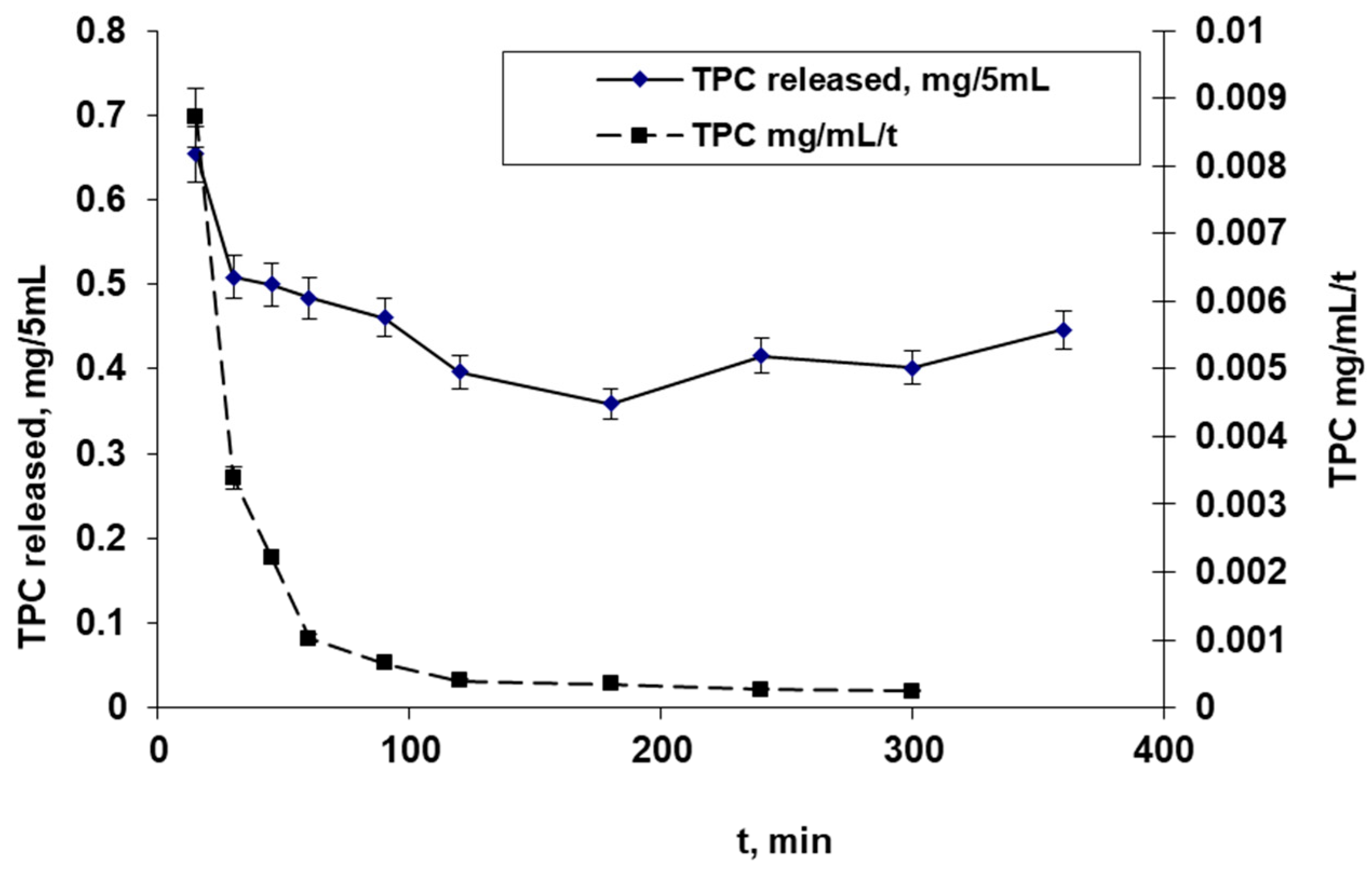
3.3.4. In Vitro Diffusion Test—Frant Cell Test
- The TPC value for the polyphenols released in the 5 mL receptor chamber is mg/mL mg;
- The TPC value for the polyphenols’ releasing speed is mg/mL/t.
4. Discussion
4.1. Microbiological Control of Cosmetic Emulsions
4.2. Analysis of Homogeneity of the Emulsion
4.3. Rheological Measurements
The Amplitude Sweep
4.4. In Vitro Diffusion Test—Frant Cell Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marchev, A.S.; Georgiev, M.I. Plant in vitro systems as a sustainable source of active ingredients for cosmeceutical application. Molecules 2020, 25, 2006. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Nasir, H.; Mohd-Setapar, S.H. Natural ingredients in cosmetics from Malaysian plants: A review. Sains Malays. 2018, 47, 951–959. [Google Scholar] [CrossRef]
- Apone, F.; Tito, A.; Arciello, S.; Carotenuto, G.; Colucci, M.G. Plant tissue cultures as sources of ingredients for skin care applications. Annu. Plant Rev. 2020, 3, 135–149. [Google Scholar]
- Martinez, A.; Estevez, J.C.; Silva-Pando, F.J. Antioxidant activity, total phenolic content and skin care properties of 35 selected plants from galicia (nw spain). Front. Life Sci. 2012, 6, 77–86. [Google Scholar] [CrossRef]
- Chutoprapat, R.; Malilas, W.; Rakkaew, R.; Udompong, S.; Boonpisuttinant, K. Collagen biosynthesis stimulation and anti-melanogenesis of Bambara groundnut (Vigna subterranea) extracts. Pharm. Biol. 2020, 58, 1023–1031. [Google Scholar] [CrossRef]
- Bezerra, P.H.A.; Stocco, B.; Bianchi, C.I.; Bianchini, F.; Figueiredo, S.A.; Fonseca, M.J.V.; Torqueti, M.R. Soybean extract modified by Aspergillus awamori stimulates a greater collagen synthesis in the intracellular matrix of human fibroblasts. J. Cosmet. Dermatol. 2022, 21, 1243–1250. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Gurumallesh, P.; Ramakrishnan, B. Evaluation of wound healing capacity of selected leaf extracts using in vitro scratch assay with l929 fibroblasts. Biosci. Biotechnol. Res. Commun. 2020, 13, 66–69. [Google Scholar] [CrossRef]
- Skarupova, D.; Vostalova, J.; Svobodova, A.R. Ultraviolet a protective potential of plant extracts and phytochemicals. Biomed. Pap.-Olomouc 2020, 164, 1–22. [Google Scholar] [CrossRef]
- Chermahini, S.H.; Majid, F.A.A.; Sarmidi, M.R. Cosmeceutical value of herbal extracts as natural ingredients and novel technologies in anti-aging. J. Med. Plants Res. 2011, 5, 3074–3077. [Google Scholar]
- Tarbiat, S.; Yener, F.G.; Kashefifahmian, A.; Mohseni, A.R. Antiaging effects of oleuropein combined with Helichrysum italicum or kumquat essentia oils in cosmetic lotions. Curr. Top. Nutraceutical Res. 2022, 20, 352–359. [Google Scholar]
- Paulo, F.; Santos, L. Microencapsulation of caffeic acid and its release using a w/o/w double emulsion method: Assessment of formulation parameters. Dry. Technol. 2019, 37, 950–961. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Mohd Setapar, S.H. Current status and future prospect of nanotechnology incorporated plant-based extracts in cosmeceuticals. In Nanotechnology for the Preparation of Cosmetics Using Plant-Based Extracts; Mohd Setapar, S.H., Ahmad, A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 235–261. [Google Scholar]
- Manful, M.E.; Ahmed, L.; Barry-Ryan, C. Cosmetic Formulations from Natural Sources: Safety Considerations and Legislative Frameworks in the European Union. Cosmetics 2024, 11, 72. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; Volume 1, pp. 285–292. [Google Scholar]
- Barbosa, A.F.; Silva, K.C.; Oliveira, M.C.D.; Carvalho, M.G.D.; Srur, A.U.S. Effects of Acmella oleracea methanolic extract and fractions on the tyrosinase enzyme. Rev. Bras. Farmacogn. 2016, 26, 321–325. [Google Scholar] [CrossRef]
- Spinozzi, E.; Ferrati, M.; Baldassarri, C.; Cappellacci, L.; Marmugi, M.; Caselli, A.; Benelli, G.; Maggi, F.; Petrelli, R. A review of the chemistry and biological activities of Acmella oleracea (“jambù”, Asteraceae), with a view to the development of bioinsecticides and acaricides. Plants 2022, 11, 2721. [Google Scholar] [CrossRef]
- Jerônimo, L.B.; Lima Santos, P.V.; Pinto, L.C.; da Costa, J.S.; de Aguiar Andrade, E.H.; Setzer, W.N.; do Rosário da Silva, J.K.; de Araújo, J.A.C.; Figueiredo, P.L.B. Acmella oleracea (L.) R.K. Jansen essential oils: Chemical composition, antioxidant, and cytotoxic activities. Biochem. Syst. Ecol. 2024, 112, 104775. [Google Scholar] [CrossRef]
- Bellumori, M.; Zonfrillo, B.; Maggini, V.; Bogani, P.; Gallo, E.; Firenzuoli, F.; Mulinacci, N.; Innocenti, M. Acmella oleracea (L.) R.K. Jansen: Alkylamides and phenolic compounds in aerial parts and roots of In Vitro seedlings. J. Pharm. Biomed. Anal. 2022, 220, 114991. [Google Scholar] [CrossRef] [PubMed]
- Abeysiri, G.R.P.I.; Dharmadasa, R.M.; Abeysinghe, D.C.; Samarasinghe, K. Screening of phytochemical, physico-chemical and bioactivity of different parts of Acmella oleraceae Murr. (Asteraceae), a natural remedy for toothache. Ind. Crops Prod. 2013, 50, 852–856. [Google Scholar] [CrossRef]
- SpSpinozzi, E.; Pavela, R.; Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Petrelli, R.; Cappellacci, L.; Fiorini, D.; Scortichini, S.; Garzoli, S.; et al. Spilanthol-rich essential oil obtained by microwave-assisted extraction from Acmella oleracea (L.) RK Jansen and its nanoemulsion: Insecticidal, cytotoxic and anti-inflammatory activities. Ind. Crops Prod. 2021, 172, 114027. [Google Scholar] [CrossRef]
- Demarne, F.; Passaro, G. Use of an Acmella oleracea Extract for the Botulinum Toxin-like Effect Thereof in an Anti-Wrinkle Cosmetic Composition. U.S. Patent 7, 531,193 B2, 2005. [Google Scholar]
- Uthpala, T.G.G.; Navaratne, S.B. Acmella oleracea Plant; Identification, Applications and Use as an Emerging Food Source—Review. Food Rev. Int. 2020, 37, 399–414. [Google Scholar] [CrossRef]
- Lalthanpuii, P.B.; Hruaitluangi, L.; Sailo, N.; Lalremsanga, H.T.; Lalchhandama, K. Nutritive value and antioxidant activity of Acmella oleracea (Asteraceae), a variety grown in Mizoram, India. Int. J. Phytopharm. 2017, 7, 42–46. [Google Scholar]
- Benelli, G.; Pavela, R.; Drenaggi, E.; Maggi, F. Insecticidal efficacy of the essential oil of jambú (Acmella oleracea (L.) R.K. Jansen) cultivated in central Italy against filariasis mosquito vectors, houseflies and moth pests. J. Ethnopharmacol. 2019, 229, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Panyadee, P.; Inta, A. Taxonomy and ethnobotany of Acmella (Asteraceae) in Thailand. Biodiversitas 2022, 23, 2177–2186. [Google Scholar] [CrossRef]
- Maxim, C.; Turcov, D.; Trifan, A.; Suteu, D.; Barna, A.S. Preliminary characterization of the phytoextracts from Acmella oleracea with therapeutic potential and applicability in active cosmetics. Sci. Study Res. 2024, 25, 169–182. [Google Scholar]
- Zinicovscaia, I.; Hramco, C.; Chaligava, O.; Yushin, N.; Grozdov, D.; Vergel, K.; Duca, G. Accumulation of Potentially Toxic Elements in Mosses Collected in the Republic of Moldova. Plants 2021, 10, 471. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasa, A.M.; Suteu, D. Antioxidants from Galium verum as ingredients for the design of new dermatocosmetic products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In Vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.S.; Ajjarapu, S.; Wu, K.-W.; Ponkshe, P.; Repka, M.A.; Murthy, S.N. Microbial Stability of Pharmaceutical and Cosmetic Products. AAPS Pharmscitech 2018, 19, 60–78. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Blaga, A.C.; Ibanescu, C.; Danu, M.; Trifan, A.; Zbranca, A.; Suteu, D. Dermatocosmetic emulsions based on the resveratrol, ferulic acid and Saffron (Crocus sativus) extract to combat skin oxidative stress-trigger factor of some potential malignant effects: Stability studies and rheological properties. Pharmaceutics 2022, 14, 2376. [Google Scholar] [CrossRef]
- Barna, A.S.; Maxim, C.; Trifan, A.; Blaga, A.C.; Cimpoesu, R.; Turcov, D.; Suteu, D. Preliminary Approaches to Cosmeceuticals Emulsions Based on N-ProlylPalmitoyl Tripeptide-56 Acetat-Bakuchiol Complex Intended to Combat Skin Oxidative Stress. J. Mol. Sci. 2023, 24, 7004. [Google Scholar] [CrossRef]
- Turcov, D.; Peptu, A.C.; Barna, A.S.; Zbranca, A.; Suteu, D. In Vitro evaluation of the dermatocosmetic emulsions based on Lady’s Bedstraw (Galium verum) alchoolic extracts. In Proceedings of the 10th IEEE International Conference on E-Health and Bioengineering—EHB 2022, Iasi, Romania, 17–18 November 2022. [Google Scholar]
- Bujor, A.; Ochiuz, L.; Sha’at, M.; Stoleriu, I.; Stamate Iliuta, M.; Luca, S.V.; Miron, A. Chemical, antioxidant and In vitro permeation and penetration studies of extracts obtained from Viburnum opulus and Crataegus pentagyna. Farmacia 2020, 68, 672–678. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Quantification of skin penetration of antioxidants of varying lipophilicity. Int. J. Cosmet. Sci. 2012, 35, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hlihor, R.M.; Rosca, M.; Hagiu-Zaleschi, L.; Simion, I.M.; Daraban, G.M.; Stoleru, V. Medicinal Plant Growth in Heavy Metals Contaminated Soils: Responses to Metal Stress and Induced Risks to Human Health. Toxics 2022, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/Convention Task Force on the Health Aspects of Air Pollution. Health Risks of Heavy Metals from Long-Range Transboundary Air Pollution; WHO Regional Office for Europe: Geneva, Switzerland, 2007; ISBN 9788490225370. [Google Scholar]
- Filote, C.; Rosca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable Application of Biosorption and Bioaccumulation of Persistent Pollutants in Wastewater Treatment: Current Practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Lalthanpuii, P.B.; Lalawmpuii, R.; Vanlaldinpuia, K.; Lalchhandama, K. Phytochemical investigations on the medicinal plant Acmella oleracea cultivated in Mizoram, India. Sci. Vis. 2016, 16, 177–183. [Google Scholar]
- Nascimento, L.E.S.; Arriola, N.D.A.; da Silva, L.A.L.; Faqueti, L.G.; Sandjo, L.P.; de Araújo, C.E.S.; Biavatti, M.W.; Barcelos-Oliveira, J.L.; Amboni, R.D.D.M.C. Phytochemical profile of different anatomical parts of jambu (Acmella oleracea (L.) RK Jansen): A comparison between hydroponic and conventional cultivation using PCA and cluster analysis. Food Chem. 2020, 332, 127393. [Google Scholar] [CrossRef]
- da Silva Borges, L.; de Souza Vieira, M.C.; Vianello, F.; Goto, R.; Lima, G.P.P. Antioxidant compounds of organically and conventionally fertilized jambu (Acmella oleracea). Biol. Agric. Hortic. 2016, 32, 149–158. [Google Scholar] [CrossRef]
- Abeysinghe, D.C.; Wijerathne, S.M.N.K.; Dharmadasa, R.M. Secondary metabolites contents and antioxidant capacities of Acmella oleraceae grown under different growing systems. World J. Agric. Res. 2014, 2, 163–167. [Google Scholar]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef] [PubMed]
- Brummer, R. Rheology of cosmetic emulsions. In Product Design and Engineering: Formulation of Gels and Pastes; Brockel, U., Meier, W., Wagner, G., Eds.; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2013; pp. 51–74. [Google Scholar]
- Tafuro, G.; Costantini, A.; Baratto, G.; Francescato, S.; Busata, L.; Semenzato, A. Characterization of polysaccharide associations for cosmetic use: Rheology and texture analysis. Cosmetics 2021, 8, 62. [Google Scholar] [CrossRef]
- Niknam, R.; Soudi, M.R.; Mousavi, M. Rheological and stability evaluation of emulsions containing fenugreek galactomannan—Xanthan gum mixtures: Effect of microwave and ultrasound treatments. Macromol 2022, 2, 361–373. [Google Scholar] [CrossRef]
- Fuhrmann, P.; Breunig, S.; Sala, G.; Sagis, L.; Stieger, M.; Scholten, E. Rheological behaviour of attractive emulsions differing in droplet-droplet interaction strength. J. Colloid Interface Sci. 2021, 607, 389–400. [Google Scholar] [PubMed]
- Huynh, A.; Garcia, A.G.; Young, L.K.; Szoboszlai, M.; Liberatore, M.W.; Baki, G. Measurements meet perceptions: Rheology-texture-sensory relations when using green, bio-derived in cosmetic emulsions. Int. J. Cosmet. Sci. 2021, 43, 11–19. [Google Scholar] [PubMed]
- Bom, S.; Fitas, M.; Martins, A.M.; Pinto, P.; Ribeiro, H.M.; Marto, J. Replacing synthetic ingredients by sustainable natural alternatives: A case study using topical O/W emulsions. Molecules 2020, 25, 4887. [Google Scholar] [CrossRef] [PubMed]
- Raposo, S.; Salgado, A.; Eccleston, G.; Urbano, M.; Ribeiro, H.M. Cold processed oil-in-water emulsions for dermatological purpose: Formulation design and structure analysis. Pharm. Dev. Technol. 2014, 19, 417–429. [Google Scholar] [CrossRef]
- Turcov, D.; Peptu, A.C.; Zbranca, A.; Suteu, D. In Vitro evaluation of the dermatocosmetic emulsions based on saffron (Crocus sativus) alchoolic extracts. Bull. IPI Secțiunea Chim. Ing. Chim 2023, 69, 39–46. [Google Scholar]
| Polyphenols Content, mg GAE/mL | Flavonoids Content, mg QE/mL | Antioxidant Activity | |
|---|---|---|---|
| DPPH (mg TE/mL) | ABTS (mg TE/mL) | ||
| 3.7986 | 4.490 | 0.21 ± 0.05 | 2.03 ± 0.12 |
| Plant | Type of Extract | Polyphenol Content | Flavonoid Content | Ref. |
|---|---|---|---|---|
| Flowering aerial parts of A. oleracea | Solvent Methanol, Soxhlet extract | 1.38 GAE mg/g | 28.7 QE mg/g | [39] |
| Leaf of A. oleracea | 80% ethanol (v/v), ultrasonic extraction | 3.19 mg GAE/g | 11.45 mg RE/g | [40] |
| Flower of A. oleracea | 80% ethanol (v/v), Ultrasonic extraction | 1.98 mg GAE/g | 5.91 mg RE/g | [40] |
| Dried extract of Flowering aerial parts of A. oleracea | Solvent Methanol, Soxhlet extract | 7.59 mg GAE/g of dried extract | indefinite | [19] |
| Fresh leaves of A. oleracea | Solvent Methanol, Ultrasonic extraction, 30 min. | 588.65 mg GAE/100 g | 9.32 mg RE/100 g | [41] |
| Fresh flower of A. oleracea | Solvent Methanol Ultrasonic extraction, 30 min. | 292.81 mg GAE/100 g | 4.10 mg RE/100 g | [41] |
| Dried leaves of A. oleracea | Solvent Methanol Vortex extraction | 10.99 mg GAE/g | 11.33 mg RE/g | [42] |
| Sample | Total Viable Microbiological Count, CFU/g | Total Viable Bacteria Count, CFU/g | Total Viable Yeast and Molds Count, CFU/g | Presence of Pathogenic Contaminants |
|---|---|---|---|---|
| Formulation 1 3% extract Acmella oleracea (F1A) | 10 | 10 | 10 | absent |
| Formulation 2 5% extract Acmella oleracea (F2A) | 0 | 0 | 10 | absent |
| Base (B1A) | 0 | 0 | 0 | absent |
| Samples | Strain (ɣ = 0.1%) | |
|---|---|---|
| G’ (Pa) | G” (Pa) | |
| B1A | 417 | 86 |
| F1A | 671 | 159 |
| F2A | 773 | 187 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxim, C.; Blaga, A.C.; Cimpoeșu, R.; Zinicovscaia, I.; Peshkova, A.; Danu, M.; Barna, A.S.; Suteu, D. Natural Antioxidants from Acmella oleracea Extract as Dermatocosmetic Actives. Sci. Pharm. 2024, 92, 52. https://doi.org/10.3390/scipharm92030052
Maxim C, Blaga AC, Cimpoeșu R, Zinicovscaia I, Peshkova A, Danu M, Barna AS, Suteu D. Natural Antioxidants from Acmella oleracea Extract as Dermatocosmetic Actives. Scientia Pharmaceutica. 2024; 92(3):52. https://doi.org/10.3390/scipharm92030052
Chicago/Turabian StyleMaxim, Claudia, Alexandra Cristina Blaga, Ramona Cimpoeșu, Inga Zinicovscaia, Alexandra Peshkova, Maricel Danu, Ana Simona Barna, and Daniela Suteu. 2024. "Natural Antioxidants from Acmella oleracea Extract as Dermatocosmetic Actives" Scientia Pharmaceutica 92, no. 3: 52. https://doi.org/10.3390/scipharm92030052
APA StyleMaxim, C., Blaga, A. C., Cimpoeșu, R., Zinicovscaia, I., Peshkova, A., Danu, M., Barna, A. S., & Suteu, D. (2024). Natural Antioxidants from Acmella oleracea Extract as Dermatocosmetic Actives. Scientia Pharmaceutica, 92(3), 52. https://doi.org/10.3390/scipharm92030052










