Cannabigerol Reduces Acute and Chronic Hypernociception in Animals Exposed to Prenatal Hypoxia-Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Substances
2.3. Experimental Model
2.3.1. Pharmacological Screening
Hot Plate Test
Locomotor Activity
2.3.2. Induction of Prenatal Hypoxic-Ischemic Insult
2.3.3. Formalin Test
2.3.4. Chronic Nociception Model
2.3.5. Thermal and Mechanical Hypernociception
2.4. Tissue Collection
2.5. Quantification of IFN-Gamma by ELISA
2.6. Gene Expression Evaluation
2.7. Statistical Analysis
3. Results
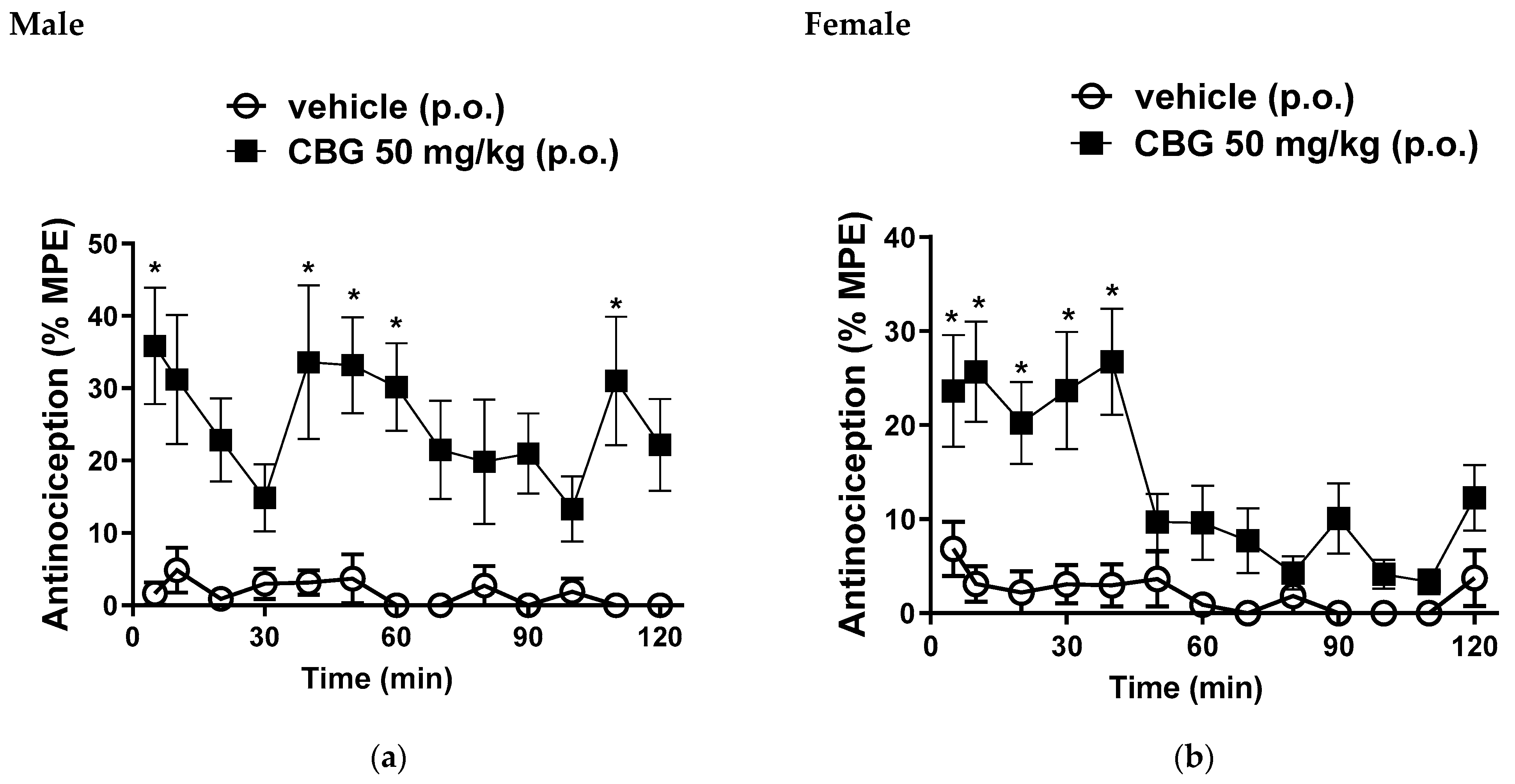
3.1. Hot Plate Test
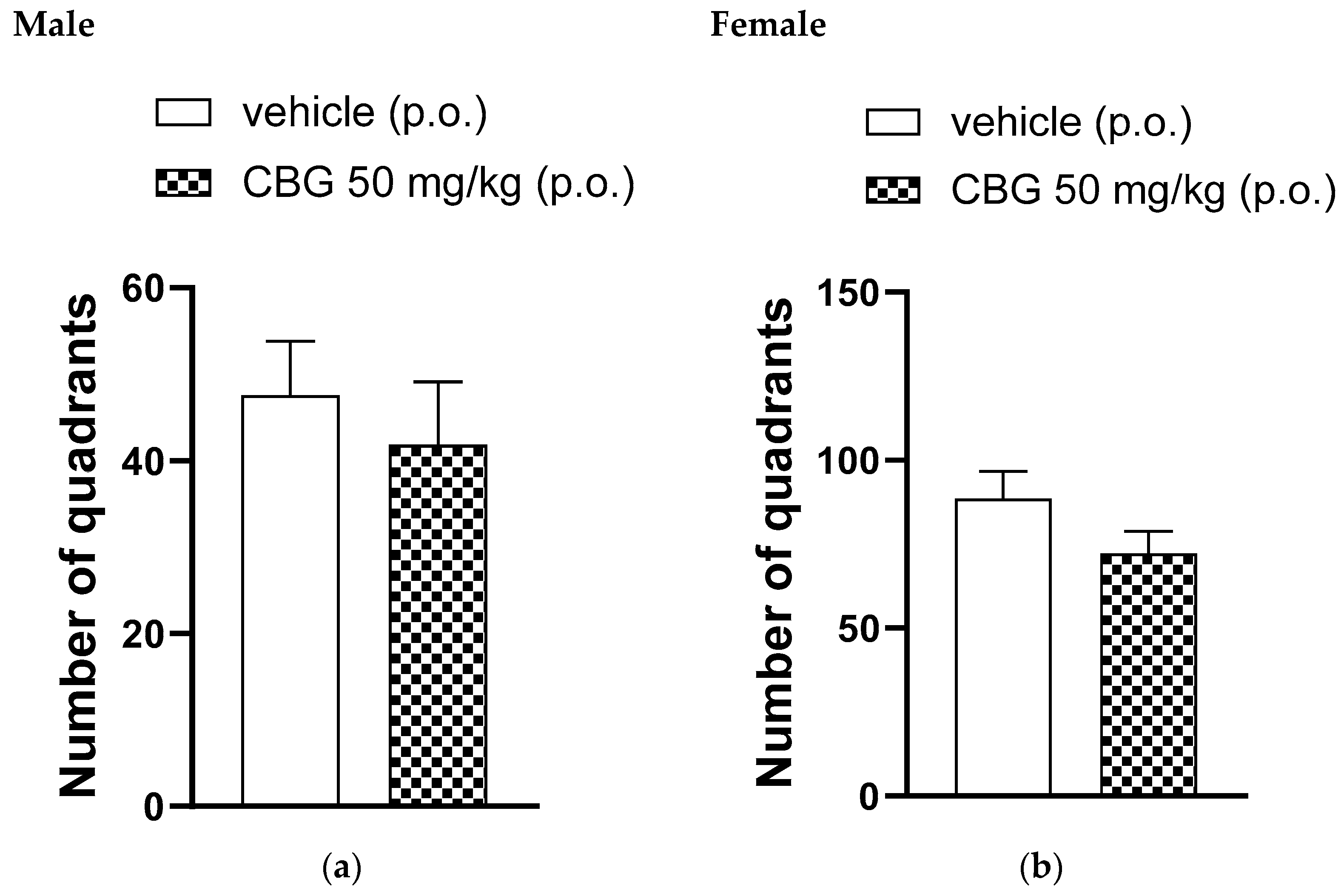
3.2. Locomotor Activity
3.3. Formalin Test
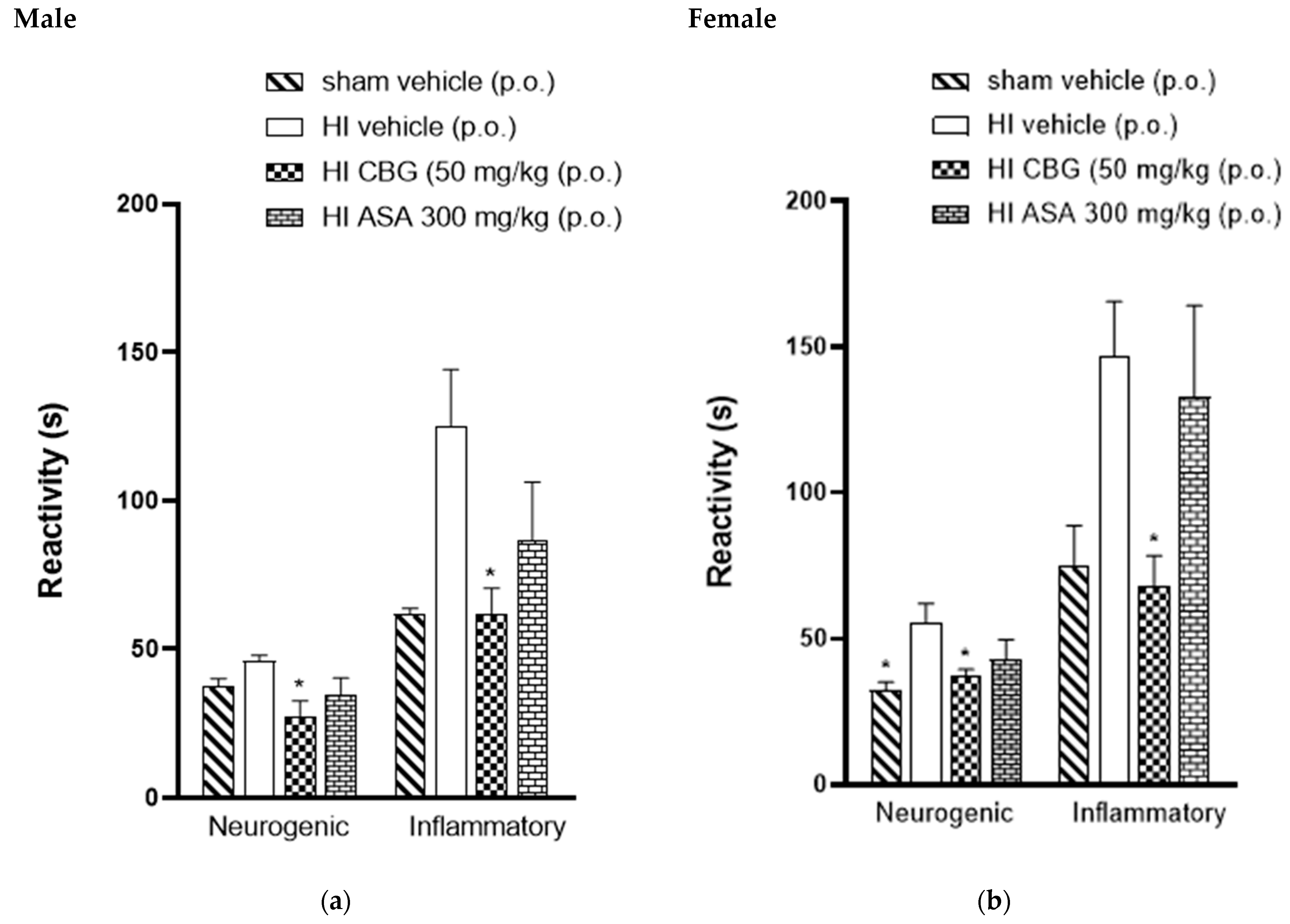
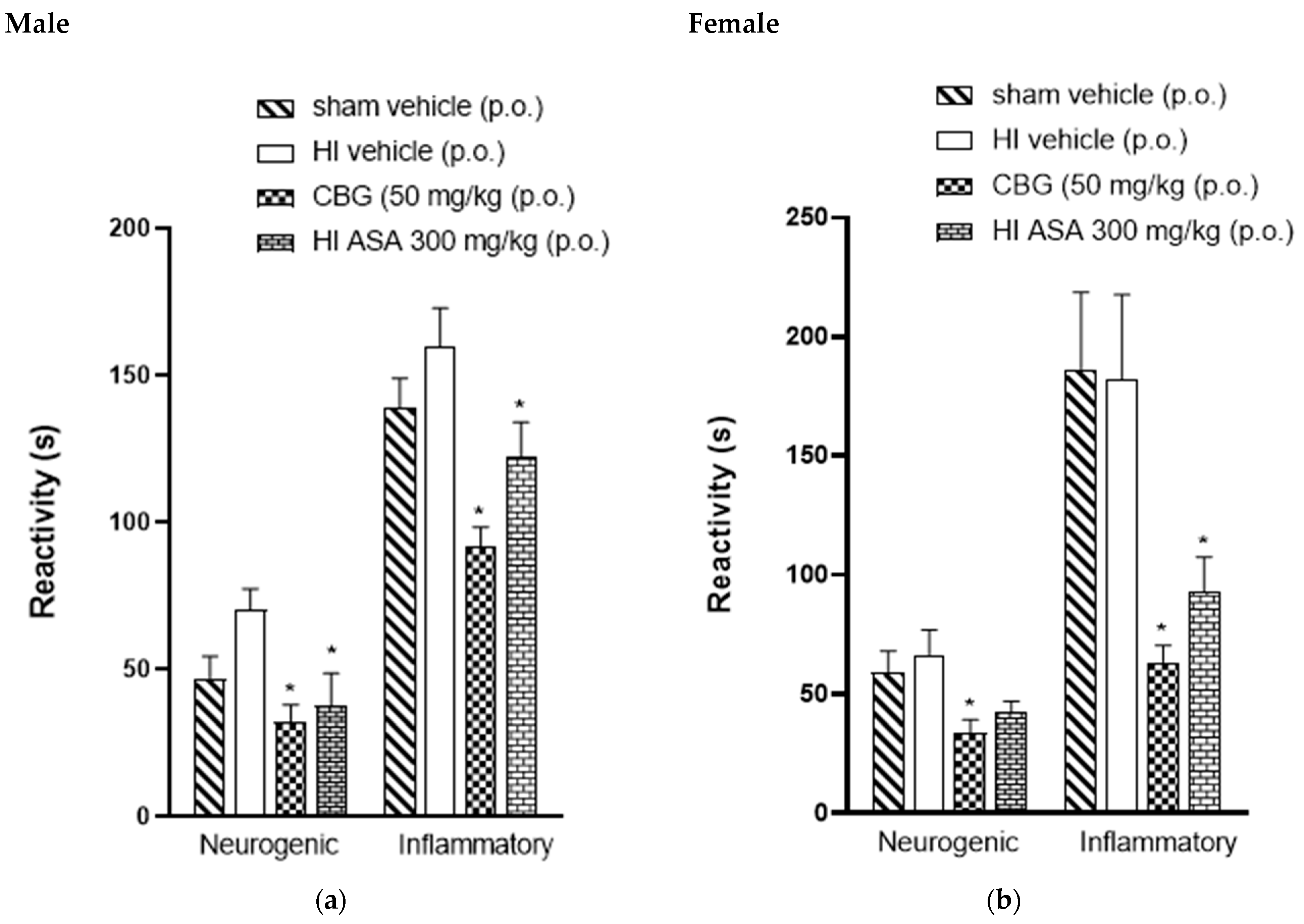
3.4. Thermal and Mechanical Hypernociception
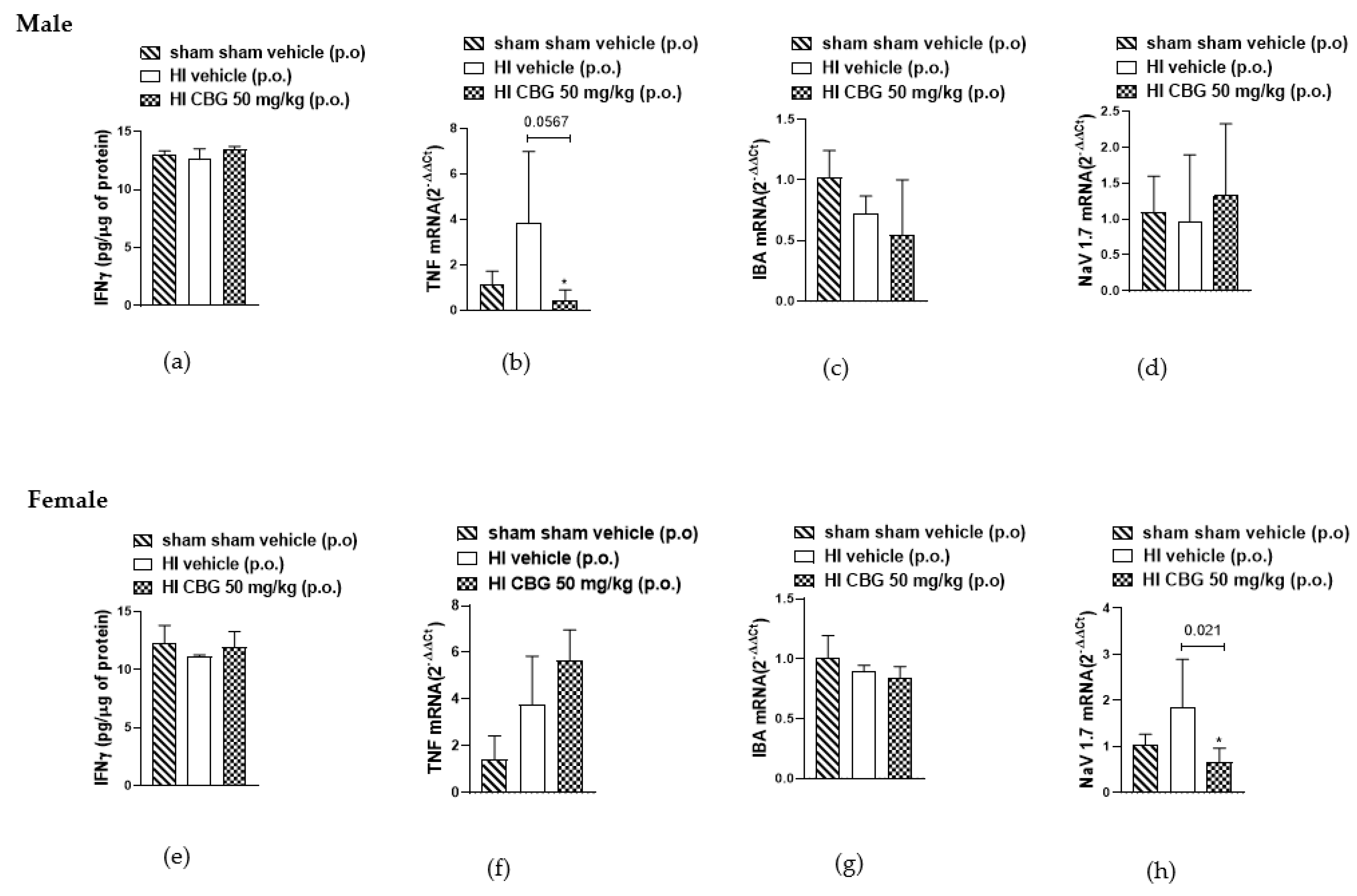
3.5. Evaluation of Inflammatory Cytokines
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolf, C.J. What is this thing called pain? J. Clin. Investig. 2010, 120, 3742–3744. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Woolf, C.J. Pain. Curr. Biol. 1999, 9, R429–R431. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.C.; Mullally, W.J. Pain Neurology. Am. J. Med. 2020, 133, 273–280. [Google Scholar] [CrossRef]
- Desantana, J.M.; Perissinotti, D.M.N.; Oliveira Junior, J.O.D.; Correia, L.M.F.; Oliveira, C.M.D.; Fonseca, P.R.B.D. Definition of pain revised after four decades. Braz. J. Pain. 2020, 3, 197–198. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Muley, M.M.; Krustev, E.; McDougall, J.J. Preclinical Assessment of Inflammatory Pain. CNS Neurosci. Ther. 2016, 22, 88–101. [Google Scholar] [CrossRef]
- Engidawork, E.; Chen, Y.; Dell’Anna, E.; Goiny, M.; Lubec, G.; Ungerstedt, U.; Andersson, K.; Herrera-Marschitz, M. Effect of perinatal asphyxia on systemic and intracerebral pH and glycolysis metabolism in the rat. Exp. Neurol. 1997, 145, 390–396. [Google Scholar] [CrossRef] [PubMed]
- de Haan, M.; Wyatt, J.S.; Roth, S.; Vargha-Khadem, F.; Gadian, D.; Mishkin, M. Brain and cognitive-behavioural development after asphyxia at term birth. Dev. Sci. 2006, 9, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Biarge, M.; Madero, R.; González, A.; Quero, J.; García-Alix, A. Perinatal morbidity and risk of hypoxic-ischemic encephalopathy associated with intrapartum sentinel events. Am. J. Obstet. Gynecol. 2012, 206, 148.e1–148.e7. [Google Scholar] [CrossRef]
- du Plessis, A.J.; Johnston, M.V. Hypoxic-ischemic brain injury in the newborn. Cellular mechanisms and potential strategies for neuroprotection. Clin. Perinatol. 1997, 24, 627–654. [Google Scholar] [CrossRef]
- Gross, J.; Andersson, K.; Chen, Y.; Müller, I.; Andreeva, N.; Herrera-Marschitz, M. Effect of perinatal asphyxia on tyrosine hydroxylase and D2 and D1 dopamine receptor mRNA levels expressed during early postnatal development in rat brain. Brain Res. Mol. Brain Res. 2005, 134, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Rumajogee, P.; Bregman, T.; Miller, S.P.; Yager, J.Y.; Fehlings, M.G. Rodent Hypoxia-Ischemia Models for Cerebral Palsy Research: A Systematic Review. Front. Neurol. 2016, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.S.; Cunha-Rodrigues, M.C.; Araujo, P.C.; de Almeida, O.M.; Barradas, P.C. Effects of prenatal hypoxia-ischemia on male rat periaqueductal gray matter: Hyperalgesia, astrogliosis and nitrergic system impairment. Neurochem. Int. 2023, 164, 105500. [Google Scholar] [CrossRef] [PubMed]
- Von Adamovich, G.M.G.; Bastos Torres, J.A.G.; Vianna, F.S.; Barradas, P.C.; Alves de Oliveira, B.F.; Villela, N.R.; De Rodrigues, M.C.C.; Montes, G.C. Evaluation of Pain Prevalence in Children Who Experienced Perinatal Hypoxia-Ischemia Events: Characteristics and Associations With Sociodemographic Factors. Cureus 2023, 15, e46359. [Google Scholar] [CrossRef] [PubMed]
- Collier, R. A short history of pain management. CMAJ 2018, 190, E26–E27. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Butler, D.S. Fifteen Years of Explaining Pain: The Past, Present, and Future. J. Pain. 2015, 16, 807–813. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Urits, I.; Charipova, K.; Gress, K.; Li, N.; Berger, A.A.; Cornett, E.M.; Kassem, H.; Ngo, A.L.; Kaye, A.D.; Viswanath, O. Adverse Effects of Recreational and Medical Cannabis. Psychopharmacol. Bull. 2021, 51, 94–109. [Google Scholar] [PubMed]
- Anand, P.; Whiteside, G.; Fowler, C.J.; Hohmann, A.G. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res. Rev. 2009, 60, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.R.; Ali, D.W. Pharmacology of Medical Cannabis. Adv. Exp. Med. Biol. 2019, 1162, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Bass, B.; Padwa, H.; Khurana, D.; Urada, D.; Boustead, A. Adult use cannabis legalization and cannabis use disorder treatment in California, 2010–2021. J. Subst. Use Addict. Treat. 2024, 162, 209345. [Google Scholar] [CrossRef]
- Pantoja-Ruiz, C.; Restrepo-Jimenez, P.; Castañeda-Cardona, C.; Ferreirós, A.; Rosselli, D. Cannabis and pain: A scoping review. Braz. J. Anesthesiol. 2022, 72, 142–151. [Google Scholar] [CrossRef]
- McPartland, J.M.; Glass, M.; Matias, I.; Norris, R.W.; Kilpatrick, C.W. A shifted repertoire of endocannabinoid genes in the zebrafish (Danio rerio). Mol. Genet. Genom. 2007, 277, 555–570. [Google Scholar] [CrossRef]
- Ndong, C.; O’Donnell, D.; Ahmad, S.; Groblewski, T. Cloning and pharmacological characterization of the dog cannabinoid CB₂ receptor. Eur. J. Pharmacol. 2011, 669, 24–31. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Wang, W.; Zhou, N. New Insights in Cannabinoid Receptor Structure and Signaling. Curr. Mol. Pharmacol. 2019, 12, 239–248. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef]
- Robaina Cabrera, C.L.; Keir-Rudman, S.; Horniman, N.; Clarkson, N.; Page, C. The anti-inflammatory effects of cannabidiol and cannabigerol alone, and in combination. Pulm. Pharmacol. Ther. 2021, 69, 102047. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Khatian, N.; Aslam, M. Effect of Ganoderma lucidum on memory and learning in mice. Clin. Phytoscience 2019, 5, 4. [Google Scholar] [CrossRef]
- Savignon, T.; Costa, E.; Tenorio, F.; Manhães, A.C.; Barradas, P.C. Prenatal hypoxic-ischemic insult changes the distribution and number of NADPH-diaphorase cells in the cerebellum. PLoS ONE 2012, 7, e35786. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Ho Kim, S.; Mo Chung, J. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]
- Lolignier, S.; Amsalem, M.; Maingret, F.; Padilla, F.; Gabriac, M.; Chapuy, E.; Eschalier, A.; Delmas, P.; Busserolles, J. Nav1.9 channel contributes to mechanical and heat pain hypersensitivity induced by subacute and chronic inflammation. PLoS ONE 2011, 6, e23083. [Google Scholar] [CrossRef]
- Ren, H.; Chen, X.; Tian, M.; Zhou, J.; Ouyang, H.; Zhang, Z. Regulation of Inflammatory Cytokines for Spinal Cord Injury Repair Through Local Delivery of Therapeutic Agents. Adv. Sci. 2018, 5, 1800529. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Jiang, C.; Andriessen, A.S.; Wang, K.; Wang, Z.; Ding, H.; Zhao, J.; Luo, X.; Lee, M.S.; Lei, Y.L.; et al. STING controls nociception via type I interferon signalling in sensory neurons. Nature 2021, 591, 275–280. [Google Scholar] [CrossRef]
- Ferrara, V.; Toti, A.; Ghelardini, C.; Di Cesare Mannelli, L. Interferon-gamma and neuropathy: Balance between pain and neuroprotection. Neural Regen. Res. 2022, 17, 2700–2701. [Google Scholar]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflamm. 2010, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Shiers, S.; Funk, G.; Cervantes, A.; Horton, P.; Dussor, G.; Hennen, S.; Price, T.J. NaV1.7 mRNA and protein expression in putative projection neurons of the human spinal dorsal horn. bioRxiv, 2023; preprint. [Google Scholar]
- Rainer, T.H.; Cheng, C.H.; Janssens, H.J.; Man, C.Y.; Tam, L.S.; Choi, Y.F.; Yau, W.H.; Lee, K.H.; Graham, C.A. Oral Prednisolone in the Treatment of Acute Gout: A Pragmatic, Multicenter, Double-Blind, Randomized Trial. Ann. Intern. Med. 2016, 164, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.; Croft, P.; Langford, R.M.; Donaldson, L.J.; Jones, G.T. Prevalence of chronic pain in the UK: A systematic review and meta-analysis of population studies. BMJ Open 2016, 6, e010364. [Google Scholar] [CrossRef]
- Taneja, A.; Della Pasqua, O.; Danhof, M. Challenges in translational drug research in neuropathic and inflammatory pain: The prerequisites for a new paradigm. Eur. J. Clin. Pharmacol. 2017, 73, 1219–1236. [Google Scholar] [CrossRef]
- Day, R.O.; Graham, G.G. The vascular effects of COX-2 selective inhibitors. Aust. Prescr. 2004, 27, 142–145. [Google Scholar] [CrossRef]
- Bally, M.; Dendukuri, N.; Rich, B.; Nadeau, L.; Helin-Salmivaara, A.; Garbe, E.; Brophy, J.M. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ 2017, 357, j1909. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Hsu, D.J.; McCarthy, E.P.; Stevens, J.P.; Mukamal, K.J. Hospitalizations, costs and outcomes associated with heroin and prescription opioid overdoses in the United States 2001-12. Addiction 2017, 112, 1558–1564. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Blanco, C.; Crane, E.; Lee, J.; Jones, C.M. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann. Intern. Med. 2017, 167, 293–301. [Google Scholar] [CrossRef]
- Corder, G.; Castro, D.C.; Bruchas, M.R.; Scherrer, G. Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci. 2018, 41, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems: A Clinical Review. JAMA 2015, 313, 2474–2483. [Google Scholar] [CrossRef] [PubMed]
- Piper, B.J.; Beals, M.L.; Abess, A.T.; Nichols, S.D.; Martin, M.W.; Cobb, C.M.; DeKeuster, R.M. Chronic pain patients’ perspectives of medical cannabis. Pain 2017, 158, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P.; Palastro, M.D.; Johnson, B.; Ditre, J.W. Cannabis and Pain: A Clinical Review. Cannabis Cannabinoid Res. 2017, 2, 96–104. [Google Scholar] [CrossRef]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef]
- Kogan, N.M.; Lavi, Y.; Topping, L.M.; Williams, R.O.; McCann, F.E.; Yekhtin, Z.; Feldmann, M.; Gallily, R.; Mechoulam, R. Novel CBG Derivatives Can Reduce Inflammation, Pain and Obesity. Molecules 2021, 26, 5601. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Evans, F.J. Cannabinoids: The separation of central from peripheral effects on a structural basis. Planta Med. 1991, 57, S60–S67. [Google Scholar] [CrossRef]
- Vogel, H.G. Drug Discovery and Evaluation: Pharmacological Assays; Springer: Berlin/Heidelberg, Germany, 2002; pp. 670–773. [Google Scholar]
- Wen, Y.; Wang, Z.; Zhang, R.; Zhu, Y.; Lin, G.; Li, R.; Zhang, J. The antinociceptive activity and mechanism of action of cannabigerol. Biomed. Pharmacother. 2023, 158, 114163. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Sepulveda, D.E.; Booth, J.L.; Zhou, S.; Graziane, N.M.; Raup-Konsavage, W.M.; Vrana, K.E. Chronic Cannabigerol as an Effective Therapeutic for Cisplatin-Induced Neuropathic Pain. Pharmaceuticals 2023, 16, 1442. [Google Scholar] [CrossRef]
- Blanton, H.L.; Barnes, R.C.; McHann, M.C.; Bilbrey, J.A.; Wilkerson, J.L.; Guindon, J. Sex differences and the endocannabinoid system in pain. Pharmacol. Biochem. Behav. 2021, 202, 173107. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Goodman, A.G.; Wiley, J.L.; Pondelick, A.M.; Craft, R.M. Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. J. Pharmacol. Exp. Ther. 2020, 373, 416–428. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Shulman, L.M.; Spritzer, M.D. Changes in the sexual behavior and testosterone levels of male rats in response to daily interactions with estrus females. Physiol. Behav. 2014, 133, 8–13. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain. 2009, 10, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, S.J.; Yang, L.; Fang, X.L.; Hu, H.F.; Zhao, M.Y.; Li, L.; Guo, Y.Y.; Shao, J.P. Voltage-gated sodium channel 1.7 expression decreases in dorsal root ganglia in a spinal nerve ligation neuropathic pain model. Kaohsiung J. Med. Sci. 2019, 35, 493–500. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, J.S.; Choi, D.K.; Koh, W.W.; Suh, J.H.; Song, J.G.; Shin, J.W.; Leem, J.G. Tumor Necrosis Factor-alpha and Apoptosis Following Spinal Nerve Ligation Injury in Rats. Korean J. Pain. 2011, 24, 185–190. [Google Scholar] [CrossRef]
- Cao, J.; Li, Z.; Zhang, Z.; Ren, X.; Zhao, Q.; Shao, J.; Li, M.; Wang, J.; Huang, P.; Zang, W. Intrathecal injection of fluorocitric acid inhibits the activation of glial cells causing reduced mirror pain in rats. BMC Anesthesiol. 2014, 14, 119. [Google Scholar] [CrossRef]
- Rhen, T.; Cidlowski, J.A. Anti-inflammatory mechanisms of glucocorticoids in chronic inflammatory diseases: A critical overview. J. Clin. Investig. 2005, 115, 1384–1392. [Google Scholar]
- Ren, K.; Dubner, R. Neuron-glia crosstalk gets serious: Role in pain hypersensitivity. Curr. Opin. Anaesthesiol. 2008, 21, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial proinflammatory cytokines mediate exaggerated pain states: Implications for clinical pain. Adv. Exp. Med. Biol. 2003, 521, 1–21. [Google Scholar] [PubMed]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001, 24, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Saika, F.; Kishioka, S. Involvement of inflammatory mediators in neuropathic pain caused by vincristine. Int. Rev. Neurobiol. 2009, 85, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kochukov, M.Y.; McNearney, T.A.; Yin, H.; Zhang, L.; Ma, F.; Ponomareva, L.; Abshire, S.; Westlund, K.N. Tumor necrosis factor-alpha (TNF-alpha) enhances functional thermal and chemical responses of TRP cation channels in human synoviocytes. Mol. Pain. 2009, 5, 49. [Google Scholar] [CrossRef]
- Sasaki, M.; Hashimoto, S.; Sawa, T.; Amaya, F. Tumor necrosis factor-alpha induces expression of C/EBP-beta in primary afferent neurons following nerve injury. Neuroscience 2014, 279, 1–9. [Google Scholar] [CrossRef]
- Hensellek, S.; Brell, P.; Schaible, H.G.; Bräuer, R.; Segond von Banchet, G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol. Cell Neurosci. 2007, 36, 381–391. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; He, H.; He, J.; Wang, J.; Li, X.; Wang, S.; Li, W.; Hou, J.; Liu, T.; et al. Sensitization of TRPV1 receptors by TNF-α orchestrates the development of vincristine-induced pain. Oncol. Lett. 2018, 15, 5013–5019. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Dib-Hajj, S.D.; Goodchild, S.J.; Ruben, P.C.; Waxman, S.G. Non-psychotropic phytocannabinoid interactions with voltage-gated sodium channels: An update on cannabidiol and cannabigerol. Front. Physiol. 2022, 13, 1066455. [Google Scholar] [CrossRef]
- Jan, T.R.; Farraj, A.K.; Harkema, J.R.; Kaminski, N.E. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol. Appl. Pharmacol. 2003, 188, 24–35. [Google Scholar] [CrossRef]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. Based Complement. Alternat Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Yang, Y.; Black, J.A.; Waxman, S.G. The Na(V)1.7 sodium channel: From molecule to man. Nat. Rev. Neurosci. 2013, 14, 49–62. [Google Scholar] [CrossRef]
- Rush, A.M.; Cummins, T.R.; Waxman, S.G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007, 579, 1–14. [Google Scholar] [CrossRef]
- Drenth, J.P.; Waxman, S.G. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J. Clin. Investig. 2007, 117, 3603–3609. [Google Scholar] [CrossRef]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007, 30, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Djouhri, L.; Newton, R.; Levinson, S.R.; Berry, C.M.; Carruthers, B.; Lawson, S.N. Sensory and electrophysiological properties of guinea-pig sensory neurones expressing Nav 1.7 (PN1) Na+ channel alpha subunit protein. J. Physiol. 2003, 546, 565–576. [Google Scholar] [CrossRef]
- Yang, S.W.; Ho, G.D.; Tulshian, D.; Bercovici, A.; Tan, Z.; Hanisak, J.; Brumfield, S.; Matasi, J.; Sun, X.; Sakwa, S.A.; et al. Bioavailable pyrrolo-benzo-1,4-diazines as Na(v)1.7 sodium channel blockers for the treatment of pain. Bioorg. Med. Chem. Lett. 2014, 24, 4958–4962. [Google Scholar] [CrossRef]
- McGowan, E.; Hoyt, S.B.; Li, X.; Lyons, K.A.; Abbadie, C. A peripherally acting Na(v)1.7 sodium channel blocker reverses hyperalgesia and allodynia on rat models of inflammatory and neuropathic pain. Anesth. Analg. 2009, 109, 951–958. [Google Scholar] [CrossRef]
- Kalezic, I.; Luo, L.; Lund, P.E.; Eriksson, A.B.; Bueters, T.; Visser, S.A. In vivo and ex vivo inhibition of spinal nerve ligation-induced ectopic activity by sodium channel blockers correlate to in vitro inhibition of NaV1.7 and clinical efficacy: A pharmacokinetic-pharmacodynamic translational approach. Pharm. Res. 2013, 30, 1409–1422. [Google Scholar] [CrossRef]
- Huang, J.; Fan, X.; Jin, X.; Jo, S.; Zhang, H.B.; Fujita, A.; Bean, B.P.; Yan, N. Cannabidiol inhibits Nav channels through two distinct binding sites. Nat. Commun. 2023, 14, 3613. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.-R.; Effraim, P.R.; Tyagi, S.; Zhao, P.; Dib-Hajj, S.D.; Waxman, S.G. Functionally-selective inhibition of threshold sodium currents and excitability in dorsal root ganglion neurons by cannabinol. Commun. Biol. 2024, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Kobayashi, K.; Noguchi, K. Laminae-specific distribution of alpha-subunits of voltage-gated sodium channels in the adult rat spinal cord. Neuroscience 2010, 169, 994–1006. [Google Scholar] [CrossRef] [PubMed]


| Forward Primer (5′->3′) | Reverse Primer (3′->5′) | |
|---|---|---|
| β-actina | CGTTGACATCCGTAAAGACCTC | TAGGAGCCAGGGCAGTAATCT |
| TNFα | TACTCCCAGGTTCTCTTCAAGG | GGAGGCTGACTTTCTCCTGGTA |
| Nav1.7 | CGATGGGTCACGATTTCCTAC | CGTGAAGAATGAGCCGAAGAT |
| Iba-1 | CGAATGCTGGAGAAACTTGG | GTTGGCTTCTGGTGTTCTTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezende, B.; Marques, K.L.; de Carvalho, F.E.A.; Gonçalves, V.M.d.S.; de Oliveira, B.C.C.A.; Nascimento, G.G.; dos Santos, Y.B.; Antunes, F.; Barradas, P.C.; Fontes-Dantas, F.L.; et al. Cannabigerol Reduces Acute and Chronic Hypernociception in Animals Exposed to Prenatal Hypoxia-Ischemia. Sci. Pharm. 2024, 92, 53. https://doi.org/10.3390/scipharm92030053
Rezende B, Marques KL, de Carvalho FEA, Gonçalves VMdS, de Oliveira BCCA, Nascimento GG, dos Santos YB, Antunes F, Barradas PC, Fontes-Dantas FL, et al. Cannabigerol Reduces Acute and Chronic Hypernociception in Animals Exposed to Prenatal Hypoxia-Ischemia. Scientia Pharmaceutica. 2024; 92(3):53. https://doi.org/10.3390/scipharm92030053
Chicago/Turabian StyleRezende, Bismarck, Kethely Lima Marques, Filipe Eloi Alves de Carvalho, Vitória Macario de Simas Gonçalves, Barbara Conceição Costa Azeredo de Oliveira, Gabriela Guedes Nascimento, Yure Bazilio dos Santos, Fernanda Antunes, Penha Cristina Barradas, Fabrícia Lima Fontes-Dantas, and et al. 2024. "Cannabigerol Reduces Acute and Chronic Hypernociception in Animals Exposed to Prenatal Hypoxia-Ischemia" Scientia Pharmaceutica 92, no. 3: 53. https://doi.org/10.3390/scipharm92030053
APA StyleRezende, B., Marques, K. L., de Carvalho, F. E. A., Gonçalves, V. M. d. S., de Oliveira, B. C. C. A., Nascimento, G. G., dos Santos, Y. B., Antunes, F., Barradas, P. C., Fontes-Dantas, F. L., & Montes, G. C. (2024). Cannabigerol Reduces Acute and Chronic Hypernociception in Animals Exposed to Prenatal Hypoxia-Ischemia. Scientia Pharmaceutica, 92(3), 53. https://doi.org/10.3390/scipharm92030053







